Abstract
Recently, ETS-related gene (ERG) gene rearrangements, phosphatase tensin homologue (PTEN) deletions and EGFR family aberrations were characterized as potential biomarkers for prostate cancer (PCa) patient management. Although ERG gene rearrangement has been identified in approximately 50% of localized prostate cancers in western countries, the prognostic significance of this critical molecular event remains unknown in Chinese patients. Using fluorescence in situ hybridization (FISH) and immunohistochemistry, we evaluated ERG, PTEN and EGFR family aberrations in a cohort of 224 Chinese prostate cancer patients diagnosed in transurethral resection of the prostate (TUR-P). Overall, ERG rearrangement was detected in 23.2% (44/190) cases, of which 54.5% (24/44) showed deletion of the 5′end of ERG. PTEN deletion was identified in 10.8% (19/176) cases. Amplification of EGFR and HER2 genes was present in 1.1% (2/178) and 5.8% (10/173) of cases, respectively. Significant correlation between ERG rearrangement and PTEN deletion was identified in this cohort. EGFR and HER2 aberrations occurred more frequently in PCas without ERG rearrangement than in those with ERG rearrangement, although this did not reach statistical significance. Overall, ERG rearrangement was associated with pre-operative PSA values (P = 0.038) and cancer-related death (P = 0.02), but not with the age, clinical T stage, Gleason score, or Ki-67 labeling index (LI). Notably, multivariate analysis including known prognostic markers revealed ERG rearrangement was an independent prognostic factor (P = 0.022). Additionally, ERG rearrangement status was helpful to identify patients with poor prognosis from PCa group with low Ki-67 LI. In summary, we reported that ERG rearrangement was associated with cancer-related death in Chinese PCa patients. Determination of ERG rearrangement status allows stratification of PCa patients into different survival categories.
Introduction
Prostate cancer (PCa) is a heterogeneous disease with a variable natural history [1], [2]. It is estimated that only a small fraction of patients suffers from potential life-threatening disease that requires aggressive treatment. Currently, the established prognostic factors (Gleason score, pathological stage and serum prostate-specific antigen (PSA)) cannot precisely distinguish clinically aggressive PCas from clinically indolent ones [3], [4]. Thus, novel prognostic biomarkers are urgently needed for PCa patient management.
Recently, recurrent gene fusions involving the ETS family of transcription factors, ERG,ETV1, ETV4, ETV5 and ELK4, fused to androgen-regulated gene TMPRSS2 or other upstream partners, have been identified in the majority of PCas in western countries [5]–[8]. Among these aberrations, ERG rearrangement, which mostly results from TMPRSS2-ERG fusion, is the most prevalent and occurs in approximately 50% of localized PCas [8]. As TMPRSS2 and ERG are located ∼3 Mb apart on chromosome 21, the rearrangement between them occurs either through insertion or by an interstitial deletion (EDel) [6]. TMPRSS2-ERG fusion leads to over-expression of ERG, which may play a critical role in PCa development [8]. To date, the prognostic significance of ERG rearrangement in PCa remains controversial. Although several studies have indicated that ERG rearrangement confers a worse prognosis [9]–[12], others found either a favorable prognostic association[13]–[17] or no association with clinical outcome [18]–[20]. Of note, most of these data are from Caucasian patients in western countries. Although the emerging data suggested the distinct prevalence of ERG rearrangement in PCas among different ethnic groups [21], [22], survival analysis of ERG aberrations is rare in Asian populations.
PTEN (phosphatase and tensin homolog deleted on chromosome 10) is a key tumor suppressor gene in PCa [23]. Deletion of the PTEN occurs in 20–70% of PCas and has been linked to rapid tumor progression and early recurrence [24]. Previously, we and others reported the significant association between PTEN deletion and ERG rearrangement both in localized and metastatic PCas [25]. Recent clinical data have suggested that PTEN deletion and ERG rearrangement could be used for prognostic stratification of PCa patients [26].
The epidermal growth factor receptor (EGFR) and HER2 belong to the EGFR family and are known to regulate cell proliferation, differentiation, angiogenesis, and survival. Amplification and over-expression of EGFR and HER2 have been described in PCa and associated with cancer progression, poor prognosis or development of androgen independence [27]. Yet so far, the link between ERG rearrangement and genetic aberrations of EGFR and HER2 remains unclear.
The Ki-67 LI is a classical proliferation marker and has been found to be a predictor of outcome for PCa patients treated with radical prostatectomy [28], [29] or radiotherapy. Ki-67 has emerged as one of the global predictive markers of treatment outcome in PCa patients.
The aim of the current study was to investigate whether ERG rearrangement was associated with a more aggressive phenotype of PCa. Herein, we systematically characterized the frequency and prognostic significance of ERG rearrangement in a large cohort of Chinese PCa patients (n>200). We further determined whether the ERG rearrangement can be utilized as a prognostic indicator and provide additional value in prognostic analysis. Additionally, the relationship of ERG gene rearrangement with other molecular markers, including PTEN deletion and genetic aberrations of EGFR and HER2, was also investigated.
Materials and Methods
Patients
A total of 224 PCa patients who underwent tumor resection by transurethral resection of prostate (TUR-P) were included in our study. The tumor samples were obtained from Qilu Hospital of Shandong University (Jinan, China), The Affiliated Hospital of Qingdao University (Qingdao, China) and Liaocheng General Hospital (Liaocheng, China) between 2003 and 2011. All of these patients were hospitalized due to symptoms of lower tract urinary obstruction. Eighty-five PCa patients in the current study had transrectal ultrasound-guided prostate biopsy and 63.5% (54/85) cases had peripheral zone cancer that extended into transition zone. None of the patients received preoperative radiation or androgen deprivation therapy. Anti-androgen flutamide therapy was followed after surgery and follow-up data were available for 190 patients, ranging from 3 to 147 months (mean 47 months). Because the number of non-PCa deaths (n = 17) was limited in this cohort, the prostate cancer-related death approached the all-cause mortality. The clinical and pathological characteristics of 190 PCa cases in our cohort are summarized in Table 1. Three tissue microarrays (TMAs) were assembled using a manual tissue arrayer; for each case, two cores (1.0 mm in diameter) were taken from each representative tumor focus and morphology was verified by three pathologists (M.Q., B.H. and X.Y.). A 4 µm section form each TMA was stained with H&E to verify the presence of tumor in PCa cases. Detailed clinical and pathological profile were obtained from medical records and maintained on a secure relational database with TMA data. Informed written consents were obtained from the PCa patients and this study was approved by the Institutional Review Board at the school of medicine of Shandong University and local ethics.
