Abstract
Rationale
Environmental stimuli or contexts previously associated with rewarding drugs contribute importantly to relapse among addicts, and research has focused on neurobiological processes maintaining those memories. Much research shows contributions of cell surface receptors and intracellular signaling pathways in maintaining associations between rewarding drugs (e.g., cocaine) and concurrent cues/contexts; these memories can be degraded at the time of their retrieval through reconsolidation interference. Much less studied is the consolidation of drug-cue memories during their acquisition.
Objective
The present experiments use the cocaine conditioned place preference (CPP) paradigm in rats to directly compare, in a consistent setting, the effects of N-methyl-D-aspartate (NMDA) glutamate receptor antagonists MK-801 and memantine on the consolidation and reconsolidation of cocaine-cue memories.
Methods
For the consolidation studies, animals were systemically administered MK-801 or memantine immediately following training sessions. To investigate the effects of these NMDA receptor antagonists on the retention of previously-established cocaine-cue memories, animals were systemically administered MK-801 or memantine immediately after memory retrieval.
Results
Animals given either NMDA receptor antagonist immediately following training sessions did not establish a preference for the cocaine-paired compartment. Post-retrieval administration of either NMDA receptor antagonist attenuated the animals' preference for the cocaine-paired compartment. Furthermore, animals given NMDA receptor antagonists post-retrieval showed a blunted response to cocaine-primed reinstatement.
Conclusions
Using two distinct NMDA receptor antagonists in a common setting, these findings demonstrate that NMDA receptor-dependent processes contribute both to the consolidation and reconsolidation of cocaine-cue memories, and they point to the potential utility of treatments that interfere with drug-cue memory reconsolidation.
Keywords: Reconsolidation, Consolidation, NMDA receptor, Conditioned place preference, Cocaine, Drug-cue memory, Addiction
Introduction
Discrete stimuli or contexts previously associated with the rewarding properties of drugs are a major cause of relapse among addicts. When repeatedly paired with exposure to rewarding drugs, these initially neutral stimuli acquire incentive motivational value, and encounters with these drug-associated cues can induce intense physiological arousal and craving in addicts (Childress et al. 1988). Because drug-paired cues evoke memories that influence drug-seeking and relapse, the development of pharmacotherapeutic treatments that disrupt the strength of drug-associated memories has potential value in the treatment of drug addiction.
Drug-cue memories are strengthened through a process of consolidation. The consolidation hypothesis posits that fragile memory traces are strengthened and converted into stable, long-term memories (McGaugh 1966; Müller and Pilzecker 1900). Consolidated memories can be sustained over time and often become labile upon reactivation, making them subject to reconsolidation. In the past fifteen years, much attention has been paid to comparing molecular processes and pathways responsible for consolidation and reconsolidation of certain kinds of memories. Some investigators have suggested that when a memory is reactivated by stimuli associated with the original learning, there is a replay of cellular events that occur during the initial consolidation (Sara 2000). For instance, protein synthesis is required for both consolidation and reconsolidation of cued and contextual fear conditioning (Debiec et al. 2002; Nader et al. 2000; Schafe and LeDoux 2000), and knockout of Zif268, a zinc finger protein, results in impairments in both consolidation and reconsolidation of an object recognition memory (Bozon et al. 2003; Jones et al. 2001). Hence, processes implicated in memory consolidation, such as activation of specific intracellular signaling cascades as well as mRNA and protein synthesis, are also involved in reconsolidation (Bozon et al. 2003; Duvarci et al. 2008; Kida et al. 2002). However, in other instances, the intracellular signaling pathways contributing to the consolidation and reconsolidation of particular memories diverge. Hippocampal CCAAT enhancer binding protein (C/EBPβ) is required for inhibitory avoidance consolidation but not reconsolidation (Taubenfeld et al. 2001). Also, the consolidation and reconsolidation of contextual fear conditioning are doubly dissociable, as consolidation involves hippocampal brain-derived neurotrophic factor (BDNF) but not Zif268 whereas reconsolidation recruits Zif268 but not BDNF (Lee et al. 2004).
Direct comparisons between consolidation and reconsolidation of drug-context memories are currently lacking. In one of the few studies of drug-cue memory consolidation, Hsu et al. (2002) demonstrated that post-training, temporary inactivation of the amygdala blocked the consolidation of amphetamine conditioned place preference (CPP). The reconsolidation of drug-cue memories has been a recent focus of interest, with studies pointing to the necessity of transcription, translation, and specific intracellular signaling pathways in the reconsolidation of these memories (Brown et al. 2007; Lee et al 2005; Milekic et al. 2006; Miller and Marshall 2005). Specific cell surface receptors (e.g., β-adrenergic receptors) are also necessary for the reconsolidation of drug-cue memories, as post-reactivation administration of propranolol was found to disrupt the reconsolidation of cocaine-context memories (Bernardi et al. 2006; Fricks-Gleason and Marshall 2008).
The involvement of N-methyl-D-aspartate (NMDA) glutamate receptor-dependent processes in drug-cue memories accords with their involvement in a variety of brain and behavioral plasticity mechanisms. Their role in long-term synaptic potentiation (LTP) makes NMDA receptors essential for the neuroplasticity proposed to underlie learning and memory processes (Cain 1997; Castellano et al. 2001; Riedel et al. 2003). Recent findings from different laboratories have supported the pivotal role for NMDA receptors in the consolidation and reconsolidation of drug-cue memories. Infusion of a NMDA receptor antagonist into the basolateral amygdala (BLA) immediately following cocaine-cue associative learning was found to block the consolidation of cocaine-cue memories (Feltenstein and See 2007), while several others have used systemic administrations of NMDA receptor antagonists to disrupt the reconsolidation of drug-cue memories (Brown et al. 2008; Kelley et al. 2007; Milton et al. 2008; Sadler et al. 2007; Wouda et al. 2010). But, direct comparisons between the influences of NMDA receptor antagonists on drug-cue memory consolidation and reconsolidation have been hampered by the use of different behavioral paradigms (i.e., self-administration and CPP), drugs and drug doses, and particular training/testing procedures in separate laboratories. The goal of the present study was to compare directly the roles of NMDA receptors in both the consolidation and reconsolidation of cocaine-cue memories. Specifically, these experiments compared the effects of systemic administrations of two different NMDA receptor antagonists, MK-801 and memantine, on the consolidation and reconsolidation of cocaine-conditioned place preference in a common setting. Two non-competitive NMDA receptor antagonists were used to determine whether the effects observed were due to the common biological consequence of blocking NMDA receptors.
Methods
Subjects
Male Sprague-Dawley rats (Charles River Labs) weighing 250-275 g upon arrival were individually housed in a temperature-controlled (21 ± 2°C) colony room with ad libitum access to food and water. Lights were maintained on 12 h light/dark cycle (lights on from 6:00 to 18:00), with all procedures performed during the light portion of the cycle. Animals were handled for two days prior to the start of experiments. All experiments were conducted in accordance with the National Institutes of Health guidelines for animals care and were approved by the Institutional Animal Care and Use Committee of the University of California, Irvine.
Drugs
Cocaine-HCl, (+)-MK-801 hydrogen maleate, and memantine-HCl were purchased from Sigma-Aldrich (St. Louis, Missouri, USA) and dissolved in saline (0.9% NaCl). All solutions were freshly prepared prior to use. Cocaine-HCl is expressed as the weight of the salt, while MK-801 and memantine are expressed as free-base. For training, cocaine-HCl was dissolved to a final concentration of 12 mg/ml and administered in a volume of 1 ml/kg body weight. The lower doses of MK-801 and memantine (0.1 and 6.5 mg/kg, respectively) and the corresponding saline groups were administered in a volume of 1 ml/kg body weight, whereas the higher doses of MK-801 and memantine (0.2 and 10 mg/kg, respectively) and the corresponding saline groups were given at 2 ml/kg body weight. All drug treatments and saline were administered intraperitoneally (i.p).
The doses of MK-801 used in these experiments are commonly used in behavioral pharmacological studies and have been previously shown to have effects on aversive and appetitive memories (Biala and Kotlinska 1999; Brown et al. 2008; Lee et al. 2006; Milton et al. 2008; Sadler et al. 2007; Wouda et al. 2010). The doses of memantine were chosen on the basis that administration of 5-10 mg/kg memantine leads to deficits in appetitive memories (Bespalov et al. 2000; Popik et al. 2006).
