Abstract
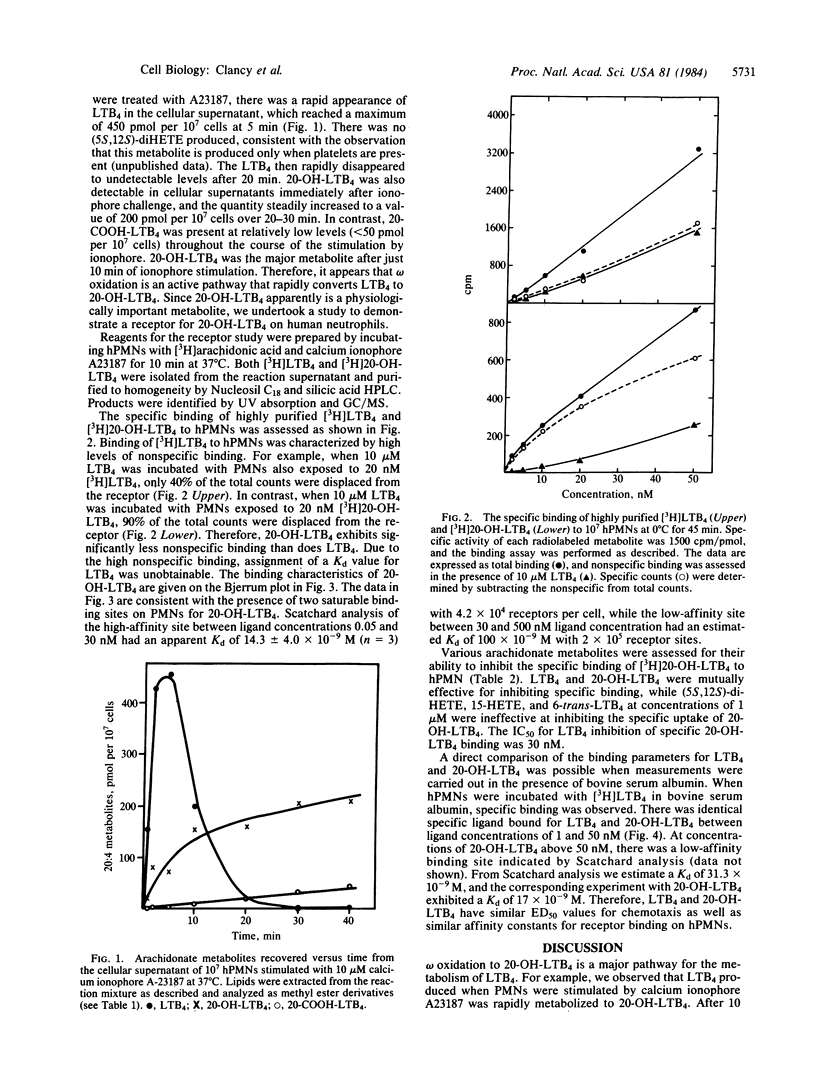
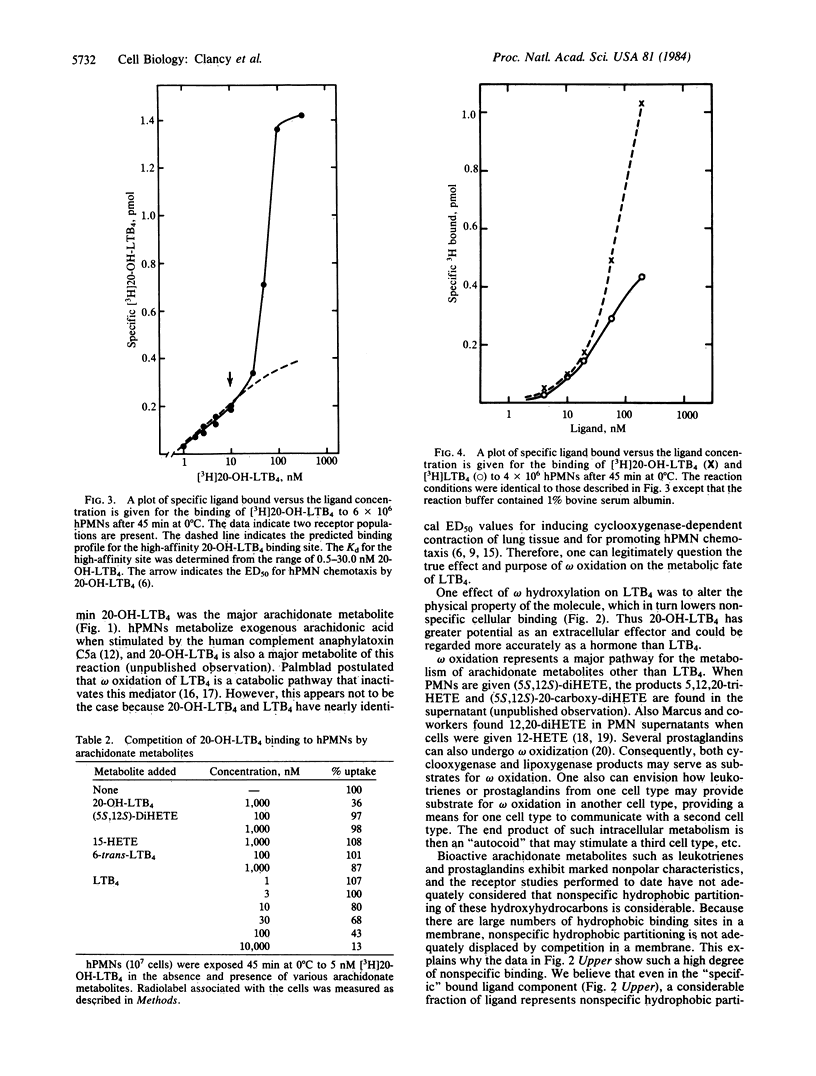
Leukotriene B4 [LTB4; (5S,12R)-5,12-dihydroxy-6,14-cis-8,10-trans-icosatetraenoic acid] and its 20-hydroxy derivative [20-OH-LTB4; (5S,12R)-5,12,20-trihydroxy-6,14-cis-8,10-trans-icosatetraenoic acid] are principal metabolites produced when human neutrophils (hPMNs) are stimulated by the calcium ionophore A23187. These compounds were purified to homogeneity by Nucleosil C18 and silicic acid HPLC and identified by UV absorption and gas chromatographic/mass spectral analyses. 20-OH-LTB4 is considerably more polar than LTB4 and interacts weakly with the hydrophobic Nucleosil C18 resin, whereas LTB4 interacts strongly, reflecting the hydrophobic C13-C20 domain in LTB4. Specific binding of highly purified [3H]LTB4 and [3H]20-OH-LTB4 to hPMNs was assessed. Binding of [3H]20-OH-LTB4 could be largely displaced by an excess of nonlabeled LTB4 or 20-OH-LTB4 but not by 15-hydroxyicosatetraenoic acid (15-HETE), (5S,12S)-5,12-dihydroxy-6,10-trans-8,14-cis-icosatetraenoic acid [(5S,12S)-diHETE], or the 6-trans stereoisomer of LTB4 at 1 microM. In contrast, [3H]LTB4 displays a high level of nonspecific binding to human PMNs, which makes assessment of the Kd for LTB4 binding unobtainable. Binding measurements for [3H]LTB4 were performed in a buffer containing bovine serum albumin, and under these conditions significantly less nonspecific binding was observed. The apparent Kd for high-affinity binding sites on human PMNs at 0 degrees C was 31.3 X 10(-9) M for LTB4 and 14.3 X 10(-9) M for 20-OH-LTB4. In addition, we observed a saturable low-affinity receptor for 20-OH-LTB4 with a Kd of approximately 100 X 10(-9) M and 2 X 10(5) receptors per cell. The data from this study suggest that omega oxidation represents a major pathway for metabolism of LTB4 as well as other arachidonate metabolites. LTB4 and 20-OH-LTB4 express similar functional activities and share common binding properties to hPMNs but differ significantly in their physical properties. It is the unique physical characteristics of 20-OH-LTB4 that suggest that arachidonate metabolites oxidized at the omega position may be more important agents in inflammation than LTB4.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borgeat P., Samuelsson B. Arachidonic acid metabolism in polymorphonuclear leukocytes: effects of ionophore A23187. Proc Natl Acad Sci U S A. 1979 May;76(5):2148–2152. doi: 10.1073/pnas.76.5.2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgeat P., Samuelsson B. Metabolism of arachidonic acid in polymorphonuclear leukocytes. Structural analysis of novel hydroxylated compounds. J Biol Chem. 1979 Aug 25;254(16):7865–7869. [PubMed] [Google Scholar]