Table 1. Clinicopathological demographics of 190 Chinese prostate cancer patients.
| Parameters | Count | Percentage (%) |
| Age(years) | ||
| <60 | 29 | 15 |
| 60–69 | 41 | 22 |
| ≥70 | 120 | 63 |
| Gleason scores | ||
| <7 | 26 | 14 |
| = 7 | 70 | 37 |
| >7 | 94 | 49 |
| cT | ||
| ≤T2 | 138 | 73 |
| T3 | 30 | 16 |
| T4 | 22 | 11 |
| Preoperative PSA levels(ng/ml) | ||
| ≤4 | 22 | 12 |
| 4–10 | 27 | 14 |
| 10–20 | 25 | 13 |
| >20 | 116 | 61 |
| Distant metastasis at diagnosis | ||
| No | 150 | 79 |
| Yes | 40 | 21 |
Fluorescence in situ Hybridization (FISH)
A previously described dual-color interphase break-apart FISH assay was performed to detect ERG rearrangement [25]. Bacterial artificial chromosomes (BACs) were obtained from the BACPAC Resource Center (Oakland, CA), and probes RP11-95I21 (5′ to ERG) and RP11-476D17 (3′ to ERG) were prepared as described [25]. The integrity and correct localization of all probes were verified by hybridization to metaphase spreads of normal peripheral lymphocytes. To detect PTEN deletion, the commercially available DNA probes for cytoband 10q23 (Spectrum Orange PTEN locus-specific probe) and region 10p11.1–q11.1 (Spectrum Green centromere of chromosome 10 probe) (LSI PTEN/CEP 10; Vysis Inc. Des Plaines,IL, USA) for chromosome identification were utilized. The PTEN genomic probe spans 368 kb and starts 166 kb from 5′ end of the gene and extends 98 kb past the 3′ end of the gene. Assessment of EGFR and HER2 gene aberrations was performed using the GLP EGFR/CSP 7 probe and GLP HER2/CSP17, respectively (GP Medical Technologies, Beijing, China).
Interphase FISH was performed as previously described [25], [30]. Slides were examined using an ImagingZ1 microscope (Carl Zeiss, Oberkochen, Germany). FISH signals were scored manually (100× oil immersion) in morphologically intact and non-overlapping nuclei by two pathologists (B.H., and M.Q.), and a minimum of 50 cancer cells from each site were recorded. Cancer sites with very weak or no signals were recorded as insufficiently hybridized. Cases lacking tumor tissue in all two cores were excluded.
To validate deletion of PTEN and amplification of EGFR and HER2, we utilized a previously documented method with minor modification [25], [31]. Briefly, based on hybridization in five control cores (data not shown), hemizygous deletion of PTEN gene was termed as >50% nuclei (mean±3 standard deviations in non-neoplastic controls) containing either one signal of locus probe and ≥2 signals of reference probe (absolute deletion), or two signals of locus probe and ≥4 signals of reference probe (relative deletion). Homozygous deletion of PTEN was exhibited by the concurrent lack of the both PTEN locus signals and the presence of control signals in >30% of cells. Specimens were considered amplified for EGFR when >10% of tumor cells displayed either EGFR: CEP 7 ratio >2 or countless tight clusters of signals of the locus probe (3–5copies). EGFR copy number gain was defined as a low copy number increase due to chromosome 7 polysomy. Similarly, specimens were considered amplified for HER2 when >10% of tumor cells displayed either HER2: CEP 17 ratio >2 or countless tight clusters of signals of the locus probe (3–5copies). HER2 copy number gains were defined as a low copy number increase due to chromosome 17 polysomy. Representative FISH images of ERG rearrangement were shown in Figure 1. Figure 2A and 2B demonstrated representative cases with PTEN deletion as well as HER2 amplification.
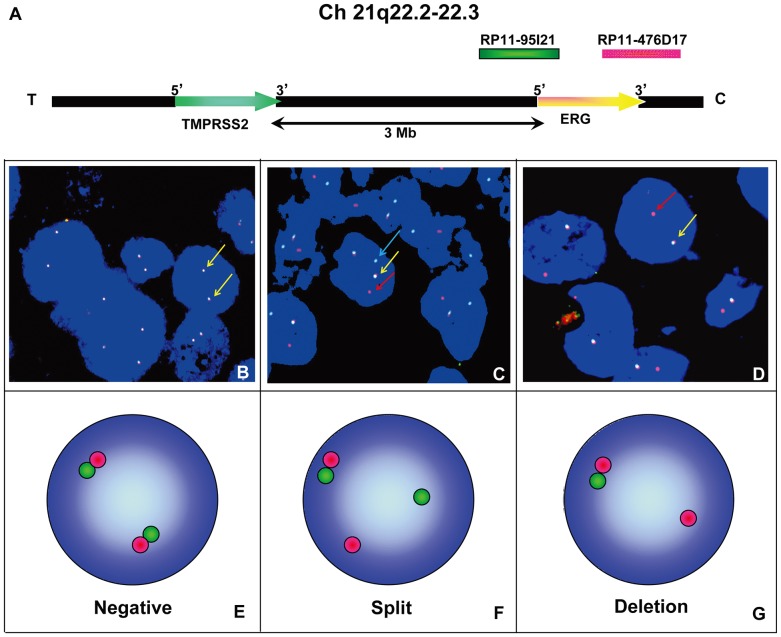
Figure 1. FISH probe design and representative images of ERG rearrangement.
(A) Schematic map of ‘TMPRSS2’ and ‘ERG’ position on 21q22.2–22.3. T and C orientate toward the telomeric and centromeric regions, respectively. BACs located 5′ and 3′ to ERG were used as probes for interphase FISH. Chromosomal coordinates are from the March 2006 build of the human genome using the UCSC Genome Browser. The TMPRSS2and ERG loci are separated by approximately 3 Mb. (B) FISH was performed using BACs as indicated with the corresponding fluorescent label on formalin-fixed paraffin-embedded tissue sections for break-apart FISH of the ERG gene. (B & E), ERG rearrangement negative case, as indicated by two pairs of co-localized green and red signals. (C & F), ERG rearrangement positive (translocation) case showed one pair of split 5′ and 3′ signals. (D & G), ERG rearrangement positive (with deletion) case showed loss of one green labeled probe 5′ to ERG.