Place Preference Apparatus
Conditioning took place in a three-chamber apparatus (Med Associates Inc.) consisting of two larger compartments (compartments [28 cm × 21 cm] separated by a smaller compartment [12 cm × 21 cm]). The two larger compartments differed in visual, olfactory, and tactile cues. One compartment had white walls and a wire mesh floor above pine shavings, while the other compartment had black and white checkered walls and a bar floor above cedar shavings. The middle compartment had two gray walls and a solid gray Polyvinyl floor above corncob bedding. Additionally, this middle compartment had a white wall and a checkered wall leading into the corresponding compartment. Guillotine doors (9 cm × 10 cm), patterned to match the outer compartments, separated the three compartments and were lowered on training days and raised on test days. The entire apparatus contained photobeams, which were used to quantify time spent in each compartment of the CPP apparatus and total distance traveled during testing.
Conditioned Place Preference
All training and testing procedures were conducted between 8:00 to 12:00 each day. The CPP procedure for all of the experiments was performed using an unbiased, counterbalanced protocol. Baseline preferences for each of the experiments were assessed by placing the animals in the center compartment of the place preference apparatus and allowing free access to all compartments for 15 min (initial test). The time spent in each of the large outer compartments was analyzed by automated software recordings of infrared beam breaks. Animals were divided into treatment groups such that each group had an equal mean time spent in each compartment. A Wilcoxon signed ranks test revealed no significant difference in time spent in the large compartments during the initial test for any of the prospective treatment groups (unbiased design). However, cocaine was always paired with the large compartment determined to be less preferred during the initial test. The training stage began one day after the initial test. Animals were given cocaine or saline on training day 1 and immediately confined to the cocaine-paired or -unpaired compartment, respectively, for 30 min. A counterbalanced design was used so that half the animals were given cocaine prior to placement in the cocaine-paired compartment and half received saline prior to placement in the cocaine-unpaired compartment on training day 1. The next day, treatment and compartment were reversed for each animal (training day 2). For experiments 1 and 2, animals received a total of two 30-min pairings with cocaine in one compartment and two 30-min pairings with saline in the other. Each pair of training sessions was followed by a preference test the next day. For experiments 3 through 5, animals received three saline and three cocaine pairings in an alternate fashion and were confined to the assigned compartment for a 30-min period. Thus, a total of six consecutive days of training were carried out, which we refer to as “cocaine-CPP conditioning.”
The preference test for cocaine-induced CPP (test 1) was administered two days after the last training day. Preference tests were conducted using the same procedures as for initial test. All preference tests were given in a drug free state, and cocaine-CPP expression was indicated by preference score (PS) values, which represent the time spent in the cocaine-paired compartment minus the time spent in the saline-paired compartment.
Experimental Designs
Experiment 1: Effect of post-training MK-801 treatment
To determine the effect of post-training MK-801 administration on the consolidation of cocaine-cue memory, MK-801 was injected immediately after cocaine-CPP training sessions and animals were subsequently tested for cocaine-CPP expression. Training took place as described above. All animals in the MK-801 group received an injection of 0.1 mg/kg MK-801, while the saline group received an injection of saline (1 ml/kg) after both pairs of cocaine and saline training sessions.
Experiment 2: Effect of post-training memantine treatment
To further investigate the potential role of NMDA receptors in the consolidation of cocaine-cue memories, we conducted this experiment identically in all respects to the experiment above, except that the NMDA receptor antagonist memantine (6.5 mg/kg) was administered after both pairs of cocaine and saline training sessions.
Experiment 3: Effect of post-reactivation MK-801 (0.1 mg/kg) treatment
To determine its effect on the stability of a previously learned cocaine-cue memory, animals were given 0.1 mg/kg MK-801 immediately after brief exposures to the cocaine-paired compartment (memory reactivation trials) and subsequently tested for cocaine-CPP expression. Animals underwent six days of cocaine-CPP conditioning and were subsequently preference-tested (test 1) two days after the last training day. One day later, all animals were confined to the cocaine-paired compartment for 3 min and administered either 0.1 mg/kg MK-801 or saline (l ml/kg) immediately afterward. The animals underwent another preference test (test 2) the next day. This two-day paradigm of confinement followed by treatment and preference test was repeated once more three days later. Reinstatement tests began two days after test 3, during which each group received a priming injection of cocaine (2.5 mg/kg or 5.0 mg/kg) immediately prior to a preference test. Both groups were given a drug-free preference test one or two days later.
Experiment 4: Reactivation-dependence of MK-801 (0.2 mg/kg) treatment
This experiment was conducted to determine whether concomitant memory-reactivation was necessary for MK-801 to disrupt subsequent preference for the cocaine-paired compartment and to investigate the dose-response relationship between this NMDA receptor antagonist and the reconsolidation of cocaine-cue memory. This experiment was similar to experiment 3 except that it used a higher dose of MK-801, employed an additional round of confinement followed by treatment, and incorporated a nonreactivated control group consisting of a separate cohort of animals that were given MK-801 in their home cage. The nonreactivated control group did not undergo memory reactivation; instead, this group was given 0.2 mg/kg MK-801 in the home cage on the days that the MK-801 treatment and corresponding saline groups were confined to the cocaine-paired compartment and given 0.2 mg/kg MK-801 or saline (l ml/kg), respectively, immediately afterward. The 0.2 mg/kg dose of MK-801 was chosen for the nonreactivated control group in order to maximize the likelihood of detecting any nonspecific, context-independent pharmacological influence on the behavioral measure (preference).
Experiment 5: Effect of post-reactivation memantine treatment
To further test the potential role of NMDA receptors in the reconsolidation of a previously learned cocaine-cue memory, we examined whether the NMDA receptor antagonist memantine would interfere with cocaine-CPP reconsolidation in a different set of animals. The experimental paradigm used was similar to experiments 3 and 4 except that, following cocaine-CPP conditioning, preference testing continued until a priori criteria for loss of preference (“no-preference criteria”) were met. Once each group met these criteria, they were tested for cocaine-primed reinstatement. The “no-preference criteria” were met when two consecutive tests occurred during which: (1) the mean PS was 25% of test 1 PS and (2) the mean PS did not differ significantly from zero (one-sample t-test, p < 0.05). Following test 1, the four-day paradigm of confinement (followed by treatment), preference test, and two days off was repeated until each group met the no-preference criteria. Reinstatement testing began two days after preference loss criteria were met by each group. A priming injection of cocaine (1.25 mg/kg) was administered immediately prior to a preference test. The following day, animals were given a priming injection of saline (1 ml/kg) and tested.
Experiment 6: Effect of memantine treatment after confinement to both the saline- and cocaine-paired compartments
This experiment was conducted to address the possibility that memantine induced an aversion to the cocaine-paired compartment in experiment 5. The experimental paradigm used was similar to experiments 3, 4, and 5, except that reactivation trials were conducted by confining the animals to each of the compartments on alternating days. After animals underwent cocaine-CPP conditioning and preference testing, they were confined to the cocaine-paired or -unpaired (referred to as saline-paired in Fig. 3b) compartment for 3 min and administered 10 mg/kg memantine or saline (1 ml/kg) immediately afterward. A counterbalanced design was used so that half the animals were confined to the cocaine-paired compartment and half were confined to the cocaine-unpaired compartment during the first reactivation trial day. The next day, animals were confined for 3 min to the alternate compartment and given 10 mg/kg memantine or saline (1 ml/kg) immediately afterward. Another preference test (test 2) was given the following day. This three-day paradigm of two confinements (each followed by treatment) and preference test was repeated twice more with two days off in between.
Figure 3. Post-reactivation memantine administration interfered with the reconsolidation of cocaine-CPP.