- Clancy R. M., Dahinden C. A., Hugli T. E. Arachidonate metabolism by human polymorphonuclear leukocytes stimulated by N-formyl-Met-Leu-Phe or complement component C5a is independent of phospholipase activation. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7200–7204. doi: 10.1073/pnas.80.23.7200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahinden C., Galanos C., Fehr J. Granulocyte activation by endotoxin. I. Correlation between adherence and other granulocyte functions, and role of endotoxin structure on biologic activity. J Immunol. 1983 Feb;130(2):857–862. [PubMed] [Google Scholar]
- Feinmark S. J., Lindgren J. A., Claesson H. E., Malmsten C., Samuelsson B. Stimulation of human leukocyte degranulation by leukotriene B4 and its omega-oxidized metabolites. FEBS Lett. 1981 Dec 21;136(1):141–144. doi: 10.1016/0014-5793(81)81233-1. [DOI] [PubMed] [Google Scholar]
- Fernandez H. N., Henson P. M., Otani A., Hugli T. E. Chemotactic response to human C3a and C5a anaphylatoxins. I. Evaluation of C3a and C5a leukotaxis in vitro and under stimulated in vivo conditions. J Immunol. 1978 Jan;120(1):109–115. [PubMed] [Google Scholar]
- Ford-Hutchinson A. W., Bray M. A., Doig M. V., Shipley M. E., Smith M. J. Leukotriene B, a potent chemokinetic and aggregating substance released from polymorphonuclear leukocytes. Nature. 1980 Jul 17;286(5770):264–265. doi: 10.1038/286264a0. [DOI] [PubMed] [Google Scholar]
- Goetzl E. J., Pickett W. C. Novel structural determinants of the human neutrophil chemotactic activity of leukotriene B. J Exp Med. 1981 Feb 1;153(2):482–487. doi: 10.1084/jem.153.2.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman D. W., Goetzl E. J. Specific binding of leukotriene B4 to receptors on human polymorphonuclear leukocytes. J Immunol. 1982 Oct;129(4):1600–1604. [PubMed] [Google Scholar]
- Granström E. On the metabolism of prostaglandin F 2 in female subjects. Structures of two C 14 metabolites. Eur J Biochem. 1972 Feb;25(3):581–589. doi: 10.1111/j.1432-1033.1972.tb01731.x. [DOI] [PubMed] [Google Scholar]
- Hamberg M., Samuelsson B. On the specificity of the oxygenation of unsaturated fatty acids catalyzed by soybean lipoxidase. J Biol Chem. 1967 Nov 25;242(22):5329–5335. [PubMed] [Google Scholar]
- Hansson G., Lindgren J. A., Dahlén S. E., Hedqvist P., Samuelsson B. Identification and biological activity of novel omega-oxidized metabolites of leukotriene B4 from human leukocytes. FEBS Lett. 1981 Jul 20;130(1):107–112. doi: 10.1016/0014-5793(81)80676-x. [DOI] [PubMed] [Google Scholar]