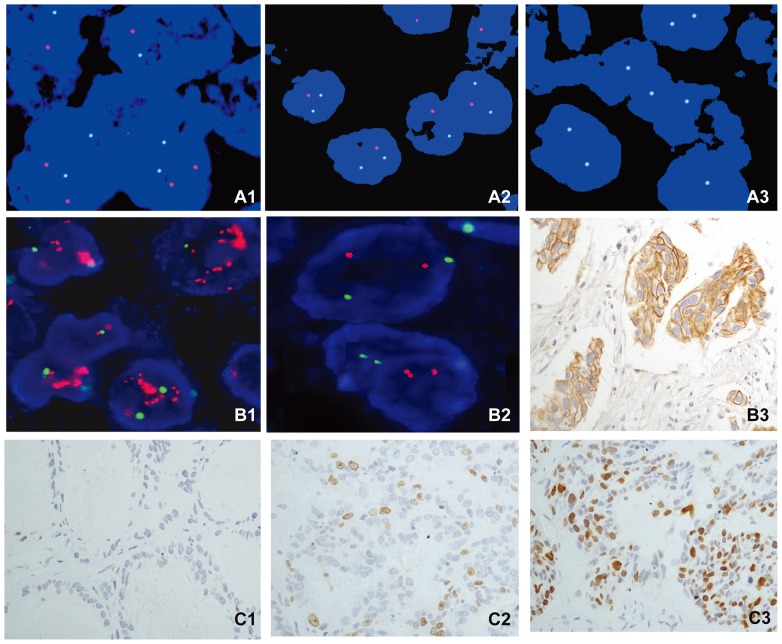
Figure 2. Representative images for IHC staining and FISH analysis of PTEN, HER2 and Ki-67 expression in PCa.
(A1–A3) FISH images of undeleted, hemizygous and homozygous PTEN deletion in PCa. A1, PTEN deletion negative case showed both paired red signals (10q23/PTEN locus) and green signals in tumor cells. A2, Representative case with PTEN hemizygous deletion showed one red signals and pairs of green signals in tumor cells. A3, Representative case with PTEN homozygous deletion showed absence of red signals but retained pairs of green signals. For all assays, at least 50 cancer cell nuclei were evaluated. (B1–B3) The detection of HER2 expression by IHC and FISH in PCa. B1, FISH analysis of representative case without HER2 amplification. B2, FISH analysis of case with HER2 amplification. B3, HER2 IHC staining shows complete membranous reactivity of strong intensity (3+) in tumor cells (original magnification, ×200). (C1–C3) The Ki-67 staining by IHC in PCa cells. C1, No staining (0) of Ki-67 in tumor cells. C2, Low Ki-67 (LI<10%) nuclear positivity in tumor cells. C3, High Ki-67(LI≥10%) nuclear positivity in tumor cells.
Immunohistochemistry
Immunohistochemistry (IHC) for PTEN, EGFR and HER2 was performed using a polymer-based method (EnvisionTM +Dual Link System-HRP). Sources and dilutions of primary antibodies were as follows: anti-PTEN (Cell signaling, 1∶100), anti-EGFR (DAKO, 1∶500), anti-HER2 (DAKO, 1∶500) and anti-Ki67 antibody (DAKO, 1∶100). Sections from TMA (4 µm) were deparaffinized and prepared by successive passages through xylene and grade concentration of ethanol as routine procedure, then antigens were retrieved by pressure cooker using a citrate buffer(0.01 M), for 8 minutes 120°C. Endogenous peroxidase activity was blocked by incubation with 0.3% hydrogen peroxide solution for 15 min. The tissue sections were incubated overnight at 4°C with primary antibodies. After a washing in PBS, the sections were treated with EnvisionTM +Dual Link System-HRP reagent at room temperature for 30 min. 3, 3′-Diaminobenzidine tetrahydrochloride was used as the chromogen for 3 minutes and the tissue sections were counterstained with haematoxylin.
The immunostaining of EGFR and HER2 was semiquantitatively evaluated based on intensity of membrane reactivity following the original DAKO Herceptest criteria with a threshold of 10% immunopositive cells. The scoring system was described elsewhere [27]. Evaluation of PTEN was based on the cytoplasmic staining intensity; the tumors were divided into three categories as previously described [25]. Grade 2 showed increased or equal staining intensity compared to the corresponding normal tissue; grade 1 had decreased staining intensity, and grade 0 demonstrated complete absence of staining. The Ki-67 labeling index (LI) was defined as the fraction of tumor cells showing any nuclear Ki-67 immunoreactivity and was considered high if 10% or more of the tumor nuclei were stained. For this purpose, 100–200 tumor cells were analyzed for each case. Representative immunohistochemical images of Ki-67 were shown in Figure 2C.
Statistical Analysis
Statistical analyses were carried out using the Statistical Package for Social Sciences, version 19.0 (SPSS), with a significance level of 0.05(two-tailed probability). Pearson’s χ2 test and Fisher’s exact test were used to evaluate the associations between ERG rearrangement and clinico-pathologic variables as well as other molecular aberrations. Kaplan-Meier analysis was utilized to assess the prognostic value of ERG rearrangement in PCa patients. The prognostic value of ERG rearrangement was further determined in univariable and multivariable analysis, including PSA values at diagnosis, Gleason score, clinical tumor stage, distant metastasis, Ki-67 LI and EGFR family gene aberrations.
Results
Frequency of ERG Rearrangement, PTEN Deletion and EGFR Family Aberrations
Overall, ERG was rearranged in 23.2% (44/190) of Chinese PCa patients, of which 54.5% (24/44) demonstrated deletion of the 5′end of ERG. Interestingly, two out of these 24 cases demonstrated two copies of the 3′-ERG signals, suggesting the duplication of ERG rearrangement. PTEN deletion was identified in 10.8% (19/176) of cases, with hemizygous and homozygous deletions present in 12 of 19 (63.2%) and 7 of 19 (36.8%) cases, respectively. Amplification of HER2 was identified in 10 of 173 (5.8%) tumors and polysomy of chromosome 17 was noted in 41 of 173 (23.8%) cases. By contrast, only 2 of 178 (1.1%) cases showed amplification of EGFR with polysomy of chromosome 7 being present in 18 of 178 (10.1%) tumors.
Relationships between ERG Rearrangement and Clinicopathologic Variables
ERG gene rearrangement was significantly associated with preoperative PSA levels in PCa patients (P = 0.038) (Table 2). The incidence of ERG rearrangement was significantly lower in patients with Low PSA level (<4 ng/ml) compared with those having medium or high PSA levels. However, no significant correlation was identified between ERG rearrangement and age, Gleason score, clinical T stage, or distant metastasis at diagnosis.