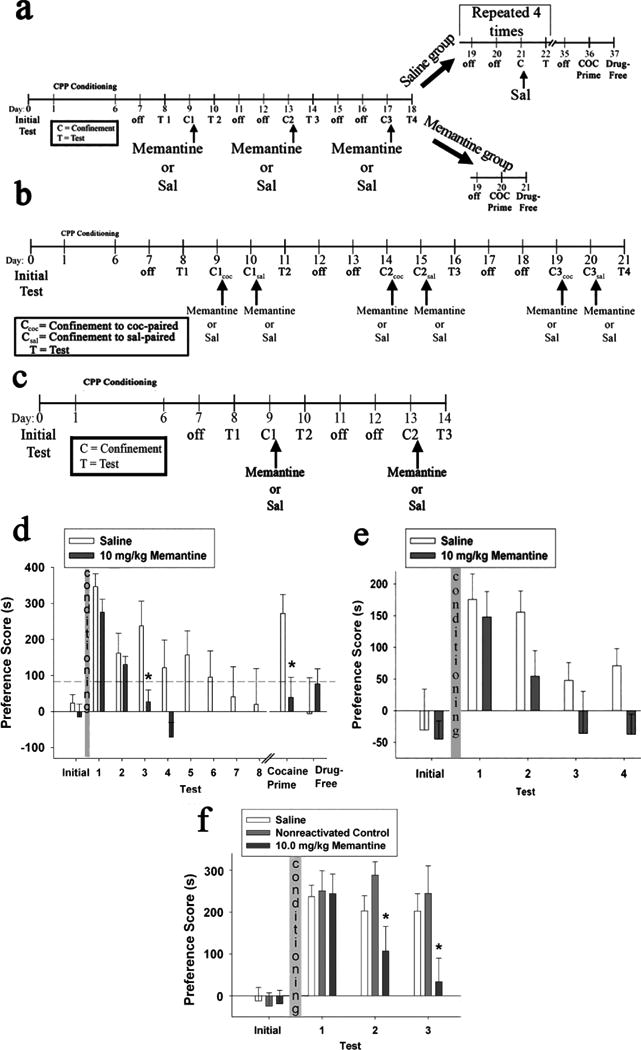
(a, b, c) Schematic representation of the experimental timelines for experiments 5, 6, and 7, respectively. (d, e, f) Cocaine-CPP expression for experiments 5, 6, and 7, respectively. Data are presented as Preference Score, as defined in Figure 1 legend. For (d) Saline group (n = 8); 10 mg/kg Memantine group (n = 10). Dashed line represents a PS less than 25% of test 1 PS, used to establish no-preference criteria. For (e) Saline group (n = 11); 10 mg/kg Memantine group (n = 13). For (f) Saline group (n = 12); Nonreactivated 10 mg/kg Memantine-treated Control group (n = 10); 10 mg/kg Memantine treatment group (n = 11). * Indicates significant difference from saline group (one-way ANOVAs, p < 0.01). Abbreviations: COC, cocaine; Sal, saline; C and Ccoc, 3 min confinement to the cocaine-paired compartment; Csal , 3 min confinement to the cocaine-unpaired compartment; T, Test.
Experiment 7: Reactivation-dependence of memantine treatment
This experiment was conducted to determine whether the ability of memantine to disrupt subsequent preference for the cocaine-paired environment dependent upon memory-reactivation. We conducted this experiment similarly to experiment 4, but the NMDA receptor antagonist memantine (10 mg/kg) was utilized instead of MK-801 and the two-day paradigm of confinement followed by treatment and preference test was carried out twice.
Statistical analysis
SPSS 17.0 for Windows (SPSS) was used for all statistical analysis. Data were analyzed using analysis of variance (ANOVAs) after being checked for normality and sphericity. Mauchly's test of sphericity was not significant (p > 0.3) for repeated measures ANOVA models. All datasets passed the test for normality (p > 0.06 by Kolmogorov-Smornov test). General linear model was used to run two-way repeated measures ANOVA (two-way ANOVA), with PS scores at tests as within-subjects variables and treatment (NMDA receptor antagonist vs. saline groups) as the between-subjects factor. When two-way ANOVAs revealed significant treatment-by-test interactions and/or main group effects, post hoc tests were conducted using one-way ANOVAs to investigate an effect of prior NMDA receptor antagonist treatment on subsequent tests. For post hoc analyses in which multiple comparisons were performed, the Bonferroni correction was used. Wilcoxon signed-ranks tests (z-values) were conducted to compare the amount of time spent in the cocaine-paired and -unpaired compartments during the initial and reinstatement tests. Statistical significance was typically declared at p ≤ 0.05, but when the Bonferroni correction was used in instances where 2 or 3 post hoc comparisons were made significance was declared at p ≤0.025 or p ≤ 0.017, respectively.
Results
Post-training NMDA receptor antagonist administration blocks the formation of cocaine- CPP
In experiment 1, systemic administrations of MK-801 immediately after training sessions impaired the formation of cocaine-CPP, whereas the saline group was able to establish a significant preference for the cocaine-paired environment (Fig. 1a). The main effect of treatment on post-training preference scores was significant (F (1, 26) = 4.73, p = 0.04). The 0.1 mg/kg MK-801 group showed a significantly lower PS than the saline group during the second post-training test (F (1, 26) = 6.25, p = 0.02).
Figure 1. Post-training NMDA receptor antagonist (MK-801 or memantine) administration significantly impaired consolidation of cocaine-CPP.
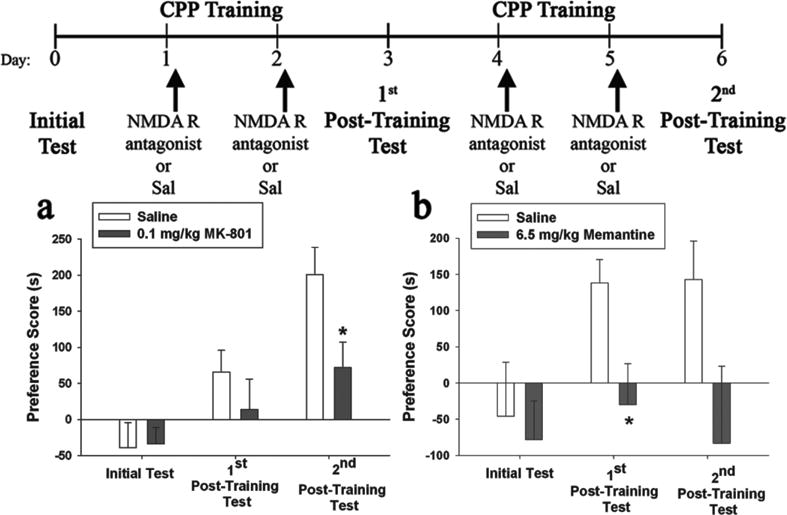
(Top) Schematic representation of the experimental timeline for both experiments 1 and 2. Cocaine-CPP expression is indicated by Preference Score (time spent in the cocaine-paired compartment minus time spent in the cocaine-unpaired compartment). Values represent mean ± SEM. (a) Saline group (n = 14); 0.1 mg/kg MK-801 group (n = 14). (b) Saline group (n = 9); 6.5 mg/kg Memantine group (n = 10). * Indicates significant difference from saline group (one-way ANOVAs, p = 0.02). Abbreviation: Sal, saline.
The results of experiment 2 further support a role for NMDA receptors in the consolidation of cocaine-cue memories by demonstrating that post-training administrations of memantine also blocked consolidation (Fig. 1b). The main effect of treatment on post-training PS was significant (F (1, 17) = 4.71, p = 0.04). During the first post-training test, the 6.5 mg/kg memantine group showed a significantly lower PS than the corresponding saline group (F (1, 17) = 6.27, p = 0.02).
Post-reactivation NMDA receptor antagonist administration attenuates an established cocaine-CPP
In experiment 3, we found that short (3 min) confinements to the cocaine-paired compartment followed immediately by 0.1 mg/kg MK-801 or saline administration resulted in a rapid decay of preference in the MK-801 group, while the saline group more strongly maintained its preference (Fig. 2c). This was supported by the results of ANOVAs comparing PS from tests 1 through 3, where a significant treatment-by-test interaction was found (F (2, 56) = 4.75, p = 0.01). After two rounds of confinement followed by 0.1 mg/kg MK-801 treatment, the MK-801 group showed a significantly lower PS than the corresponding saline group on test 3 (F (1, 28) = 6.81, p = 0.01). By test 3, the MK-801 group's preference was abolished (z = -0.91, p = 0.36).
