Abstract
Anoectochilus roxburghii was grown under different shade treatments–50%, 30%, 20%, and 5% of natural irradiance–to evaluate its photosynthetic characteristics, chloroplast ultrastructure, and physiology. The highest net photosynthetic rates and stomatal conductance were observed under 30% irradiance, followed in descending order by 20%, 5%, and 50% treatments. As irradiance decreased from 50% to 30%, electron transport rate and photochemical quenching increased, while non-photochemical quenching indexes declined. Reductions in irradiance significantly increased Chl a and Chl b contents and decreased Chl a/b ratios. Chloroplast ultrastructure generally displayed the best development in leaves subjected to 30% irradiance. Under 50% irradiance, leaf protein content remained relatively stable during the first 20 days of treatment, and then increased rapidly. The highest peroxidase and superoxide dismutase levels, and the lowest catalase activities, were observed in plants subjected to the 50% irradiance treatment. Soluble sugar and malondialdehyde contents were positively correlated with irradiance levels. Modulation of chloroplast development, accomplished by increasing the number of thylakoids and grana containing photosynthetic pigments, is an important shade tolerance mechanism in A. roxburghii.
Introduction
Anoectochilus roxburghii, a member of the Orchidaceae, is a valued plant species in many Asian countries, where it is used for ornamental, culinary, and medicinal purposes. Because of its unique medicinal properties, such as its notable curative effects of clearing heat and cooling blood, eliminating dampness, and detoxification, A. roxburghii has been called “the king of medicine” [1], [2]. Recent research has demonstrated that the entire plant possesses medicinal properties, such as antioxidant, anti-inflammatory, and antitumor activities [3], [4]. A. roxburghii has been traditionally harvested mainly from wild populations. The species has become endangered, however, as a result of human overexploitation coupled with its specific environmental growth requirements. As a consequence, artificial cultivation is beginning to be carried out in various locations in China.
Light has long been known to be the most important factor influencing plant growth, with changes in irradiance having impacts on plant growth, morphology, and anatomy, various aspects of physiology and cellular biochemistry, and, ultimately, flowering time and plant productivity [5]–[7]. In the light reactions of photosynthesis, light energy is used to produce ATP and NADPH, which are then used for carbon fixation to carbohydrates and production of oxygen during the light-independent phase. Shading effects are not just about the plants’ growth and development, but through which, it also has a major impact on plant photosynthesis. Normal plant growth needs optimal light irradiance because excessively high and low irradiances would result in photoinhibition and light deficiency respectively, and therein the growth of plant was restricted severely. Under high irradiance conditions, photoinhibition takes place: the photosynthetic apparatus absorbs excessive light energy, resulting in the inactivation or impairment of the chlorophyll-containing reaction centers of chloroplasts and consequent depression of photosynthetic activity [8], [9]. In contrast, under low irradiance conditions, insufficient ATP is produced to allow for carbon fixation and carbohydrate biosynthesis. This leads to a reduction in plant growth.
Chloroplasts are the sole organelles of photosynthesis. Although many authors have reported a close relationship among photosynthesis, chlorophyll content, and chlorophyll fluorescence in different species under shading conditions, little work has been performed on the association between photosynthesis-related parameters and chloroplast ultrastructure and physiology [10]. A comprehensive understanding of changes in chloroplast ultrastructure and physiology during leaf development under different irradiance conditions is needed.
The objectives of the present study were to quantify the influences of different shading levels on photosynthetic characteristics, chloroplast ultrastructure, and physiology of A. roxburghii, to determine optimum light intensity for plant growth, and to consequently expand our understanding of its shade-tolerance abilities and mechanisms. In addition, we wished to establish a sound foundation for improving the cultivation and breeding of this important plant species.