Table 2. Association of clinicopathologic variables and molecular biomarkers with ERG rearrangement.
| Variable | ERG rearrangement according to FISH (%) | P | |
| Not rearranged(n/%) | Rearranged (n/%) | ||
| All cases | 146(76.8) | 44(23.2) | |
| age(years) | |||
| ≤65 | 21(67.8) | 10(32.2) | 0.173 |
| >65 | 117(79.0) | 31(21.0) | |
| Pre PSA(ng/ml) | |||
| <4 | 15(93.8) | 1(6.2) | 0.038 |
| 4–10 | 11(64.7) | 6(35.3) | |
| >10 | 103(79.8) | 26(20.2) | |
| Gleason score | |||
| <7 | 17(73.9) | 6(26.1) | 0.430 |
| 7 | 51(76.1) | 16(23.9) | |
| >7 | 78(83.0) | 16(17.0) | |
| Clinical tumor stage | |||
| ≤cT2 | 101(78.3) | 28(21.7) | 0.607 |
| ≥cT3 | 29(74.4) | 10(25.6) | |
| Metastasis | |||
| No | 97(80.8) | 23(19.1) | 0.585 |
| Yes | 37(77.1) | 11(22.9) | |
| Ki-67 | |||
| <10% | 127(80.5) | 31(19.5) | 0.385 |
| ≥10% | 19(73.1) | 7(26.9) | |
| PTEN deletion | |||
| Not deleted | 130(80.3) | 27(19.7) | 0.0008 |
| Deleted | 7(36.8) | 12(63.2) | |
| EGFR amplification | |||
| Not amplified | 139(79.0) | 37(21.0) | 0.883 |
| Amplified | 2(100.0) | 0(0.0) | |
| EGFR IHC | |||
| 0 and 1+ | 120(82.3) | 25(17.7) | 0.779 |
| 2+ and 3+ | 25(80.6) | 6(19.7) | |
| HER2 amplification | |||
| Not amplified | 129(79.1) | 34(20.9) | 0.671 |
| Amplified | 9(90.0) | 1(10.0) | |
| HER2 IHC | |||
| 0 and 1+ | 131(76.6) | 40(23.4) | 0.541 |
| 2+ and 3+ | 11(100.0) | 0(0.0) | |
Values not available for all 190 cases.
Association of ERG Rearrangement with Other Molecular Markers
As deletion of PTEN and amplifications of EGFR and HER2 are relevant genomic aberrations in PCa, we next explored the association of ERG rearrangement with these molecular events in our cohort. As shown in Table 2, the ERG rearrangement was present in approximately 63.2% (12/19) of PCa patients with PTEN deletion (hemizygous or homozygous). Likewise, PTEN deletion occurred more frequently in cases that harbored ERG rearrangement (30.8%, 12/39) as compared with those ERG rearrangement negative cases (5.1%, 7/137). Overall, a significant association between PTEN deletion and ERG rearrangement was observed in Chinese PCa cohort (P = 0.0008). Of note, 46/182 (25.2%) PCa cases revealed decreased PTEN protein expression by immunohistochemistry. Concordance between PTEN deletion status and PTEN protein expression was also identified in our cohort (data not shown).
Amplification of EGFR was identified only in two PCa cases, both of which were negative for ERG rearrangement. Similarly, 9 out of 10 (90.0%) PCa cases with HER2 amplification were absent for ERG rearrangement. ERG rearrangement was more often present in PCa cases without HER2 amplification (34/163, 20.9%) than in HER2-amplified tumors (1/10, 10.0%) (P = 0.149).
Immunohistochemical overexpressions of EGFR and HER2 were identified in 17.6% (31/176) and 6.0% (11/181) of cases, respectively. HER2 protein overexpression was significantly correlated to amplification of HER2 (P<0.01). However, there was no correlation between EGFR protein expression and gene amplification (data not shown). ERG rearrangement was neither associated with EGFR nor HER2 protein expression.
Survival Analysis of ERG Rearrangement in Relation to Cancer-related Death
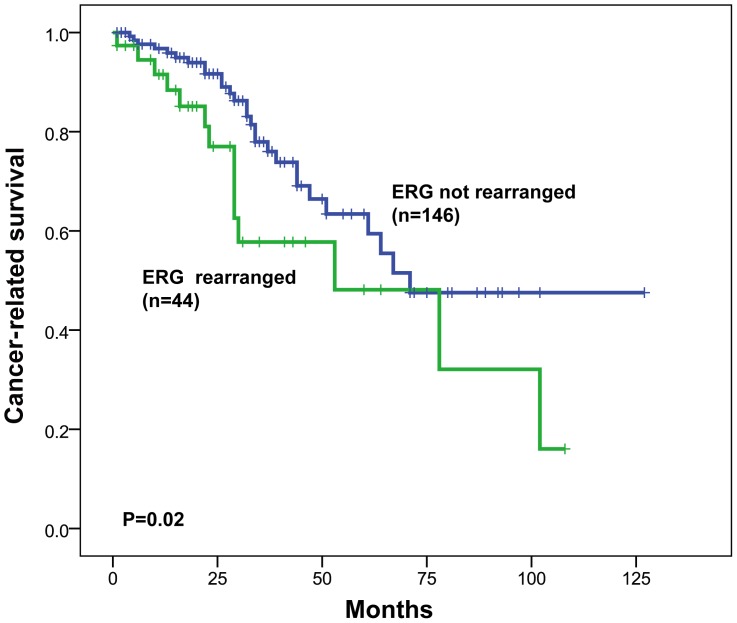
To determine whether the presence of ERG rearrangement was a prognostic factor for PCa, we compared cancer-related death rates between patients with or without ERG rearrangement. On the basis of the Kaplan-Meier survival estimates, the group of patients with ERG rearrangement had a much greater rate of mortality than patients who lacked the gene rearrangement (P = 0.02) (Figure 3).
Figure 3. Kaplan-Meier survival curves for PCa patients with and without ERG rearrangement.
The cancer-related survival rates were compared between patients with and without ERG rearrangement using the log-rank test.
ERG rearrangement status was shown to be a significant prognostic predictor of prostate cancer-related survival [HR (95% CI): 3.368 (1.261–8.955), P = 0.015] in univariate analysis (Table 3). PSA values at diagnosis (P = 0.009), Gleason score (P<0.001), clinical tumor stage (P = 0.011), distant metastasis (P = 0.006), Ki-67 LI (P = 0.002), EGFR amplification (P = 0.023), and HER2 amplification (P = 0.001) were also significantly related to cancer-related survival in univariate analysis. Notably, in a multivariate analysis that included known prognostic markers, ERG rearrangement status remained a significant predictor (P = 0.022) with a hazard ratio of 2.099 (95% CI: 1.112–3.962) (Table 3).