Figure 2. Post-reactivation MK-801 administration interfered with the reconsolidation of cocaine-CPP.
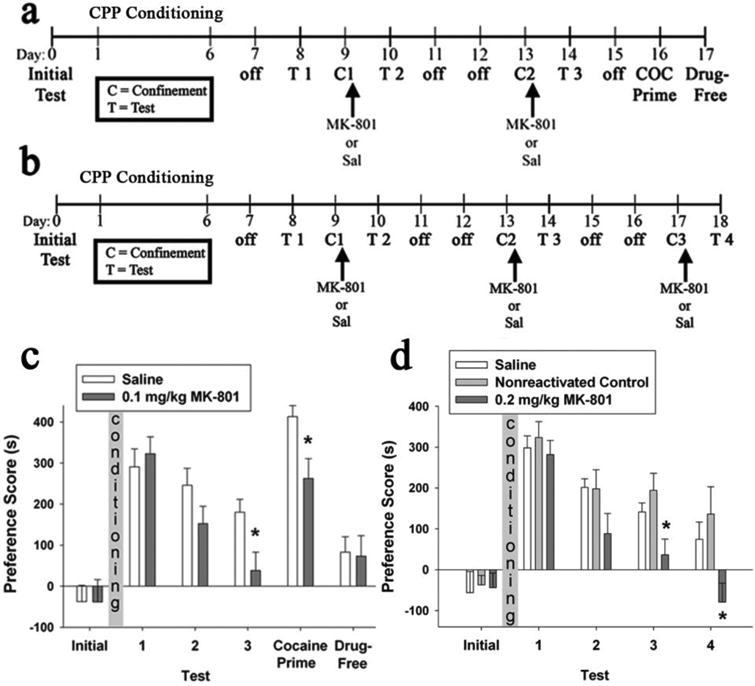
(a, b) Schematic representation of the experimental timelines for experiments 3 and 4. (c, d) Cocaine-CPP expression for experiments 3 and 4. Data are presented as Preference Score, as defined in Figure 1 legend. (c) Saline group (n = 15); 0.1 mg/kg MK-801 group (n = 15). (d) Saline group (n = 9); Nonreactivated 0.2 mg/kg MK-801-treated Control group (n = 9); 0.2 mg/kg MK-801 treatment group (n = 9). * Indicates significant difference from saline group (one-way ANOVAs, p ≤ 0.01). Abbreviations: COC, cocaine; Sal, saline; C, 3 min confinement to the cocaine-paired compartment; T, Test.
MK-801 treatment post-confinement also blunted subsequent cocaine-primed reinstatement. In response to the cocaine priming injection, both groups showed a significant preference for the cocaine-paired compartment (saline group: z = -3.41, p < 0.01; MK-801 group: z = -3.18; p < 0.01). However, the 0.1 mg/kg MK-801 group displayed a significantly lower PS than the corresponding saline group during the cocaine-primed reinstatement test (F (1, 28) = 7.46, p = 0.01). This significant group difference depended upon the cocaine priming injection, since testing the animals the following day in a drug-free state yielded no significant preference or group difference (F (1, 28) = 0.024, p = 0.88) (Fig. 2c).
In experiment 4, we found that systemic administration of 0.2 mg/kg MK-801 immediately following brief confinement to the cocaine-paired compartment, but not when given in the absence of a reactivation session, attenuated the animals' previously established preference for the cocaine-paired compartment (Fig. 2d). The nonreactivated control group, which received MK-801 in the home cage and was not exposed to the CPP apparatus on these days, was included to assess whether the behavioral effect of MK-801 was a consequence of non-associative drug effects. For this experiment, analysis was performed by comparing the PS values of the MK-801 treatment group with the values of a combined control group, which included both the saline and nonreactivated MK-801 controls. The use of the combined control was supported by our a priori prediction that the nonreactivated MK-801-treated group would be unaffected by 0.2 mg/kg MK-801, a prediction confirmed by a two-way ANOVA showing no main effects or interaction with test session between the saline and nonreactivated control conditions (F (1,16) = 0.62, p = 0.44; F (3,48) = 0.44, p = 0.73; respectively). ANOVA comparing PS of the reactivated MK-801 group and combined control group across tests 1 through 4 revealed a significant main effect of treatment (F (1, 25) = 9.40, p < 0.01) and a significant treatment-by-test interaction (F (3, 75) = 2.72, p = 0.05). Similar to experiment 3, after two rounds of confinement followed by 0.2 mg/kg MK-801 treatment, the MK-801 group showed a significantly lower PS than the combined control group on test 3 (F (1, 25) = 9.15, p < 0.01). Such a significant group difference was also attained after three rounds of confinement followed by 0.2 mg/kg MK-801 treatment, on test 4 (F (1, 25) = 8.31, p < 0.01) (Fig. 2d). These results suggest that the attenuation of preference as a result of post-reactivation MK-801 administrations is unlikely to result from non-associative effects of this drug. Examination of the effects of MK-801 in experiments 3 and 4 (Fig. 2c, d) suggests no obvious differences in the effects of the 0.1 mg/kg and 0.2 mg/kg MK-801 doses.
In experiment 5, we used another NMDA receptor antagonist, memantine, to test the role of NMDA receptors in the reconsolidation of previously established cocaine-CPP. As shown in Fig. 3d, after repeated confinement followed by 10 mg/kg memantine or saline administrations, the memantine group's preference decayed more rapidly than that of the saline group. ANOVAs were used to compare PS across tests 1 through 4, where a significant main effect of treatment (F (1, 16) = 6.46, p = 0.02) and a significant treatment-by-test interaction (F (3, 48) = 3.12, p = 0.04) were found. After two rounds of confinement followed by memantine treatment, the memantine group's preference was completely abolished and we observed a significant difference (F (1,16) = 8.73, p < 0.01) between the PS values of the memantine group compared to the saline group on test 3. The memantine group met the pre-established preference loss criteria during test 4 (day 18), whereas the corresponding saline group did not meet these criteria until test 8 (day 34). Once each group met these criteria, they underwent a cocaine-primed reinstatement test (memantine group: day 20; saline group: day 36). In response to the cocaine priming injection, the saline group showed a significant preference for the cocaine-paired compartment (z = - 2.52, p = 0.01), but the memantine group exhibited no significant reinstatement of preference (z = - 0.051, p = 0.96) and this group's PS was significantly lower than that of the saline group (F (1, 16) = 8.98, p < 0.01). When tested in a drug-free state the following day, there was no significant group difference (F (1, 16) = 0.68, p = 0.42), indicating that the cocaine-primed reinstatement of the saline group on the previous day was indeed attributable to the cocaine priming injection (Fig. 3d).
In experiment 6, we found that systemic administration of 10 mg/kg memantine immediately following brief confinement to both the cocaine-paired and -unpaired compartments attenuated the animals' previously established preference (Fig. 3e). ANOVAs comparing PS during tests 1 through 4 showed a significant main effect of treatment (F (1, 22) = 4.19, p = 0.05). After three rounds of confinement to both compartments on consecutive days followed by memantine treatment and subsequent testing, the memantine group showed a strong trend towards a significantly lower PS than the corresponding saline group on test 4 (F (1, 22) = 6.46, p = 0.019), and the difference between the saline and memantine groups was similar to that observed in experiment 5. Because we observed that the memantine-induced loss of cocaine-CPP occurred just as it did when memantine was administered only after confinement to the cocaine-paired compartment, we conclude that an influence of memantine on reactivation-dependent memory processes provides a more suitable explanation for the results than does conditioned aversion.