Materials and Methods
Plant Materials and Growth Conditions
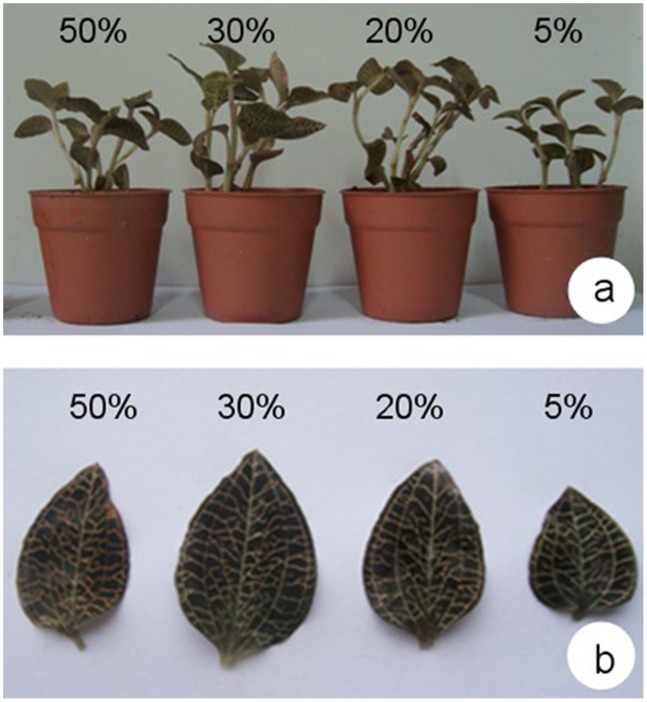
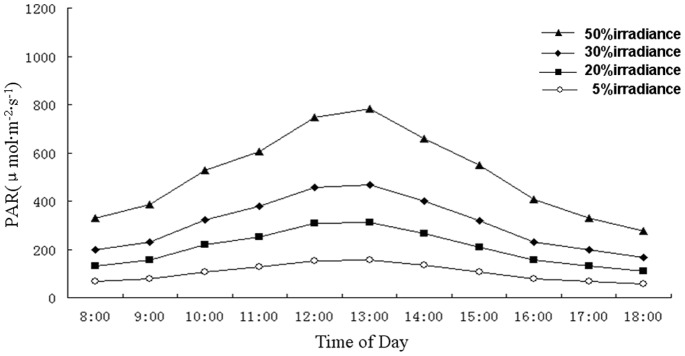
A. roxburghii plants were obtained from Lin’an commercial plantations and were maintained in a greenhouse at the Baicaoyuan test site of Zhejiang A & F University, China (30°15′N, 119°43′E). The photoperiod (day/night) and air relative humidity in the greenhouse were 14/10 h and 75% respectively. Plants were subjected to four different shade treatments for 40 days, beginning on June 1, 2012. Treatments consisted of 50%, 30%, 20%, or 5% natural irradiance, and were conducted in net houses (2.5 m high, 3 m long, and 2 m wide) covered with one or two layers of commercial plastic shading nets. Each treatment involved 10 pots with three replications (Figure 1). Diurnal variations of photosynthetically active radiation (400–700 nm wavelengths) were measured under all shade treatments with a TES-1332 digital lux meter (TES, Taiwan). Recorded data are displayed in Figure 2. All plants were kept well-irrigated and protected from bacterial pathogens and weed competition.
Figure 1. The appearance of whole plants (a) and leaves (b) exposed to 40 d of various levels of shading.
Figure 2. Curves of diurnal variation of photosynthetically active radiation (PAR) under 50%, 30%, 20% and 5% light irradiances during one day in June 2012 in Lin’an, China.
Photosynthetic Parameters
Photosynthetic parameters were investigated using a LI-6400XT CO2/H2O porometer (Li-Cor, Lincoln, NE, USA). The parameters measured were net photosynthetic rate (Pn, µmol m−2 s−1), stomatal conductance (Gs, mmol m−2 s−1), and intercellular CO2 concentration (Ci, µmol mol−1). During the treatment period, data were recorded between 9∶30 and 11∶30 am on days 0, 10, 20, 30, and 40. Air cuvette irradiance, temperature, and CO2 concentration were maintained at 1000 µmol m−2 s−1, 30°C and 380 µmol l−1 respectively, and the assimilation was recorded following a 10 min acclimation period [5]. At each conducted time point, five representative plants were randomly selected from each treatment and analyzed for the above parameters.
Chlorophyll Fluorescence
Chlorophyll fluorescence of the same leaves used for determination of photosynthetic parameters was measured with a MINIPAM fluorometer (Walz, Effeltrich, Germany). Leaves were light-adapted for approximately 15 min prior to measurements of effective quantum yield of photochemical energy conversion (Yield) and photochemical (qP) and nonphotochemical (NPQ) quenching of chlorophyll fluorescence. The effective quantum yield of photochemical energy conversion at steady-state photosynthesis was calculated as Yield = (Fm′ − Fs)/Fm′, where Fs and Fm′ were fluorescence at steady-state photosynthesis and maximum fluorescence in the light, respectively. qP was calculated as (Fm − Fm′)/(Fm′ − F0), and NPQ was calculated as (Fm − Fm′)/Fm′ [32]. The relative rate of electron transport through PSII (ETR) was calculated as Yield × photosynthetically active radiation × 0.84 × 0.5 [33].
Chlorophyll Content
Following measurement of chlorophyll fluorescence as described above, leaves were collected for determination of chlorophyll content (Chl a, Chl b, and Chl a+b). Chlorophyll pigments were extracted by grinding leaves in 80% acetone in the dark at room temperature, with their concentrations expressed as mg g−1 FW based on the equations of Porra [34].
Chloroplast Ultrastructure
To examine chloroplast ultrastructure of mesophyll cells, sampled leaves described above were immediately fixed in 2.5% (v/v) glutaraldehyde (0.1 mol l−1 phosphate buffer, pH 7.2) for at least 48 h after removal from plants. The samples were then immersed in 1% (v/v) osmium acid for post-fixation, resin embedding, and ultrathin sectioning for transmission electron microscopy (H7650, Hitachi, Tokyo, Japan).
Physiological and Biochemical Assays
Approximately 0.5 g leaf samples were collected on treatment days 0, 10, 20, 30, and 40 frozen immediately at −80°C. Protein content was determined based on the Bradford method [35], with bovine serum albumin employed as a standard. Crude enzyme preparation for superoxide dismutase (SOD) and peroxidase (POD) assays involved tissue homogenization in 5 ml of 100 mM potassium phosphate buffer (pH 7.0) following the procedure of He et al. [36]. Assays used for POD, SOD, and catalase (CAT) were those described by Argandona et al. [37] and Yin et al. [38]. Soluble sugar (SS) content was determined using anthrone colorimetry as described by Li [39], and malondialdehyde (MDA) content was measured by the thiobarbituric acid method according to Deng et al. [40].