Table 3. Univariate and multivariate analysis of variables associated with survival in PCa patients.
| Parameter | Univariate analysis | Multivariate analysis | ||
| HR(95%CI) | P | HR(95%CI) | P | |
| age(years)a | 0.588(0.328–1.053) | 0.074 | – | – |
| Pre-PSA | 0.601(0.410–0.880) | 0.009 | Nonsignificancance | |
| Gleason score | 2.297(1.455–3.625) | <0.001 | 4.680(2.020–10.483) | <0.001 |
| Clincial tumor stage | 2.011(1.177–3.435) | 0.011 | Nonsignificancance | |
| Metastasis | 2.106(1.240–3.577) | 0.006 | 2.897(1.236–6.789) | 0.014 |
| Ki-67 | 2.592(1.435–4.682) | 0.002 | 2.641(1.084–6.435) | 0.019 |
| HER2amplification | 6.687(2.253–19.844) | 0.001 | Nonsignificancance | |
| HER2 IHC | 3.240(0.998–10.527) | 0.05 | Nonsignificancance | |
| EGFRamplification | 5.255(1.259–21.929) | 0.023 | Nonsignificancance | |
| ERG rearrangement | 3.368(1.261–8.955) | 0.015 | 2.099(1.112–3.962) | 0.022 |
HR = hazard ratio; CI = confidence interval; PSA = prostate-specific antigen.
not included in multivariate analysis.
Prognostic Relevance of ERG Rearrangement and Ki-67 LI
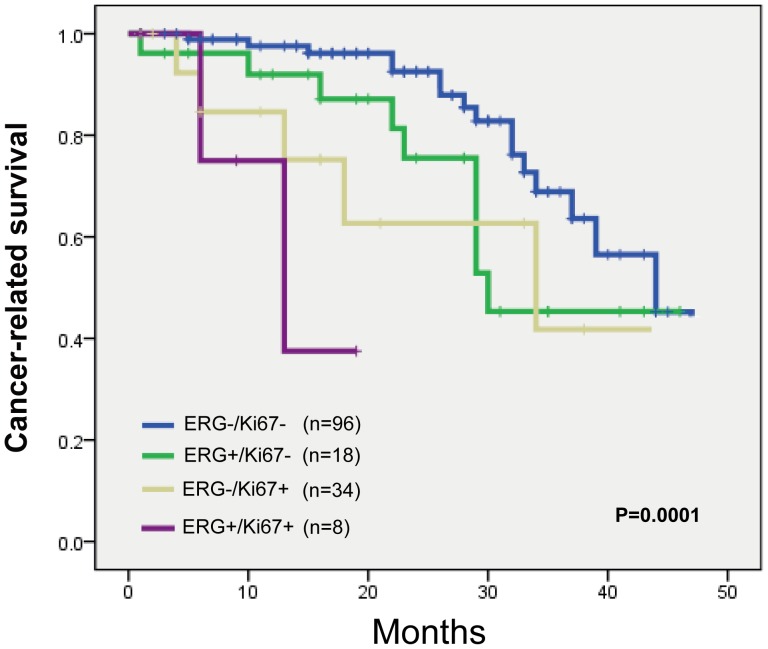
We next determined whether combining markers further improved prognostic value. Since Ki-67 is a known strong prognosticator in PCa and has independent predictive value for cancer-related survival in our cohort, we directly compared the prognostic effects of ERG rearrangement and Ki-67 LI in combination. For this analysis, we grouped all cancers according to their ERG status (not rearranged vs. rearranged) and the Ki-67 Label index status (LI <10% vs LI>10%). Cox regression analyses were therefore conducted using the group with low Ki-67 LI and no ERG aberration as the reference. As shown in Figure 4, the largest group, which comprised those who had no ERG rearrangement and low Ki-67 LI, had a greater cancer-related survival when compared with the three other groups. Notably, the subset of patients with ERG rearrangement and high Ki-67 LI had the worst cancer-related survival.
Figure 4. Kaplan-Meier curves illustrating cancer related survival among PCa patients.
The patients were stratified by ERG rearrangement and Ki-67 LI in combination and log-rank test was performed.
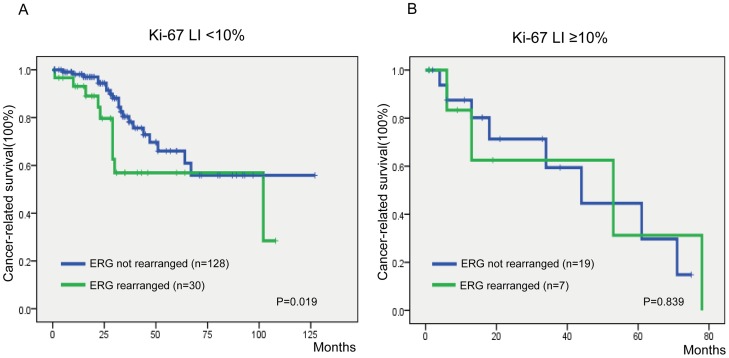
We further determined whether ERG rearrangement status could be utilized in improving risk stratification of PCa patients with low Ki-67 LI. Kaplan-Meier analysis showed that ERG rearrangement status was a prognostic factor in the group of patients with low Ki-67 LI (P = 0.019) (Figure 5A). The median survival of PCa patients with and without ERG rearrangement was 69 and 89 months, respectively. However, ERG rearrangement status lost its predictive value of outcome in those with high Ki-67 LI (Figure 5B). By contrast, ERG rearrangement status was not helpful in identifying high-risk PCa patients with low Gleason score (data not shown).
Figure 5. Kaplan-Meier survival analysis of PCa patients in relation to ERG rearrangement status.
(A) low ki-67 LI (<10%) subgroup, (B) high ki-67 LI (≥10%) subgroup.
Discussion
This is one of the largest series of PCa patients (n>200) reported so far in China analyzing ERG rearrangement. Our cohort comprises men treated with TUR-P and all of the study patients had symptoms of lower tract urinary obstruction, therefore representing a select subgroup of clinically recognized PCas. The patients with incidental PCas were excluded from our study. Although more and more PSA-screed PCa patients have been identified in western countries, there are limited data regarding the clinical phenotype or natural history of PCa. Of note, our cohort included a subset of patients with high grade PCas. This differed from most Western patients who were found to have PCa due to PSA screening and were often treated with radical prostatectomy.