In experiment 7, we found that systemic administration of 10 mg/kg memantine immediately following brief confinement to the cocaine-paired compartment, but not when administered in the absence of a reactivation session, attenuated the animals' previously established preference for the cocaine-paired compartment (Fig. 3f). The nonreactivated control group, which received memantine in the home cage and was not exposed to the CPP apparatus on these days, was included to assess whether the behavioral effect of memantine was a consequence of non-associative drug effects. For this experiment, analysis was performed by comparing the PS values of the memantine treatment group with the values of a combined control group, which included both the saline and nonreactivated memantine controls. The use of the combined control was supported by our a priori prediction that the nonreactivated memantine-treated group would not be affected by 10 mg/kg memantine, a prediction that was confirmed by a two-way ANOVA showing no main effects or interaction with test session between the saline and nonreactivated control conditions (F (1,20) = 0.90, p = 0.35; F (2,40) = 0.74, p = 0.49; respectively). ANOVA comparing PS of the reactivated memantine group and combined control group across tests 1 through 3 revealed a significant main effect of treatment (F (1, 31) = 4.70, p = 0.04) and a significant treatment-by-test interaction (F (2, 62) = 7.98, p < 0.01). In this experiment, after only one round of confinement followed by 10 mg/kg memantine treatment, the memantine group showed a significantly lower PS than the combined control group on test 2 (F (1, 31) = 5.97, p = 0.02). Such a significant group difference was also attained after two rounds of confinement followed by memantine treatment, on test 3 (F (1, 31) = 8.26, p < 0.01) (Fig. 3f). These results indicate that the attenuation of preference as a result of post-reactivation memantine administrations is unlikely to result from non-associative effects of this drug.
Discussion
By directly comparing the influences of two distinct non-competitive NMDA receptor antagonists on the consolidation and reconsolidation of cocaine-cue memories within a single setting, the present experiments point to a common requirement for NMDA receptor activation in the establishment and maintenance of these associations. In experiments 1 and 2, animals were administered MK-801 or memantine systemically during cocaine-CPP training. Unlike saline-treated animals, NMDA receptor antagonist-treated animals did not establish a preference for the cocaine-paired compartment (Fig. 1a, b), leading to the conclusion that the consolidation of cocaine-cue memories is NMDA receptor-dependent. In experiments 3 through 7, once cocaine-CPP was established, animals were systemically administered MK-801 or memantine immediately following sessions of short-duration confinement to the previously cocaine-paired compartment. Not only did post-reactivation NMDA receptor antagonist administration attenuate the animals' preference for the cocaine-paired compartment (Fig. 2c, d; Fig. 3d-f), it also blunted subsequent cocaine-primed reinstatement (Fig. 2c; Fig. 3d). Regardless of whether number of confinements and tests (Fig. 2c) or preference loss level (Fig. 3d) was controlled for, cocaine-primed reinstatement was significantly less in rats given post-reactivation NMDA antagonists than in animals receiving post-reactivation saline. This blunted reinstatement, which occurred two days after the NMDA receptor antagonist was last administered, suggests that NMDA receptor blockade did not simply make the stored information briefly inaccessible; it interfered with the reconsolidation of the drug-cue memory.
Studies have shown that impairments in drug-cue memory reconsolidation can result from pre-reactivation NMDA receptor antagonist administration (Brown et al. 2008; Kelley et al. 2007), suggesting that normal NMDA receptor function during drug-cue exposure is critical to the maintenance of such memories. Conversely, others have demonstrated that NMDA receptor antagonism interferes with the reconsolidation of drug-cue memories even when these receptors are blocked post-reactivation (Popik et al. 2006; Sadler et al. 2007). Our work extends these findings by demonstrating that two distinct NMDA receptor antagonists disrupt the reconsolidation of drug-cue memories when given after memory reactivation. The present design of administering NMDA receptor antagonists after memory reactivation affords significant benefits because it eliminates the potentially confounding influences of these drugs on attention, arousal, or other processes during the memory reactivation period (Gold and Van Buskirk 1976; Introini-Collison et al.1992; McGaugh 1992).
In interpreting the consolidation experiments, we have considered the possibility that the post-training administration of NMDA receptor antagonists might have resulted in significant levels of these antagonists in the animals the next day, when subsequent training or testing was performed. The short half-lives of both MK-801 and memantine make it unlikely that the presence of these drugs in the animals the day after administration could have interfered with our results. The half-life of MK-801 in the rat is approximately 49 min–2.05 h (Hucker et al. 1983; Schwartz and Wasterlain 1991; Wegener et al. 2011; Vezzani et al. 1989); even assuming a half-life of 2.05 h, 0.05% of the original dose of MK-801 should be present in the animal 24 h after its administration. The half-life of memantine in the rat is approximately 2.3–3.3 h (Spanagel et al. 1994), and assuming a half-life of 3.3 h, 0.78% of the original memantine dose is expected to be present in the animal 24 h after its administration. Because less than 1% of each drug remains in the rat 24 h after its administration, at the time when the subsequent training or testing session occurs, the effects of post-training administration of these NMDA receptor antagonists are almost certainly due to their actions on memory consolidation and unrelated to interference with learning or performance the next day.
Also novel to the present study is the comparison between two non-competitive NMDA receptor antagonists, both of which interfered with the consolidation and reconsolidation of cocaine-cue memories. MK-801 and memantine are open-channel blockers that block NMDA receptors only when these channels are activated (Chen et al. 1992). This mechanism of action contributes to their efficacy in blocking NMDA receptors at a time when activation of these receptors is necessary to support the establishment and maintenance of memories. Despite these common features, MK-801 and memantine interact with the NMDA receptor complex in different ways. One of the most important distinctions is the receptor binding kinetics of these two antagonists. In comparison to memantine, which is a low-affinity blocker with rapid blocking kinetics (Ribeiro Do Couto et al. 2004; Tzschentke and Schmidt 1999), MK-801 is a high-affinity blocker with slower kinetics, and this is reflected in its marked use- and weaker voltage-dependency (Parsons et al. 1999). Further, these compounds differ in their binding site. While memantine is believed to bind at or near the Mg2+ binding site (Chen and Lipton 1997; Chen et al. 1992), MK-801 binds to the PCP site inside the pore of the receptor (Moring et al. 1994; Sakurada et al. 1993). Despite differing pharmacological profiles, these two drugs produced the same behavioral effects of blocking consolidation and interfering with the reconsolidation of cocaine-cue memories. Therefore, we conclude that these behavioral effects are attributable to the drugs' common effect of blocking NMDA receptors.
Our present results point to the common involvement of glutamate NMDA receptors in both the consolidation and reconsolidation of cocaine-cue memories. Once consolidated, the original cocaine-cue memory can be reactivated to a labile state via reactivation and made subject to reconsolidation in order to persist. The finding that NMDA receptor antagonism impairs the consolidation of cocaine-cue memories during cocaine-CPP training reinforces our conclusion that the attenuation of a previously-established preference is the result of an interference with the reconsolidation process due to NMDA receptor antagonism. In keeping with this hypothesis, Rose and Rankin (2006) showed, in C. elegans, that interfering with the reconsolidation of a memory caused a reversal in the training-induced change in glutamate receptor subunit levels that correlated with the original consolidation of the memory. This finding not only implies that reactivation can induce memory lability, but it demonstrates that interfering with a reactivated memory can also alter the cellular correlate of that memory. In this case, the changes in glutamate receptor subunit levels produced by the original learning are subject to reconsolidation interference.
The NMDA receptor is an upstream activator of many of the molecular signaling cascades required to support consolidation and reconsolidation. Studies examining the underlying mechanisms of consolidation and reconsolidation have yielded conflicting results as to whether consolidation and reconsolidation depend on similar or distinct molecular mechanisms. Many groups have found that consolidation and reconsolidation depend on similar processes such as protein synthesis and the activation of transcription factors protein kinase A (PKA), cyclic AMP-response element binding protein (CREB), Zif 268, as well as MAPK activation (Kelly et al. 2003; Kida et al. 2002; Koh and Bernstein 2003; Nader et al. 2000). However, others have identified distinct molecular processes underlying consolidation and reconsolidation. Lee et al. (2004) suggest a double dissociation between BDNF, which is selectively required for consolidation, and Zif 268, which is selectively involved in the reconsolidation of contextual fear memory. Further, the expression of the transcription factor C/EBPβ in the hippocampus is required for the consolidation, but not the reconsolidation, of inhibitory avoidance memory (Taubenfeld et al. 2001). Our present results, which strongly suggest that both the consolidation and reconsolidation of cocaine-cue memories are NMDA receptor-dependent, are not incompatible with evidence that these memory processes can be mediated through distinctive molecular pathways. Post-NMDA receptor activation, there may be a divergence in the signaling cascades activated in order to support consolidation versus reconsolidation processes (Lee et al. 2004; Taubenfeld et al. 2001; Tronel et al. 2005).