Statistical Analysis
One-way analysis of variance (ANOVA) was carried out using SPSS software version 16.0 (SPSS, Chicago, IL, USA). Duncan’s multiple range test was employed to detect differences between means (with P set to 0.05).
Results
Photosynthesis
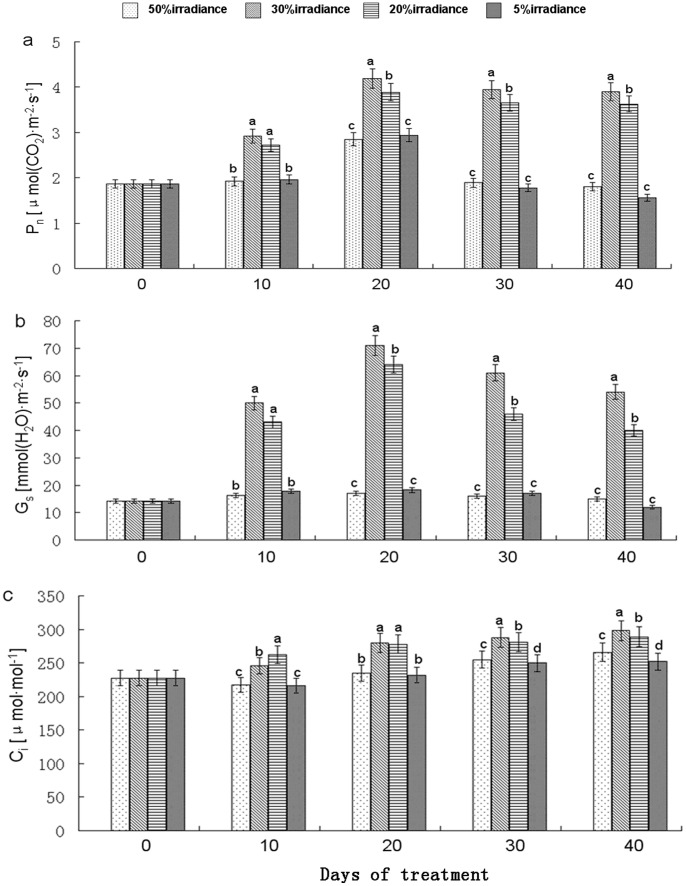
Pn values of A. roxburghii varied significantly (P<0.05) among light intensities and treatment periods (Figure 3a). The Pn value was always highest at 30% irradiance, followed (in descending order) at most time points by 20%, 5%, and 50% irradiance treatments. Pn values significantly increased during the first 20 days of treatment, with the highest Pn value measured at 30% irradiance on day 20. Subtle changes were observed within treatments between days 30 and 40, but Pn values under 50% irradiance were always higher than those under 5% irradiance.
Figure 3. Net photosynthetic rate (Pn) (a), stomatal conductance (Gs) (b) and intercellular CO2 concentration (Ci) (c).
The values represented mean ± SE, and different letters mark significant differences among shade treatments on the same day (P<0.05).
Gs values varied significantly among various light levels and exposure periods (Figure 3b). Gs values of plants subjected to 50% and 5% irradiance were always lower than those of 30%- and 20%-irradiance treated plants; on days 10 to 30, 50%-irradiance treated plants always exhibited the lowest values. The highest Gs values under 30%, 20%, and 5% irradiance were observed on the 20th day of treatment.
Ci values displayed slight increases over time (Figure 3c). On treatment day 10, Ci values from the 20% irradiance treatment were higher than those from other treatments. The highest values, however, were observed under 30% irradiance on days 20–40, and were higher than values measured during other treatments. Values measured from plants under 5% irradiance treatment were always the lowest.