Overall, the frequency of ERG rearrangement was 23.2% in our cohort and this was comparable with that previously reported by Mao et al [21] and Ren et al [32] in Chinese PCa patients. In consistent with these findings, Kimura et al [17] and Lee et al [33] reported the prevalence of TMPRSS2-ERG gene fusion was 16.3% (15/92) in Japanese and 20.9% (53/254) in Korean PCa patients, respectively. Previously, Mosquera et al [34] detected TMPRSS2-ERG fusion in 100 Caucasian and non-Caucasian PCa patients undergoing prostate biopsy. They reported that the incidence was significantly different in Caucasians (44/85, 52%) and in non-Caucasians (2/15, 13%). Most recently, using a multicolor FISH assay, Magi-Galluzzi et al [35] found that TMPRSS2-ERG gene fusion was present in 50% (21/42) of Caucasians, 31.3% (20/64) of African-Americans and 15.9% (7/44) of Japanese (P = 0.003). Collectively, these studies highlighted the low prevalence of TMPRSS2-ERG gene fusions in PCa patients in Asia compared with western countries and this disparity at least partially resulted from different genetic background rather than the effects of lifestyle or diet. Of note, the difference may also reflect previous findings that the fusion is less common in transition zone tumors (from which most tumors found in TUR-P samples) than in peripheral zone tumors [17], [36], [37]. Additionally, cohort design and consideration of multifocality might have impacts on the ERG rearrangement frequency [38].
So far, the prognostic significance of ERG rearrangement in PCa remains contradictory. A series of retrospective studies that sought an association between TMPRSS2-ERG and outcome following PSA screened radical prostatectomy gave mixed results. Several published studies have shown that PCa patients with the TMPRSS2-ERG gene fusion conferred a higher risk of recurrence, whereas others reported a significant association with a favorable prognosis or a null relationship with clinical outcome. Among patients managed with watchful waiting, TMPRSS2-ERG seemed to be associated with worse outcomes. In a meta-analysis including 227 men diagnosed with TUR-P, men with fusion-positive tumors were 1.37 (95% CI, 0.53–3.51) times as likely to experience distant metastases or die from PCa as those negative for the fusion [18]. Discrepancies in the reported prognostic significance of ERG rearrangements can be due to cohort design (multifocality and zonal origin of the tumor), fusion detection technique, and are also liable to the primary end point of the study (i.e., biochemical recurrence, overall survival). Therefore, further standardized studies are needed to address this issue. In the current study, we found that ERG rearrangement was significantly associated with prostate cancer-related death in Chinese PCa patients. More importantly, ERG rearrangement was suggested to be an independent predictor of overall survival in multivariate analyses. It is notable that biochemical recurrence is an imprecise predictor of prostate cancer death. Although PSA might serve as a surrogate endpoint for overall survival, the majority of men with PSA biochemical failure will die of other causes. Ward et al found that in a population of 3897 radical prostatectomy patients, only 8.3% of the men with PSA biochemical failure died of PCa [39]. Therefore, prostate cancer-related death as the primary end point might be more reliable for prognostic analysis. In total, our data supported the concept that if left untreated or lack of initial therapy, TMPRSS2-ERG PCa will run a more aggressive clinical course than fusion-negative cancer.
To date, Ki-67 has been widely utilized as a prognostic biomarker in malignancy including PCa. Its independent predictive value for PCa related survival has been confirmed in our study. In line with Antonarakis’s report [40], no significant association between ERG rearrangement and Ki-67 LI status was identified. One explanation is that ERG rearrangement has different effects on proliferation and invasion in vitro, respectively, Ki-67 is a well-known proliferation marker [41]–[43]. By contrast, in 2008, Tomlins et al reported that alternation of ERG gene expression significantly affect invasion in vitro but has no effect on cellular proliferation [41]. However, when stratifying for Ki-67 status, ERG rearrangement was a prognostic factor for cancer- related survival only in PCa patients with low Ki-67 LI. A major clinical challenge in PCa management is the inability to readily distinguish indolent from aggressive tumors in patients who present with low Gleason grade, low tumor volume or low Ki-67 LI. Our data suggested that determination of ERG rearrangement status could be helpful in stratification of PCa patients with low Ki-67 LI into different survival categories.
Although gene fusion is a key molecular event in PCa development and TMPRSS2-ERG fusion may induce high grade prostatic neoplasia (HGPIN), it is not sufficient to generate a fully transformed phenotype in vitro and in vivo [41], [44]. Several independent groups have suggested ERG may cooperate with other genetic aberrations to promote PCa development and progression, such as PTEN haploinsufficiency, enhanced androgen receptor (AR) signaling, overexpression of SOX9 and aberrant phosphoinositide 3-kinase (PI3K) pathway [45]–[47]. Most recently, TMPRSS2-ERG was shown to mediate Epithelial to Mesenchymal Transition (EMT) through the induction of WNT signaling pathway via FZD4 as well as ZEB1/ZEB2 axis [48], [49]. Previously, we and others have suggested the significant association between PTEN deletion and ERG rearrangement both in localized and metastatic PCas in western countries. In this study, we confirmed significant association between PTEN deletion and ERG rearrangement in Chinese PCa cohort (P = 0.0008). Thus our data highlighted a possible cooperative role of both ERG and PTEN aberrations in a subset of Chinese PCa cases.
Genetic aberrations of HER2 and EGFR were associated with advanced-stages disease, metastasis and shorten survival in PCa progression. Previous studies have shown the rarity of EGFR/HER2 amplifications in PCa. Schlomm et al [50] reported that amplification of EGFR was present only in 6 of 2,446 PCa cases (0.25%). Similarly, Baek et al [51] found no amplification of the EGFR or HER2 genes in 66 PCa specimens. In our cohort, amplification of HER2 was present in 5.8% of Chinese PCa cases. Although not reaching statistic significance, ERG rearrangement seemed to be more often present in PCa cases without HER2 amplification than in HER2-amplified tumors. Therefore, HER2 genetic aberration might play a role in a subset of Chinese PCa patients without ERG rearrangement.
It should be noted that a small proportion of tumors showing ERG arrangement may harbor a fusion between ERG and genes other than TMPRSS2, including SLC45A3 or NDRG1. On the other hand, it has been suggested that cancers harboring gene fusions occurring by deletion have worse prognosis than those occurring by translocation. However, we did not find significant associations between ERG rearrangement by translocation or positive by deletion cancers and outcomes in Chinese PCa patients.
In total, for the first time, we reported that ERG rearrangement was associated with cancer-related death in Chinese PCa patients. Determination of ERG rearrangement status allows stratification of PCa patients into different survival categories.