Alternatives to the explanation that these drugs disrupted the reconsolidation of cocaine-context memory have been considered. It is possible that other non-associative effects of the NMDA receptor antagonists are responsible for the observed interference; however, this appears unlikely since animals treated with either MK-801 or memantine in the home cage maintained their preference for the cocaine-paired compartment similarly to the saline group. In support of this, Brown et al. (2008) addressed the possibility that non-associative effects of MK-801 are attributable to their subsequent effects on drug-cue preferences. They found that MK-801 given in the home cage resulted in normal cocaine-primed reinstatement, whereas MK-801 given prior to a reactivation session suppressed subsequent cocaine-primed reinstatement in a self-administration model. These findings support the conclusion that the administration of either MK-801 or memantine at the time that the cocaine-context memory is reactivated is required for subsequent loss of preference for the cocaine-paired compartment. Further, in the present study a control experiment was conducted to address the possibility that memantine induced an aversion to the cocaine-paired compartment. The paradigm used in this control experiment (experiment 6) was identical to that of experiment 5 except that memantine (or saline) was administered after brief (3 min) confinement to both compartments on alternating days and animals were subsequently tested for preference. If memantine has aversive properties, this aversion should become equally associated with each of the two compartments and the rats should show no change in their preference for these compartments. However, we observed that memantine-induced preference loss occurred just as it did when memantine was given only after confinement to the cocaine-paired compartment. Therefore, the attenuation of preference seen after post-reactivation administration of memantine is not attributable to the formation of an aversive association.
The possibility that extinction learning occurred during reactivation trials and that our results are due to the facilitation of extinction rather than interference with reconsolidation by NMDA receptor antagonist administration cannot be ruled out. Several additional lines of evidence argue against the facilitation of extinction by the pharmacological agents used in this study. First, short (3 min) reactivation trials (Inda et al. 2011) and a spaced learning paradigm (Mueller et al. 2002; Mueller and Stewart 2000; Paolone et al. 2009) were used to minimize extinction learning. Second, even in animals equated for preference loss, cocaine-primed reinstatement was blunted by memantine treatment. It is important to acknowledge that when extinction of both appetitive and aversive tasks is pharmacologically enhanced, their reinstatement by primers is weakened (Ledgerwood et al. 2004; Malvaez et al. 2010; Paolone et al. 2009). Despite the limitation that reconsolidation interference and enhanced extinction can both diminish reinstatement, primed reinstatement remains a useful means by which memories that undergo extinction can be distinguished from memories that are diminished by other processes (Brown et al. 2008; Fricks-Gleason and Marshall 2008; Inda et al. 2011; Kelley et al. 2007). Importantly, in the interpretation of the effects of cocaine reinstatement, a considerable body of research has shown that NMDA receptor blockade impairs, not facilitates, memory processes. For example, blocking NMDA receptors impairs the original consolidation of long-term object recognition memory (Akirav and Maroun 2006) and the extinction of fear memories (Liu et al. 2009). With regard to drug-cue memories, blocking NMDA receptors has been shown to hinder cocaine-associated memory consolidation (Feltenstein and See 2007) as well as block the extinction of amphetamine-CPP (Hsu and Packard 2008). Because, in the present study, NMDA receptor blockade impaired the consolidation of cocaine-cue memories, it is most likely that the attenuation of a previously-established preference observed in NMDA receptor antagonist-treated animals reflects interference with the reconsolidation of these memories. For similar reasons, the effects of NMDA receptor antagonism observed here are unlikely to represent facilitated extinction learning.
The present experiments did not seek to determine the neural locus of the effects of NMDA receptor antagonists on the consolidation or reconsolidation of cocaine-cue memories. However, the amygdala is a likely candidate because its integrity appears to be necessary for both aversive and appetitive memories. Fear memory consolidation is modulated by the amygdala (Ota et al. 2008; Schafe et al. 2000), and post-training intra-basolateral amygdala (BLA) infusions of anesthetics or a NMDA receptor antagonist have been shown to block the consolidation of food-, amphetamine-, and cocaine-associated memories (Feltenstein and See 2007; Hsu et al. 2002; Schroeder and Packard 2000). Previous studies suggest that fear memory reconsolidation depends on the lateral amygdala specifically (Debiec et al. 2006; Debiec and LeDoux 2004). Additionally, Milton et al. (2008) directly investigated the role of NMDA receptors in the BLA and found that they are necessary for the reconsolidation of drug-associated memories.
In summary, administration of NMDA receptor antagonists during and after cocaine-CPP training resulted in impaired consolidation (establishment) and reconsolidation (maintenance) of cocaine-cue memories, respectively. Although studies have independently investigated the role of NMDA receptors in the consolidation (Feltenstein and See 2007; Hsu et al. 2002) and reconsolidation (Brown et al. 2008; Itzhak 2008; Kelley et al. 2007; Milton et al. 2008; Sadler et al. 2007; von der Goltz et al. 2009; Wouda et al. 2010) of drug-associated memories, this is the first study in which the consolidation and reconsolidation of drug-cue memory is investigated within a common setting, using internally consistent methodology. Our findings identify a point of commonality between the consolidation and reconsolidation of cocaine-cue memories: i.e., NMDA receptor-dependency. Therapeutically, it may be difficult to intervene during the consolidation of drug-cue memories, which drive drug-seeking behavior and relapse, in drug abusers who are becoming addicted. However, these studies identify NMDA receptors as a potential target for pharmacotherapeutic agents aimed at interfering with the reconsolidation of drug-cue memories, encouraging further work on the development of treatments for established addictions.
Acknowledgments
The authors thank Dr. Norbert J. Fortin for his help with statistical analysis and Anna J. Khalaj for her comments. Research supported by PHS grant DA 021807 to JFM.
Footnotes
Conflict of interest The authors have no conflicts of interest to declare.