Chlorophyll Fluorescence
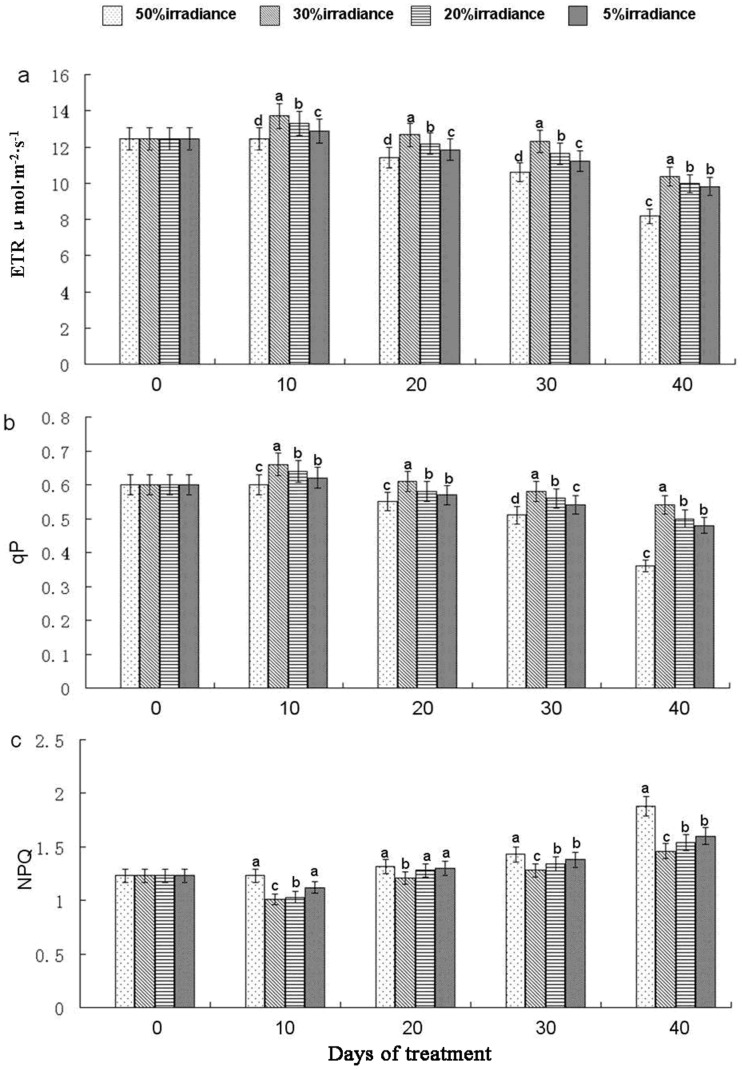
The 50% irradiance treatment resulted in a significant (P<0.05) reduction in apparent ETR and qP, and an increase in NPQ on the 40th day of treatment (Figure 4a–c). On day 10, the highest ETR value was recorded in leaves under 30% irradiance, while the lowest was found in plants grown under 50% irradiance. The trend observed for qP upon variation in light intensities and treatment times was similar to that of ETR. The highest and lowest qP values were observed in plants under 30% and 50% irradiance treatments, respectively. The highest NPQ values were always measured in 50% irradiance plants.
Figure 4. Electron transport rate (ETR) (a), photochemical quenching (qP) (b) and nonphotochemical quenching (NPQ) (c).
The values represented mean ± SE, and different letters mark significant differences among shade treatments on the same day (P<0.05).
Chlorophyll Contents
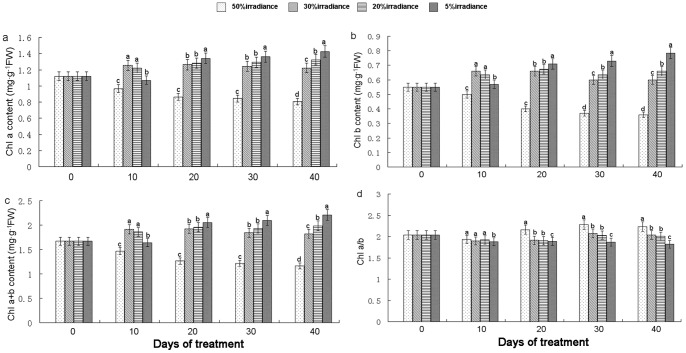
Chlorophyll content was significantly affected by different light intensities (Figure 5). Chl a and Chl b contents were increased and Chl a/b was decreased on days 20–40 of reduced irradiance treatments. The highest Chl a, Chl b, and Chl a+b contents were observed in 5%-treated plants on day 40. The shade treatments caused a decrease in Chl a/b over time, with the highest Chl a/b values observed in plants under 50% irradiance on day 40.
Figure 5. The Chl a content (a), Chl b content (b), Chl a+b content (c) and Chl a/b (d).
The values represented mean ± SE, and different letters mark significant differences among shade treatments on the same day (P<0.05).
Chloroplast Ultrastructure
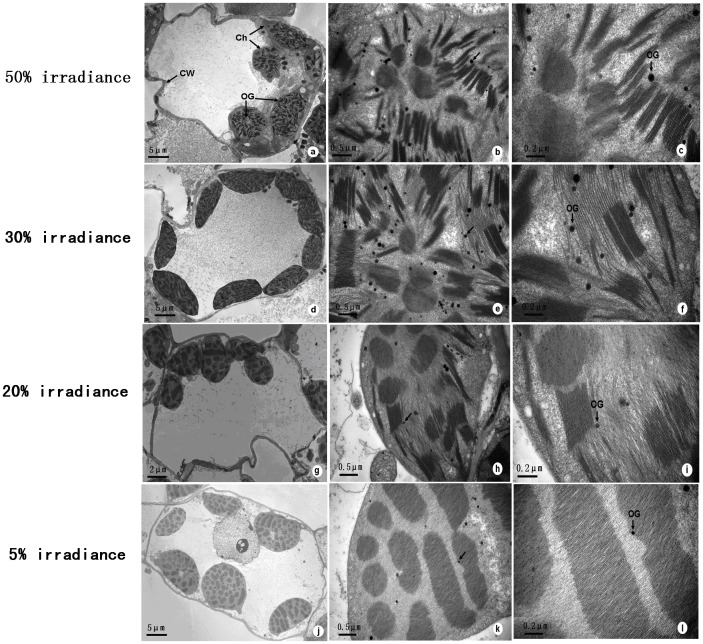
Chloroplast sizes and numbers were obviously influenced by light levels of A. roxburghii (Figure 6). The number of chloroplasts, grana, and grana lamellae generally increased as light irradiance was reduced. Most chloroplasts in leaves grown under 30% and 20% irradiance conditions exhibited normal ultrastructural organization, possessing a typical arrangement of grana and stroma thylakoids. Grana of plants grown under 30%, 20%, and 5% shading generally contained more thylakoids than those from plants grown under 50% irradiance. In addition, the number and size of osmiophilic globules was reduced in plants under 30% and 20% shading treatments compared with those from leaves grown under 50% and 5% treatments.