Funding Statement
This research was supported by the National Natural Science Foundation of China (grant numbers 81072110 and 81171951); Research Foundation of Health Department of Shandong (grant number 2011HZ037). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Shen MM, Abate-Shen C (2010) Molecular genetics of prostate cancer: new prospects for old challenges. Genes Dev 24: 1967–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lu-Yao GL, Albertsen PC, Moore DF, Shih W, Lin Y, et al. (2009) Outcomes of localized prostate cancer following conservative management. JAMA 302: 1202–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Albertsen PC, Hanley JA, Gleason DF, Barry MJ (1998) Competing risk analysis of men aged 55 to 74 years at diagnosis managed conservatively for clinically localized prostate cancer. JAMA 280: 975–980. [DOI] [PubMed] [Google Scholar]
- 4. Barry MJ, Albertsen PC, Bagshaw MA, Blute ML, Cox R, et al. (2001) Outcomes for men with clinically nonmetastatic prostate carcinoma managed with radical prostactectomy, external beam radiotherapy, or expectant management: a retrospective analysis. Cancer 91: 2302–2314. [DOI] [PubMed] [Google Scholar]
- 5. Tomlins SA, Rhodes DR, Perner S, Dhanasekaran SM, Mehra R, et al. (2005) Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science 310: 644–648. [DOI] [PubMed] [Google Scholar]
- 6. Perner S, Demichelis F, Beroukhim R, Schmidt FH, Mosquera JM, et al. (2006) TMPRSS2:ERG fusion-associated deletions provide insight into the heterogeneity of prostate cancer. Cancer Res 66: 8337–8341. [DOI] [PubMed] [Google Scholar]
- 7. Helgeson BE, Tomlins SA, Shah N, Laxman B, Cao Q, et al. (2008) Characterization of TMPRSS2:ETV5 and SLC45A3:ETV5 gene fusions in prostate cancer. Cancer Res 68: 73–80. [DOI] [PubMed] [Google Scholar]
- 8. Kumar-Sinha C, Tomlins SA, Chinnaiyan AM (2008) Recurrent gene fusions in prostate cancer. Nat Rev Cancer 8: 497–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nam RK, Sugar L, Yang W, Srivastava S, Klotz LH, et al. (2007) Expression of the TMPRSS2:ERG fusion gene predicts cancer recurrence after surgery for localised prostate cancer. Br J Cancer 97: 1690–1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Attard G, Clark J, Ambroisine L, Fisher G, Kovacs G, et al. (2008) Duplication of the fusion of TMPRSS2 to ERG sequences identifies fatal human prostate cancer. Oncogene 27: 253–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Perner S (2010) [Dangerous liaisons in prostate cancer. Clinical and biological implications of recurrent gene fusions]. Pathologe 31 Suppl 2121–125. [DOI] [PubMed] [Google Scholar]
- 12. Demichelis F, Fall K, Perner S, Andren O, Schmidt F, et al. (2007) TMPRSS2:ERG gene fusion associated with lethal prostate cancer in a watchful waiting cohort. Oncogene 26: 4596–4599. [DOI] [PubMed] [Google Scholar]
- 13. Saramaki OR, Harjula AE, Martikainen PM, Vessella RL, Tammela TL, et al. (2008) TMPRSS2:ERG fusion identifies a subgroup of prostate cancers with a favorable prognosis. Clin Cancer Res 14: 3395–3400. [DOI] [PubMed] [Google Scholar]
- 14. Winnes M, Lissbrant E, Damber JE, Stenman G (2007) Molecular genetic analyses of the TMPRSS2-ERG and TMPRSS2-ETV1 gene fusions in 50 cases of prostate cancer. Oncol Rep 17: 1033–1036. [PubMed] [Google Scholar]
- 15. Hermans KG, Boormans JL, Gasi D, van Leenders GJ, Jenster G, et al. (2009) Overexpression of prostate-specific TMPRSS2(exon 0)-ERG fusion transcripts corresponds with favorable prognosis of prostate cancer. Clin Cancer Res 15: 6398–6403. [DOI] [PubMed] [Google Scholar]
- 16. Boormans JL, Porkka K, Visakorpi T, Trapman J (2011) Confirmation of the association of TMPRSS2(exon 0):ERG expression and a favorable prognosis of primary prostate cancer. Eur Urol 60: 183–184. [DOI] [PubMed] [Google Scholar]
- 17. Kimura T, Furusato B, Miki J, Yamamoto T, Hayashi N, et al. (2012) Expression of ERG oncoprotein is associated with a less aggressive tumor phenotype in Japanese prostate cancer patients. Pathol Int 62: 742–748. [DOI] [PubMed] [Google Scholar]
- 18. Pettersson A, Graff RE, Bauer SR, Pitt MJ, Lis RT, et al. (2012) The TMPRSS2:ERG rearrangement, ERG expression, and prostate cancer outcomes: a cohort study and meta-analysis. Cancer Epidemiol Biomarkers Prev 21: 1497–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. FitzGerald LM, Agalliu I, Johnson K, Miller MA, Kwon EM, et al. (2008) Association of TMPRSS2-ERG gene fusion with clinical characteristics and outcomes: results from a population-based study of prostate cancer. BMC Cancer 8: 230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gopalan A, Leversha MA, Satagopan JM, Zhou Q, Al-Ahmadie HA, et al. (2009) TMPRSS2-ERG gene fusion is not associated with outcome in patients treated by prostatectomy. Cancer Res 69: 1400–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mao X, Yu Y, Boyd LK, Ren G, Lin D, et al. (2010) Distinct genomic alterations in prostate cancers in Chinese and Western populations suggest alternative pathways of prostate carcinogenesis. Cancer Res 70: 5207–5212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Miyagi Y, Sasaki T, Fujinami K, Sano J, Senga Y, et al. (2010) ETS family-associated gene fusions in Japanese prostate cancer: analysis of 194 radical prostatectomy samples. Mod Pathol 23: 1492–1498. [DOI] [PubMed] [Google Scholar]
- 23. Di Cristofano A, Pandolfi PP (2000) The multiple roles of PTEN in tumor suppression. Cell 100: 387–390. [DOI] [PubMed] [Google Scholar]
- 24. Bertram J, Peacock JW, Fazli L, Mui AL, Chung SW, et al. (2006) Loss of PTEN is associated with progression to androgen independence. Prostate 66: 895–902. [DOI] [PubMed] [Google Scholar]
- 25. Han B, Mehra R, Lonigro RJ, Wang L, Suleman K, et al. (2009) Fluorescence in situ hybridization study shows association of PTEN deletion with ERG rearrangement during prostate cancer progression. Mod Pathol 22: 1083–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yoshimoto M, Joshua AM, Cunha IW, Coudry RA, Fonseca FP, et al. (2008) Absence of TMPRSS2:ERG fusions and PTEN losses in prostate cancer is associated with a favorable outcome. Mod Pathol 21: 1451–1460. [DOI] [PubMed] [Google Scholar]