References
- Akirav I, Maroun M. Ventromedial prefrontal cortex is obligatory for consolidation and reconsolidation of object recognition memory. Cereb Cortex. 2006;16:1759–1765. doi: 10.1093/cercor/bhj114. [DOI] [PubMed] [Google Scholar]
- Bernardi RE, Lattal KM, Berger SP. Postretrieval propranolol disrupts a cocaine conditioned place preference. Neuroreport. 2006;17:1443–1447. doi: 10.1097/01.wnr.0000233098.20655.26. [DOI] [PubMed] [Google Scholar]
- Bespalov AY, Zvartau EE, Balster RL, Beardsley PM. Effects of N-methyl-D-aspartate receptor antagonists on reinstatement of cocaine-seeking behavior by priming injections of cocaine or exposures to cocaine-associated cues in rats. Behav Pharmacol. 2000;11:37–44. doi: 10.1097/00008877-200002000-00004. [DOI] [PubMed] [Google Scholar]
- Biala G, Kotlinska J. Blockade of the acquisition of ethanol-induced conditioned place preference by N-methyl-D-aspartate receptor antagonists. Alcohol Alcohol. 1999;34:175–82. doi: 10.1093/alcalc/34.2.175. [DOI] [PubMed] [Google Scholar]
- Bozon B, Davis S, Laroche S. A requirement for the immediate early gene zif268 in reconsolidation of recognition memory after retrieval. Neuron. 2003;40:695–701. doi: 10.1016/S0896-6273(03)00674-3. [DOI] [PubMed] [Google Scholar]
- Brown TE, Forquer MR, Cocking DL, Jansen HT, Harding JW, Sorg BA. Role of matrix metalloproteinases in the acquisition and reconsolidation of cocaine-induced conditioned place preference. Learn Mem. 2007;14:214–223. doi: 10.1101/lm.476207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TE, Lee BR, Sorg BA. The NMDA antagonist MK-801 disrupts reconsolidation of a cocaine-associated memory for conditioned place preference but not for self-administration in rats. Learn Mem. 2008;15:857–865. doi: 10.1101/lm.1152808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain DP. LTP, NMDA, genes and learning. Curr Opin Neurobiol. 1997;7:235–242. doi: 10.1016/S0959-4388(97)80012-8. [DOI] [PubMed] [Google Scholar]
- Castellano C, Cestari V, Ciamei A. NMDA receptors and learning and memory processes. Curr Drug Targets. 2001;2:273–283. doi: 10.2174/1389450013348515. [DOI] [PubMed] [Google Scholar]
- Chen HS, Lipton SA. Mechanism of memantine block of NMDA-activated channels in rat retinal ganglion cells: uncompetitive antagonism. J Physiol. 1997;499:27–46. doi: 10.1113/jphysiol.1997.sp021909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HS, Pellegrini JW, Aggarwal SK, Lei SZ, Warach S, Jensen FE, Lipton SA. Open-channel block of N-methyl-D-aspartate (NMDA) responses by memantine: therapeutic advantage against NMDA receptor-mediated neurotoxicity. J Neurosci. 1992;12:4427–4436. doi: 10.1523/JNEUROSCI.12-11-04427.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childress A, Ehrman R, McLellan AT, O'Brien C. Conditioned craving and arousal in cocaine addiction: a preliminary report. NIDA Res Monogr. 1988;81:74–80. [PubMed] [Google Scholar]
- Debiec J, Doyere V, Nader K, LeDoux JE. Directly reactivated, but not indirectly reactivated, memories undergo reconsolidation in the amygdala. Proc Natl Acad Sci U S A. 2006;103:3428–3433. doi: 10.1073/pnas.0507168103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debiec J, LeDoux JE. Disruption of reconsolidation but not consolidation of auditory fear conditioning by noradrenergic blockade in the amygdala. Neuroscience. 2004;129:267–272. doi: 10.1016/j.neuroscience.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Debiec J, LeDoux JE, Nader K. Cellular and systems reconsolidation in the hippocampus. Neuron. 2002;36:527–38. doi: 10.1016/S0896-6273(02)01001-2. [DOI] [PubMed] [Google Scholar]
- Duvarci S, Nader K, LeDoux JE. De novo mRNA synthesis is required for both consolidation and reconsolidation of fear memories in the amygdala. Learn Mem. 2008;15:747–755. doi: 10.1101/lm.1027208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltenstein MW, See RE. NMDA receptor blockade in the basolateral amygdala disrupts consolidation of stimulus-reward memory and extinction learning during reinstatement of cocaine-seeking in an animal model of relapse. Neurobiol Learn Mem. 2007;88:435–444. doi: 10.1016/j.nlm.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fricks-Gleason AN, Marshall JF. Post-retrieval beta-adrenergic receptor blockade: effects on extinction and reconsolidation of cocaine-cue memories. Learn Mem. 2008;15:643–648. doi: 10.1101/lm.1054608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold PE, Van Buskirk R. Enhancement and impairment of memory processes with post-trial injections of adrenocorticotrophic hormone. Behav Biol. 1976;16:387–400. doi: 10.1016/S0091-6773(76)91539-X. [DOI] [PubMed] [Google Scholar]
- Hsu E, Packard MG. Medial prefrontal cortex infusions of bupivacaine or AP-5 block extinction of amphetamine conditioned place preference. Neurobiol Learn Mem. 2008;89:504–12. doi: 10.1016/j.nlm.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Hsu EH, Schroeder JP, Packard MG. The amygdala mediates memory consolidation for an amphetamine conditioned place preference. Behav Brain Res. 2002;129:93–100. doi: 10.1016/S0166-4328(01)00376-X. [DOI] [PubMed] [Google Scholar]
- Hucker HB, Hutt JE, White SD, Arison BH, Zacchei AG. Disposition and metabolism of (+)-5-methyl-10,11-dihydro-5H-dibenzo[a,d] cyclohepten-5,10-imine in rats, dogs, and monkeys. Drug Metab Dispos. 1983;11:54–8. [PubMed] [Google Scholar]
- Inda MC, Muravieva EV, Alberini CM. Memory retrieval and the passage of time: from reconsolidation and strengthening to extinction. J Neurosci. 2011;31:1635–1643. doi: 10.1523/JNEUROSCI.4736-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Introini-Collison I, Saghafi D, Novack GD, McGaugh JL. Memory-enhancing effects of post-training dipivefrin and epinephrine: involvement of peripheral and central adrenergic receptors. Brain Res. 1992;572:81–86. doi: 10.1016/0006-8993(92)90454-H. [DOI] [PubMed] [Google Scholar]
- Itzhak Y. Role of the NMDA receptor and nitric oxide in memory reconsolidation of cocaine-induced conditioned place preference in mice. Ann N Y Acad Sci. 2008;1139:350–7. doi: 10.1196/annals.1432.051. [DOI] [PubMed] [Google Scholar]
- Jones MW, Errington ML, French PJ, Fine A, Bliss TV, Garel S, Charnay P, Bozon B, Laroche S, Davis S. A requirement for the immediate early gene Zif268 in the expression of late LTP and long-term memories. Nat Neurosci. 2001;4:289–96. doi: 10.1038/85138. [DOI] [PubMed] [Google Scholar]
- Kelley JB, Anderson KL, Itzhak Y. Long-term memory of cocaine-associated context: disruption and reinstatement. Neuroreport. 2007;18:777–780. doi: 10.1097/WNR.0b013e3280c1e2e7. [DOI] [PubMed] [Google Scholar]
- Kelly A, Laroche S, Davis S. Activation of mitogen-activated protein kinase/extracellular signal-regulated kinase in hippocampal circuitry is required for consolidation and reconsolidation of recognition memory. J Neurosci. 2003;23:5354–5360. doi: 10.1523/JNEUROSCI.23-12-05354.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kida S, Josselyn SA, Pena de Ortiz S, Kogan JH, Chevere I, Masushige S, Silva AJ. CREB required for the stability of new and reactivated fear memories. Nat Neurosci. 2002;5:348–355. doi: 10.1038/nn819. [DOI] [PubMed] [Google Scholar]
- Koh MT, Bernstein IL. Inhibition of protein kinase A activity during conditioned taste aversion retrieval: interference with extinction or reconsolidation of a memory? Neuroreport. 2003;14:405–407. doi: 10.1097/00001756-200303030-00021. [DOI] [PubMed] [Google Scholar]
- Ledgerwood L, Richardson R, Cranney J. D-cycloserine and the facilitation of extinction of conditioned fear: consequences for reinstatement. Behav Neurosci. 2004;118:505–13. doi: 10.1037/0735-7044.118.3.505. [DOI] [PubMed] [Google Scholar]
- Lee JL, Di Ciano P, Thomas KL, Everitt BJ. Disrupting reconsolidation of drug memories reduces cocaine-seeking behavior. Neuron. 2005;47:795–801. doi: 10.1016/j.neuron.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Lee JL, Everitt BJ, Thomas KL. Independent cellular processes for hippocampal memory consolidation and reconsolidation. Science. 2004;304:839–843. doi: 10.1126/science.1095760. [DOI] [PubMed] [Google Scholar]