Figure 6. The chloroplast ultrastructure observed in the leaves of Anoectochilus roxburghii at 40 DOT.
(a), (b), (c) the plants under 50% irradiance treatment; (d), (e), (f) the plants under 30% irradiance treatment; (g), (h), (i) the plants under 20% irradiance treatment; (j), (k), (l) the plants under 5% irradiance treatment. Notice the differences of the number of SG (indicated by arrow heads) and the number of grana lamella (indicated by arrows) between different irradiances. Abbreviation: Ch, chloroplast; CW, cell wall; OG, osmiophilic globules.
Physiological and Biochemical Assays
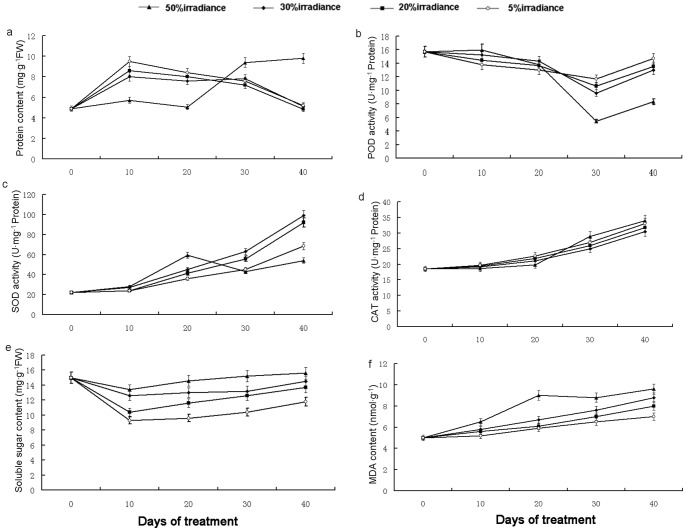
Under 50% irradiance conditions, leaf protein content remained relatively stable during the first 20 days of treatment, but increased rapidly thereafter. The other shaded plants reacted rather differently, however: during the first 10 days of treatment, leaf protein content increased, and subsequently declined (Figure 7a). A pronounced fall in leaf POD activity occurred after 20 days of treatment. On days 30–40, POD activity in 50% irradiance plants was significantly lower than in other shade-treated plants. Up to day 20, SOD activity in 50% irradiance plants was higher than in other shade-grown plants (Figure 5c). By day 40, relative SOD levels were reversed, with the highest expression (at 30% irradiance) being approximately double that of the 50% irradiance treatment. Shading had little influence on CAT activity up to the 20th day of treatment, but significant increases were observed thereafter. Up to day 20, 50% irradiance plants displayed the lowest CAT activity; on days 30–40, however, they exhibited the highest CAT activity levels. SS contents were positively correlated with the irradiance levels (Fig. 7e). SS content decreased at all levels of shade treated plants. Similarly, the response of MDA contents were also positively correlated with the irradiance levels. But its contents increased at all levels of treatments.
Figure 7. Protein content (a), POD activity (b), SOD activity (c), CAT activity (d), Soluble sugar content (e) and MDA content (f).
Discussion
Photosynthetic Response of A. roxburghii to Shading
Ci changed only slightly in A. roxburghii grown under different shade treatments, with the data suggesting that CO2 concentration was not the main factor reducing photosynthetic rate in leaves of plants under 50% and 5% irradiance treatments (Figure 3c). At the same time, values of Gs decreased significantly in plants grown under these treatments (Figure 3b, d). Under the high light environment, the observed reductions in Gs indicate that stomatal closure was due to light saturation and functioned to decrease water loss. When net CO2 assimilation became light saturated, transpiration constantly decreased with the declined photosynthetic photon flux density [11]. Thus under 5% irradiance treatment, plants also exhibit a similar behaviour as that in high light condition, that is, closing stomata due to water saturation to adapt to low light.
Chlorophyll Fluorescence Response of A. roxburghii to Shading
Chlorophyll fluorescence measurement is a mainstay of studies of photosynthetic regulation and plant responses to the environment because of its sensitivity, convenience, and nonintrusive characteristics [12], [13]. ETR represents the relative quantity of electrons passing through PSII during steady-state photosynthesis [14]. Exposure to the high irradiance conditions of 50% irradiance resulted in a greatly reduction in ETR value (Fig. 4a). Reductions in ETR may be due to the loss of chlorophyll via and the reduction in the efficiency of excitation capture, which most likely as a result of photoinhibition [15]. qP is an indicator of the proportion of open PSII reaction centers [16]. A high qP is advantageous for the separation of electric charge in the reaction center, and is also beneficial to electron transport and PSII yield [17], [18]. In this experiment, observed differences in qP values revealed that A. roxburghii had significant differences in PSII electron transport activities when plants were grown under varied shade treatments. Electric charge separation in the reaction center, electron transport ability, and quantum yield of PSII were enhanced under 30% irradiance and weakened under 50% irradiance. NPQ is a reflection of the amount of unused energy from photosynthetic electron transport that is dissipated harmlessly as heat energy from PSII antennae [19], [20]. The low NPQ measured for 30% irradiance treatment plants indicates that these plants were able to effectively reduce irradiance heat and efficiently utilize the energy absorbed by PSII antenna pigments [17]. In contrast, the high NPQ observed in plants under 50% irradiance demonstrates that the energy absorbed in the physiologically usable range of light was much higher than the quantity photochemically usable, which would cause inhibition of photosynthetic capacity.