- 27. Di Lorenzo G, Tortora G, D’Armiento FP, De Rosa G, Staibano S, et al. (2002) Expression of epidermal growth factor receptor correlates with disease relapse and progression to androgen-independence in human prostate cancer. Clin Cancer Res 8: 3438–3444. [PubMed] [Google Scholar]
- 28. Bubendorf L, Sauter G, Moch H, Schmid HP, Gasser TC, et al. (1996) Ki67 labelling index: an independent predictor of progression in prostate cancer treated by radical prostatectomy. J Pathol 178: 437–441. [DOI] [PubMed] [Google Scholar]
- 29. Bettencourt MC, Bauer JJ, Sesterhenn IA, Mostofi FK, McLeod DG, et al. (1996) Ki-67 expression is a prognostic marker of prostate cancer recurrence after radical prostatectomy. J Urol 156: 1064–1068. [PubMed] [Google Scholar]
- 30. Han B, Mehra R, Dhanasekaran SM, Yu J, Menon A, et al. (2008) A fluorescence in situ hybridization screen for E26 transformation-specific aberrations: identification of DDX5-ETV4 fusion protein in prostate cancer. Cancer Res 68: 7629–7637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Korshunov A, Sycheva R, Gorelyshev S, Golanov A (2005) Clinical utility of fluorescence in situ hybridization (FISH) in nonbrainstem glioblastomas of childhood. Mod Pathol 18: 1258–1263. [DOI] [PubMed] [Google Scholar]
- 32. Ren S, Peng Z, Mao JH, Yu Y, Yin C, et al. (2012) RNA-seq analysis of prostate cancer in the Chinese population identifies recurrent gene fusions, cancer-associated long noncoding RNAs and aberrant alternative splicings. Cell Res 22: 806–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee K, Chae JY, Kwak C, Ku JH, Moon KC (2010) TMPRSS2-ERG gene fusion and clinicopathologic characteristics of Korean prostate cancer patients. Urology 76: 1268 e1267–1213. [DOI] [PubMed]
- 34. Mosquera JM, Mehra R, Regan MM, Perner S, Genega EM, et al. (2009) Prevalence of TMPRSS2-ERG fusion prostate cancer among men undergoing prostate biopsy in the United States. Clin Cancer Res 15: 4706–4711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Magi-Galluzzi C, Tsusuki T, Elson P, Simmerman K, LaFargue C, et al. (2011) TMPRSS2-ERG gene fusion prevalence and class are significantly different in prostate cancer of Caucasian, African-American and Japanese patients. Prostate 71: 489–497. [DOI] [PubMed] [Google Scholar]
- 36. Falzarano SM, Navas M, Simmerman K, Klein EA, Rubin MA, et al. (2010) ERG rearrangement is present in a subset of transition zone prostatic tumors. Mod Pathol 23: 1499–1506. [DOI] [PubMed] [Google Scholar]
- 37. Guo CC, Zuo G, Cao D, Troncoso P, Czerniak BA (2009) Prostate cancer of transition zone origin lacks TMPRSS2-ERG gene fusion. Mod Pathol 22: 866–871. [DOI] [PubMed] [Google Scholar]
- 38. Braun M, Scheble VJ, Menon R, Scharf G, Wilbertz T, et al. (2011) Relevance of cohort design for studying the frequency of the ERG rearrangement in prostate cancer. Histopathology 58: 1028–1036. [DOI] [PubMed] [Google Scholar]
- 39. Ward JF, Blute ML, Slezak J, Bergstralh EJ, Zincke H (2003) The long-term clinical impact of biochemical recurrence of prostate cancer 5 or more years after radical prostatectomy. J Urol 170: 1872–1876. [DOI] [PubMed] [Google Scholar]
- 40. Antonarakis ES, Keizman D, Zhang Z, Gurel B, Lotan TL, et al. (2012) An immunohistochemical signature comprising PTEN, MYC, and Ki67 predicts progression in prostate cancer patients receiving adjuvant docetaxel after prostatectomy. Cancer 118: 6063–6071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tomlins SA, Laxman B, Varambally S, Cao X, Yu J, et al. (2008) Role of the TMPRSS2-ERG gene fusion in prostate cancer. Neoplasia 10: 177–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pathmanathan N, Balleine RL (2013) Ki67 and proliferation in breast cancer. J Clin Pathol 66: 512–516. [DOI] [PubMed] [Google Scholar]
- 43. Perner S, Mosquera JM, Demichelis F, Hofer MD, Paris PL, et al. (2007) TMPRSS2-ERG fusion prostate cancer: an early molecular event associated with invasion. Am J Surg Pathol 31: 882–888. [DOI] [PubMed] [Google Scholar]
- 44. Klezovitch O, Risk M, Coleman I, Lucas JM, Null M, et al. (2008) A causal role for ERG in neoplastic transformation of prostate epithelium. Proc Natl Acad Sci U S A 105: 2105–2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Carver BS, Tran J, Gopalan A, Chen Z, Shaikh S, et al. (2009) Aberrant ERG expression cooperates with loss of PTEN to promote cancer progression in the prostate. Nat Genet 41: 619–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sun C, Dobi A, Mohamed A, Li H, Thangapazham RL, et al. (2008) TMPRSS2-ERG fusion, a common genomic alteration in prostate cancer activates C-MYC and abrogates prostate epithelial differentiation. Oncogene 27: 5348–5353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Cai C, Wang H, He HH, Chen S, He L, et al. (2013) ERG induces androgen receptor-mediated regulation of SOX9 in prostate cancer. J Clin Invest 123: 1109–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zong Y, Xin L, Goldstein AS, Lawson DA, Teitell MA, et al. (2009) ETS family transcription factors collaborate with alternative signaling pathways to induce carcinoma from adult murine prostate cells. Proc Natl Acad Sci U S A 106: 12465–12470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Leshem O, Madar S, Kogan-Sakin I, Kamer I, Goldstein I, et al. (2011) TMPRSS2/ERG promotes epithelial to mesenchymal transition through the ZEB1/ZEB2 axis in a prostate cancer model. PLoS One 6: e21650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Schlomm T, Kirstein P, Iwers L, Daniel B, Steuber T, et al. (2007) Clinical significance of epidermal growth factor receptor protein overexpression and gene copy number gains in prostate cancer. Clin Cancer Res 13: 6579–6584. [DOI] [PubMed] [Google Scholar]
- 51. Baek KH, Hong ME, Jung YY, Lee CH, Lee TJ, et al. (2012) Correlation of AR, EGFR, and HER2 Expression Levels in Prostate Cancer: Immunohistochemical Analysis and Chromogenic In Situ Hybridization. Cancer Res Treat 44: 50–56. [DOI] [PMC free article] [PubMed] [Google Scholar]