- Lee JL, Milton AL, Everitt BJ. Reconsolidation and extinction of conditioned fear: inhibition and potentiation. J Neurosci. 2006;26:10051–10056. doi: 10.1523/JNEUROSCI.2466-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JL, Li M, Dang XR, Wang ZH, Rao ZR, Wu SX, Li YQ, Wang W. A NMDA receptor antagonist, MK-801 impairs consolidating extinction of auditory conditioned fear responses in a Pavlovian model. PLoS One. 2009;4:e7548. doi: 10.1371/journal.pone.0007548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malvaez M, Sanchis-Segura C, Vo D, Lattal KM, Wood MA. Modulation of chromatin modification facilitates extinction of cocaine-induced conditioned place preference. Biol Psychiatry. 2010;67:36–43. doi: 10.1016/j.biopsych.2009.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaugh JL. Time-dependent processes in memory storage. Science. 1966;153:1351–1358. doi: 10.1126/science.153.3742.1351. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. Affect, neuromodulatory systems, and memory storage. In: Christianson S, editor. The Handbook of Emotion and Memory: Current Research and Theory. Erlbaum Associates; New Jersey: 1992. pp. 245–268. [Google Scholar]
- Milekic MH, Brown SD, Castellini C, Alberini CM. Persistent disruption of an established morphine conditioned place preference. J Neurosci. 2006;26:3010–3020. doi: 10.1523/JNEUROSCI.4818-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CA, Marshall JF. Molecular substrates for retrieval and reconsolidation of cocaine-associated contextual memory. Neuron. 2005;47:873–884. doi: 10.1016/j.neuron.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Milton AL, Lee JL, Butler VJ, Gardner R, Everitt BJ. Intra-amygdala and systemic antagonism of NMDA receptors prevents the reconsolidation of drug-associated memory and impairs subsequently both novel and previously acquired drug-seeking behaviors. J Neurosci. 2008;28:8230–8237. doi: 10.1523/JNEUROSCI.1723-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moring J, Niego LA, Ganley LM, Trumbore MW, Herbette LG. Interaction of the NMDA receptor noncompetitive antagonist MK-801 with model and native membranes. Biophys J. 1994;67:2376–2386. doi: 10.1016/S0006-3495(94)80724-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller D, Perdikaris D, Stewart J. Persistence and drug-induced reinstatement of a morphine-induced conditioned place preference. Behav Brain Res. 2002;136:389–397. doi: 10.1016/S0166-4328(02)00297-8. [DOI] [PubMed] [Google Scholar]
- Mueller D, Stewart J. Cocaine-induced conditioned place preference: reinstatement by priming injections of cocaine after extinction. Behav Brain Res. 2000;115:39–47. doi: 10.1016/S0166-4328(00)00239-4. [DOI] [PubMed] [Google Scholar]
- Müller GE, Pilzecker A. Experimentelle Beitrage zur Lehre vom Gedachtnis. Z Psychol. 1900;1:1–300. [Google Scholar]
- Nader K, Schafe GE, Le Doux JE. Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature. 2000;406:722–726. doi: 10.1038/35021052. [DOI] [PubMed] [Google Scholar]
- Ota KT, Pierre VJ, Ploski JE, Queen K, Schafe GE. The NO-cGMP-PKG signaling pathway regulates synaptic plasticity and fear memory consolidation in the lateral amygdala via activation of ERK/MAP kinase. Learn Mem. 2008;15:792–805. doi: 10.1101/lm.1114808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolone G, Botreau F, Stewart J. The facilitative effects of D-cycloserine on extinction of a cocaine-induced conditioned place preference can be long lasting and resistant to reinstatement. Psychopharmacology (Berl) 2009;202:403–409. doi: 10.1007/s00213-008-1280-y. [DOI] [PubMed] [Google Scholar]
- Parsons CG, Danysz W, Quack G. Memantine is a clinically well tolerated N-methyl-D-aspartate (NMDA) receptor antagonist--a review of preclinical data. Neuropharmacology. 1999;38:735–767. doi: 10.1016/S0028-3908(99)00019-2. [DOI] [PubMed] [Google Scholar]
- Popik P, Wrobel M, Bisaga A. Reinstatement of morphine-conditioned reward is blocked by memantine. Neuropsychopharmacology. 2006;31:160–170. doi: 10.1038/sj.npp.1300760. [DOI] [PubMed] [Google Scholar]
- Ribeiro Do Couto B, Aguilar MA, Manzanedo C, Rodríguez-Arias M, Miñarro J. Effects of NMDA receptor antagonists (MK-801 and memantine) on the acquisition of morphine-induced conditioned place preference in mice. Prog Neuropsychopharmacol Biol Psych. 2004;28:1035–1043. doi: 10.1016/j.pnpbp.2004.05.038. [DOI] [PubMed] [Google Scholar]
- Riedel G, Platt B, Micheau J. Glutamate receptor function in learning and memory. Behav Brain Res. 2003;140:1–47. doi: 10.1016/S0166-4328(02)00272-3. [DOI] [PubMed] [Google Scholar]
- Rose JK, Rankin CH. Blocking memory reconsolidation reverses memory-associated changes in glutamate receptor expression. J Neurosci. 2006;26:11582–11587. doi: 10.1523/JNEUROSCI.2049-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadler R, Herzig V, Schmidt WJ. Repeated treatment with the NMDA antagonist MK-801 disrupts reconsolidation of memory for amphetamine-conditioned place preference. Behav Pharmacol. 2007;18:699–703. doi: 10.1097/FBP.0b013e3282effb81. [DOI] [PubMed] [Google Scholar]
- Sakurada K, Masu M, Nakanishi S. Alteration of Ca2+ permeability and sensitivity to Mg2+ and channel blockers by a single amino acid substitution in the N-methyl-D-aspartate receptor. J Biol Chem. 1993;268:410–415. [PubMed] [Google Scholar]
- Sara SJ. Retrieval and reconsolidation: toward a neurobiology of remembering. Learn Mem. 2000;7:73–84. doi: 10.1101/lm.7.2.73. [DOI] [PubMed] [Google Scholar]
- Schafe GE, Atkins CM, Swank MW, Bauer EP, Sweatt JD, LeDoux JE. Activation of ERK/MAP kinase in the amygdala is required for memory consolidation of Pavlovian fear conditioning. J Neurosci. 2000;20:8177–8187. doi: 10.1523/JNEUROSCI.20-21-08177.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafe GE, LeDoux JE. Memory consolidation of auditory pavlovian fear conditioning requires protein synthesis and protein kinase A in the amygdala. J Neurosci. 2000;20:RC96. doi: 10.1523/JNEUROSCI.20-18-j0003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder JP, Packard MG. Differential effects of intra-amygdala lidocaine infusion on memory consolidation and expression of a food conditioned place preference. Psychobiology. 2000;28:486–491. [Google Scholar]
- Schwartz PH, Wasterlain CG. Cardiac arrest and resuscitation alters the pharmacokinetics of MK-801 in the rat. Res Commun Chem Pathol Pharmacol. 1991;73:181–95. [PubMed] [Google Scholar]
- Spanagel R, Eilbacher B, Wilke R. Memantine-induced dopamine release in the prefrontal cortex and striatum of the rat--a pharmacokinetic microdialysis study. Eur J Pharmacol. 1994;262:21–6. doi: 10.1016/0014-2999(94)90023-X. [DOI] [PubMed] [Google Scholar]
- Taubenfeld SM, Milekic MH, Monti B, Alberini CM. The consolidation of new but not reactivated memory requires hippocampal C/EBPβ. Nat Neurosci. 2001;4:813–818. doi: 10.1038/90520. [DOI] [PubMed] [Google Scholar]
- Tronel S, Milekic MH, Alberini CM. Linking new information to a reactivated memory requires consolidation and not reconsolidation mechanisms. PLoS Biol. 2005;3:e293. doi: 10.1371/journal.pbio.0030293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzschentke TM, Schmidt WJ. Memantine does not substantially affect brain stimulation reward: comparison with MK-801. Brain Res. 1999;845:192–198. doi: 10.1016/S0006-8993(99)01963-0. [DOI] [PubMed] [Google Scholar]
- Vezzani A, Serafini R, Stasi MA, Caccia S, Conti I, Tridico RV, Samanin R. Kinetics of MK-801 and its effect on quinolinic acid-induced seizures and neurotoxicity in rats. J Pharmacol Exp Ther. 1989;249:278–83. [PubMed] [Google Scholar]
- von der Goltz C, Vengeliene V, Bilbao A, Perreau-Lenz S, Pawlak CR, Kiefer F, Spanagel R. Cue-induced alcohol-seeking behaviour is reduced by disrupting the reconsolidation of alcohol-related memories. Psychopharmacology (Berl) 2009;205:389–97. doi: 10.1007/s00213-009-1544-1. [DOI] [PubMed] [Google Scholar]
- Wegener N, Nagel J, Gross R, Chambon C, Greco S, Pietraszek M, Gravius A, Danysz W. Evaluation of brain pharmacokinetics of (+)MK-801 in relation to behaviour. Neurosci Lett. 2011;503:68–72. doi: 10.1016/j.neulet.2011.08.012. [DOI] [PubMed] [Google Scholar]
- Wouda JA, Diergaarde L, Riga D, van Mourik Y, Schoffelmeer AN, De Vries TJ. Disruption of Long-Term Alcohol-Related Memory Reconsolidation: Role of beta-Adrenoceptors and NMDA Receptors. Front Behav Neurosci. 2010;4:179. doi: 10.3389/fnbeh.2010.00179. [DOI] [PMC free article] [PubMed] [Google Scholar]