Chlorophyll Content Response of A. roxburghii to Shading
Leaf chlorophyll content is an important determinant of photosynthetic rate [18] and dry matter production [21]. Naidu et al. [22] have suggested that reduced rates of photosynthesis may be due to reduced levels of chlorophyll, particularly Chl a, which is more directly involved in determining photosynthetic activity [23]. Decreases in Chl a and Chl b contents may thus reflect destruction of pigments by excessive irradiance. We observed significant (P<0.05) decreases in chlorophyll contents (Chl a, Chl b, and Chl a+b) under 50% irradiance conditions, suggesting that high irradiance may seriously impair the photosynthetic system. Plants grown under shaded conditions are known to optimize their light absorption efficiency by increasing pigment density per unit leaf area [24]. The reductions we observed in Chl a/b ratios in leaves of 30%, 20% and 5% irradiance plants were due primarily to significant (P<0.05) increases in Chl b content, most likely as a result of changes in the organization of both light harvesting and electron transport components [25]. The marked increase in leaf Chlorophyll content under 5% irradiance conditions demonstrates the ability of plants to maximize their light-harvesting capacity under low-light growth conditions [26].
Chloroplast Ultrastructure Response of A. roxburghii to Shading
Leaves from 30%, 20%, and 5% shade treatments possessed grana containing more thylakoids than those of leaves grown under high (50%) irradiance conditions, and, as a consequence, had higher photosynthetic rates and pigment contents. Leaves grown in 30% irradiance environments exhibited better-developed chloroplasts, grana, and stroma lamellae. This result implies that 30% irradiance treatments were somewhat conducive to plant growth. Modulation of chloroplast development through increases in numbers of thylakoids, grana, and grana lamellae may be an important shade-tolerance mechanism in A. roxburghii. The number and size of osmiophilic globules can also be used as an indicator of photosynthetic efficiency [27], [28]. Chloroplasts in leaves of 30%- and 20%-treated plants contained the fewest and smallest osmiophilic globules; this result also suggests that moderate shading, to some extent, was beneficial, whereas 50% and 5% shade treatments were harmful to plant growth.
Physiological and Biochemical Response of A. roxburghii to Shading
Performances of leaf protein content and antioxidant enzyme activities revealed that these traits were under strong genetic control, whereas SS and MDA contents were largely determined by the degree of shading. Exposure to high (50%) irradiance conditions greatly increased total protein content. Activity profiles of the various antioxidant enzymes were not uniform. After 40 days of shading, POD and SOD levels were significantly higher in 30%, 20%, and 5% irradiance plants than in 50% irradiance plants, whereas CAT activity remained lower. During these experiments, we carefully maintained consistent moisture availability and temperature conditions. Consequently, it is clear that POD, SOD, and CAT activity levels were not only markedly affected by the degree of imposed shading, but were also heavily influenced by the developmental status of the experimental plants. As SSs are an important carbon source and osmoregulator of plant growth, SS levels reflect plant nutritional status [29]. In our study, plant SS content was lowest under high irradiance treatments, and was responsive to shading severity. This result suggests that A. roxburghii responds differently to light with respect to carbohydrate metabolism. MDA content is considered to be an indicator of cellular membrane lipid peroxidation [30], [31]. The significantly high MDA content observed in our study under high irradiance indicates the occurrence of damage due to excessive irradiance.
Conclusions
Our study, which measured photosynthetic characteristics associated with light stress sensitivity in A. roxburghii, has contributed to an understanding of this species’ shade tolerance. Our results demonstrate that shading is necessary for its normal growth, although different degrees and durations of shading treatments significantly influence photosynthetic activity, chlorophyll content, chlorophyll fluorescence, chloroplast ultrastructure, and physiological and biochemical indexes. Plants subjected to 50% irradiance conditions suffer photoinhibition because of excess light exposure, whereas those grown under 5% irradiance suffer from light deficiency. A. roxburghii adapts to shade conditions through increased levels of chloroplasts, grana, and grana lamellae, and higher POD and SOD activitities.
Acknowledgments
We thank Dr. Jie Zhang for assistance in improving the written English. In addition, we express our gratitude to the anonymous reviewers who carefully reviewed our manuscript and put forth many valuable suggestions.