- Kreisle R. A., Parker C. W. Specific binding of leukotriene B4 to a receptor on human polymorphonuclear leukocytes. J Exp Med. 1983 Feb 1;157(2):628–641. doi: 10.1084/jem.157.2.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindgren J. A., Hansson G., Claesson H. E., Samuelsson B. Formation of novel biologically active leukotrienes by omega-oxidation in human leukocyte preparations. Adv Prostaglandin Thromboxane Leukot Res. 1982;9:53–60. [PubMed] [Google Scholar]
- Marcus A. J., Safier L. B., Ullman H. L., Broekman M. J., Islam N., Oglesby T. D., Gorman R. R. 12S,20-dihydroxyicosatetraenoic acid: a new icosanoid synthesized by neutrophils from 12S-hydroxyicosatetraenoic acid produced by thrombin- or collagen-stimulated platelets. Proc Natl Acad Sci U S A. 1984 Feb;81(3):903–907. doi: 10.1073/pnas.81.3.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmblad J., Malmsten C. L., Udén A. M., Rådmark O., Engstedt L., Samuelsson B. Leukotriene B4 is a potent and stereospecific stimulator of neutrophil chemotaxis and adherence. Blood. 1981 Sep;58(3):658–661. [PubMed] [Google Scholar]
- Palmblad J., Udén A. M., Lindgren J. A., Rådmark O., Hansson G., Malmsten C. L. Effects of novel leukotrienes on neutrophil migration. FEBS Lett. 1982 Jul 19;144(1):81–84. doi: 10.1016/0014-5793(82)80573-5. [DOI] [PubMed] [Google Scholar]
- Powell W. S. Properties of leukotriene B4 20-hydroxylase from polymorphonuclear leukocytes. J Biol Chem. 1984 Mar 10;259(5):3082–3089. [PubMed] [Google Scholar]
- Schiffmann E., Corcoran B. A., Wahl S. M. N-formylmethionyl peptides as chemoattractants for leucocytes. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1059–1062. doi: 10.1073/pnas.72.3.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sklar L. A., Jesaitis A. J., Painter R. G., Cochrane C. G. The kinetics of neutrophil activation. The response to chemotactic peptides depends upon whether ligand-receptor interaction is rate-limiting. J Biol Chem. 1981 Oct 10;256(19):9909–9914. [PubMed] [Google Scholar]
- Sklar L. A., Jesaitis A. J., Painter R. G. The neutrophil N-formyl peptide receptor: dynamics of ligand-receptor interactions and their relationship to cellular responses. Contemp Top Immunobiol. 1984;14:29–82. doi: 10.1007/978-1-4757-4862-8_2. [DOI] [PubMed] [Google Scholar]
- Wong P. Y., Westlund P., Hamberg M., Granström E., Chao P. H., Samuelsson B. Omega-hydroxylation of 12-L-hydroxy-5,8,10,14-eicosatetraenoic acid in human polymorphonuclear leukocytes. J Biol Chem. 1984 Feb 25;259(4):2683–2686. [PubMed] [Google Scholar]