Funding Statement
This work was supported by the National Natural Science Foundation of China (Grant No. 81303167), the Zhejiang Provincial Natural Science Foundation (Grant No. LY13H280010) and the National College Students’ innovation and entrepreneurship training program (Grant No. 201310341020). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Zhang YH, Cai JY, Ruan HL, Pi HF, Wu JZ (2007) Antihyperglycemic activity of kinsenoside, a high yielding constituent from Anoectochilus roxburghii in streptozotocin diabetic rats. J Ethnopharm 114: 141–145. [DOI] [PubMed] [Google Scholar]
- 2. Du XM, Nobuto I, Norihiro F, Jun HS, Shoyama YH (2008) Pharmacologically active compounds in the Anoectochilus and Goodyera species. J Nat Med-Tokyo 62: 132–148. [DOI] [PubMed] [Google Scholar]
- 3. He CN, Wang CL, Guo SX, Xiao PG (2004) Advances on chemical compositions and pharmacological studies of Anoectochilus Blume (Orchidaceae). Chinese Pharm J 39: 81–84. [Google Scholar]
- 4. Zhang HY, Pan X (2009) Advances on chemical compositions and pharmacological studies of Anoectochilus roxburghii (Wall.) Lindl. Strait Pharm J 29: 82–84. [Google Scholar]
- 5. Dai YJ, Shen ZG, Liu Y, Wang LL, Hannaway D, et al. (2009) Effects of shade treatments on the photosynthetic capacity, chlorophyll fluorescence, and chlorophyll content of Tetrastigma hemsleyanum Diels et Gilg. Environ Exp Bot 65: 177–182. [Google Scholar]
- 6. Favaretto VF, Martinez CA, Soriani HH, Furriel RPM (2011) Differential responses of antioxidant enzymes in pioneer and late-successional tropical tree species grown under sun and shade conditions. Environ Exp Bot 70: 20–28. [Google Scholar]
- 7. Deng YM, Li CC, Shao QS, Ye XQ, She JM (2012) Differential responses of double petal and multi petal jasmine to shading: I. Photosynthetic characteristics and chloroplast ultrastructure. Plant Physiol Bioch 55: 93–102. [DOI] [PubMed] [Google Scholar]
- 8. Bertaminia M, Muthuchelianb K, Rubinigga M, Zorera R, Velascoa R, et al. (2006) Low-night temperature increased the photoinhibition of photosynthesis in grapevine (Vitis vinifera L. cv. Riesling) leaves. Environ Exp Bot 57: 25–31. [Google Scholar]
- 9. Chen THH, Murata N (2011) Glycinebetaine protects plants against abiotic stress: mechanisms and biotechnological applications. Plant Cell Environ 34: 1–20. [DOI] [PubMed] [Google Scholar]
- 10. Raveh E, Nerd A, Mizrahi Y (1998) Responses of two hemiepiphytic fruit crop cacti to different degrees of shade. Sci Hortic-Amsterdam 73: 151–164. [Google Scholar]
- 11. Schapendonk AHCM, Dijkstra P, Groenwold J, Pot CS, Van de Geijn S (1997) Carbon balance and water use efficiency of frequently cut Lolium perenne L. swards at elevated carbon dioxide. Global Change Biol 3: 207–216. [Google Scholar]
- 12.Schreiber U, Bilger W, Neubauer C (1995) Chlorophyll florescence as a nonintrusive indicator for rapid assessment of in vivo photosynthesis. In: Schulze, E.D., Caldwell, M.M. (Eds.), Ecophysiology of Photosynthesis. Springer-Verlag, Berlin, 49–70.
- 13. Rascher U, Liebig M, Lüttge U (2000) Evaluation of instant light-response curves of chlorophyll fluorescence parameters obtained with a portable chlorophyll. Plant Cell Environ 23: 1397–1405. [Google Scholar]
- 14. Tezara W, Martianez D, Rengifo E, Herrera A (2003) Photosynthetic responses of the tropical spiny shrub Lycium nodosum (Solanaceae) to drought, soil salinity and saline spray. Ann Bot-London 92: 757–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Flowers MD, Fiscus EL, Burkey KO, Booker FL, Dubois JJB (2007) Photosynthesis, chlorophyll fluorescence, and yield of snap bean (Phaseolus vulgaris L.) genotypes differing in sensitivity to ozone. Environ Exp Bot 61: 190–198. [Google Scholar]
- 16. Maxwell K, Johnson GN (2000) Chlorophyll fluorescence-a practical guide. J Exp Bot 51: 659–668. [DOI] [PubMed] [Google Scholar]
- 17. Guo HX, Liu WQ, Shi YC (2006) Effects of different nitrogen forms on photosynthetic rate and the chlorophyll fluorescence induction kinetics of flue-cured tobacco. Photosynthetica 44: 140–142. [Google Scholar]
- 18. Mao LZ, Lu HF, Wang Q, Cai MM (2007) Comparative photosynthesis characteristics of Calycanthus chinensis and Chimonanthus praecox . Photosynthetica 45: 601–605. [Google Scholar]
- 19. Muller P, Li XP, Niyogi KK (2001) Non-photochemical quenching. A response to excess light energy. Plant Physiol 125: 1158–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Veres S, Tóth VR, Láposi R, Oláh V, Lakatos G, et al. (2006) Carotenoid composition and photochemical activity of four sandy grassland species. Photosynthetica 44: 255–261. [Google Scholar]
- 21. Ghosh PK, Bandyopadhyay KK, Manna MC, Mandal KG, Misra AK, et al. (2004) Comparative effectiveness of cattle manure, poultry manure, phosphocompost and fertilizer-NPK on three cropping systems in vertisols of semi-arid tropics. II. Dry matter yield, nodulation, chlorophyll content and enzyme activity. Bioresource Technol 95: 85–93. [DOI] [PubMed] [Google Scholar]
- 22. Naidu RA, Krishnan M, Nayudu MV, Gnanam A (1984) Studies on peanut green mosaic virus infected peanut (Arachis hypogaea L.) leaves. II. Chlorophyll-protein complexes and polypeptide composition of thylakoid membranes. Physiol Plant Pathol 25: 191–198. [Google Scholar]
- 23. Sestak Z (1996) Liminations for finding linear relationship between chlorophyll content and photosynthetic activity. Biol Plantarum 8: 336–346. [Google Scholar]
- 24. Wittmann C, Aschan G, Pfanz H (2001) Leaf and twig photosynthesis of young beech (Fagus sylvatica) and aspen (Populus tremula) trees grown under different light regime. Basic Appl Ecol 2: 145–154. [Google Scholar]
- 25. Schiefthaler U, Russell AW, Bolhàr-Nordenkampf HR, Critchley C (1997) Photoregulation and photodamage in Schefflera arboricola leaves adapted to different light environments. Aust J Plant Physiol 26: 485–494. [Google Scholar]
- 26. Lei TT, Tabuchi R, Kitao M, Koike T (1996) The functional relationship between chlorophyll content, leaf reflectance, and light capturing efficiency of Japanese forest species under natural shade and open light regimes. Physiol Plantarum 96: 411–418. [Google Scholar]
- 27. Helle G, Schleser GH (2004) Beyond CO2-fixation by Rubisco–an interpretation of 13C/12C variations in tree rings from novel intra-seasonal studies on broad-leaf trees. Plant Cell Environ 27: 367–380. [Google Scholar]
- 28. Liu YB, Li XR, Liu ML, Cao B, Tan HJ, et al. (2012) Responses of three different ecotypes of reed (Phragmites communis Trin.) to their natural habitats: leaf surface micro- morphology, anatomy, chloroplast ultrastructure and physio-chemical characteristics. Plant Physiol Bioch 51: 159–167. [DOI] [PubMed] [Google Scholar]
- 29. Dong CJ, Wang XL, Shang QM (2011) Salicylic acid regulates sugar metabolism that confers tolerance to salinity stress in cucumber seedlings. Sci Hortic-Amsterdam 129: 629–636. [Google Scholar]
- 30. Sharma P, Dubey RS (2007) Involvement of oxidative stress and role of antioxida-tive defense system in growing rice seedlings exposed to toxic concentrations of aluminum. Plant Cell Rep 26: 2027–2038. [DOI] [PubMed] [Google Scholar]
- 31. Deng YM, Chen SM, Chen FD, Cheng X, Zhang F (2011) The embryo rescue derived intergeneric hybrid between chrysanthemum and Ajania przewalskii shows enhanced cold tolerance. Plant Cell Rep 30: 2177–2186. [DOI] [PubMed] [Google Scholar]
- 32. Genty B, Briantais JM, Baker NR (1989) The relationship between quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Bba-Gen Subjects 990: 87–92. [Google Scholar]
- 33. Schreiber U, Gademann R, Ralph PJ, Larkum AWD (1997) Assessment of photosynthetic performance of Prochloron in Lissoclinum patella in hospite by chlorophyll fluorescence measurements. Plant Cell Physiol 38: 945–951. [Google Scholar]
- 34. Porra RJ (2002) The chequered history of the development and use of simultaneous equations for the accurate determination of chlorophylls a and b. Photosynth Res 73: 149–156. [DOI] [PubMed] [Google Scholar]
- 35. Bradford MM (1976) A rapid and sensitive method for the quantitation of micro-gram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254. [DOI] [PubMed] [Google Scholar]
- 36. He JP, Chen FD, Chen SM, Lv GS, Deng YM, et al. (2011) Chrysanthemum leaf epidermal surface morphology and antioxidant and defense enzyme activity in response to aphid infestation. J Plant Physiol 168: 687–693. [DOI] [PubMed] [Google Scholar]
- 37. Argandona VH, Chaman M, Cardemil L, Munoz O, Zuniga GE, et al. (2001) Ethylene production and peroxidase activity in aphid-infested barley. J Chem Ecol 27: 53–68. [DOI] [PubMed] [Google Scholar]
- 38. Yin DM, Chen SM, Chen FD, Guan ZY, Fang WM (2010) Morpho-anatomical and physiological responses of two Dendranthema species to waterlogging. Environ Exp Bot 68: 122–130. [Google Scholar]
- 39.Li HS (2000) Principles and Techniques of Plant Physiological Biochemical Experi-ment. Higher Education Press, Beijing, 194–197.
- 40. Deng YM, Shao QS, Li CC, Ye XQ, Tang RS (2012) Differential responses of double petal and multi petal jasmine to shading: II. Morphology, anatomy and physiology. Sci Hortic-Amsterdam 144: 19–28. [Google Scholar]