Abstract
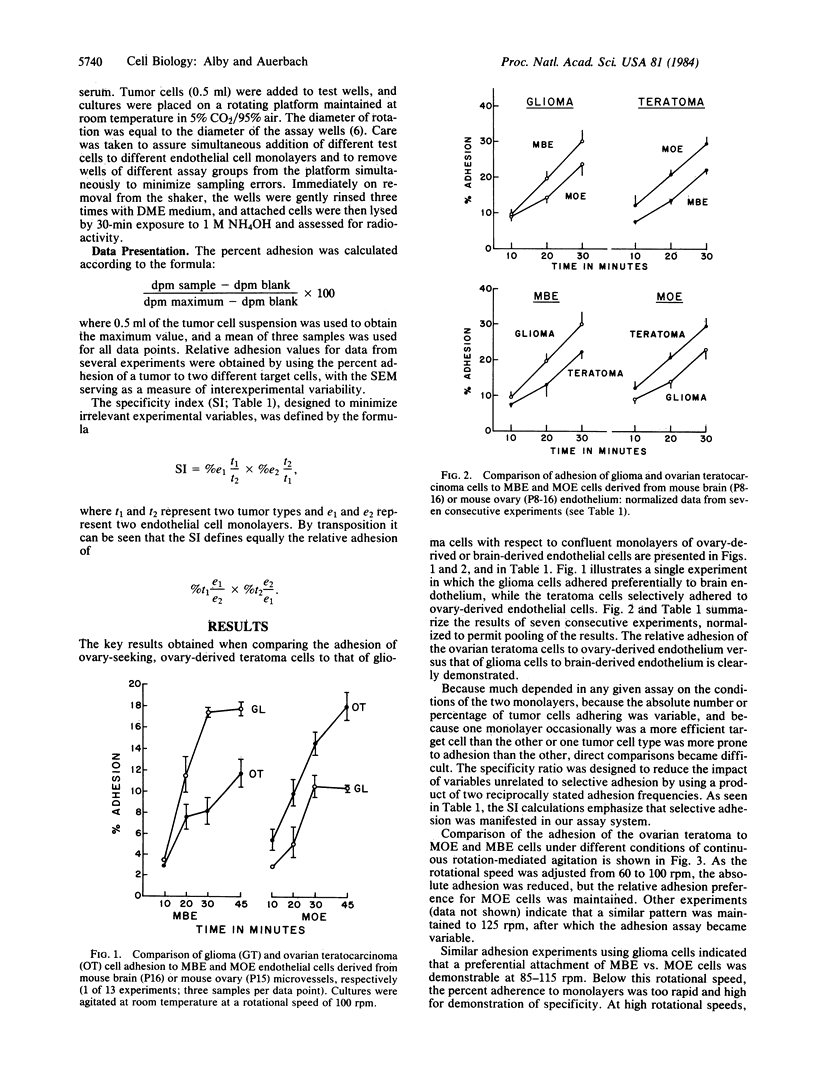
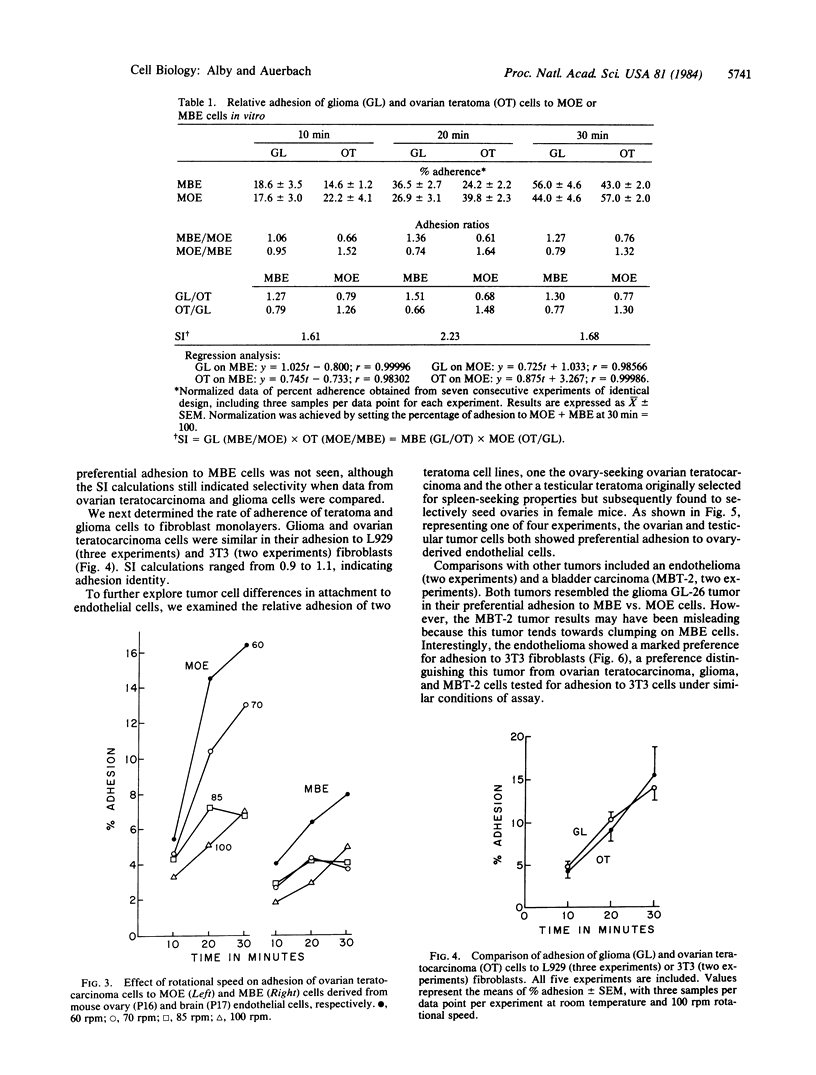
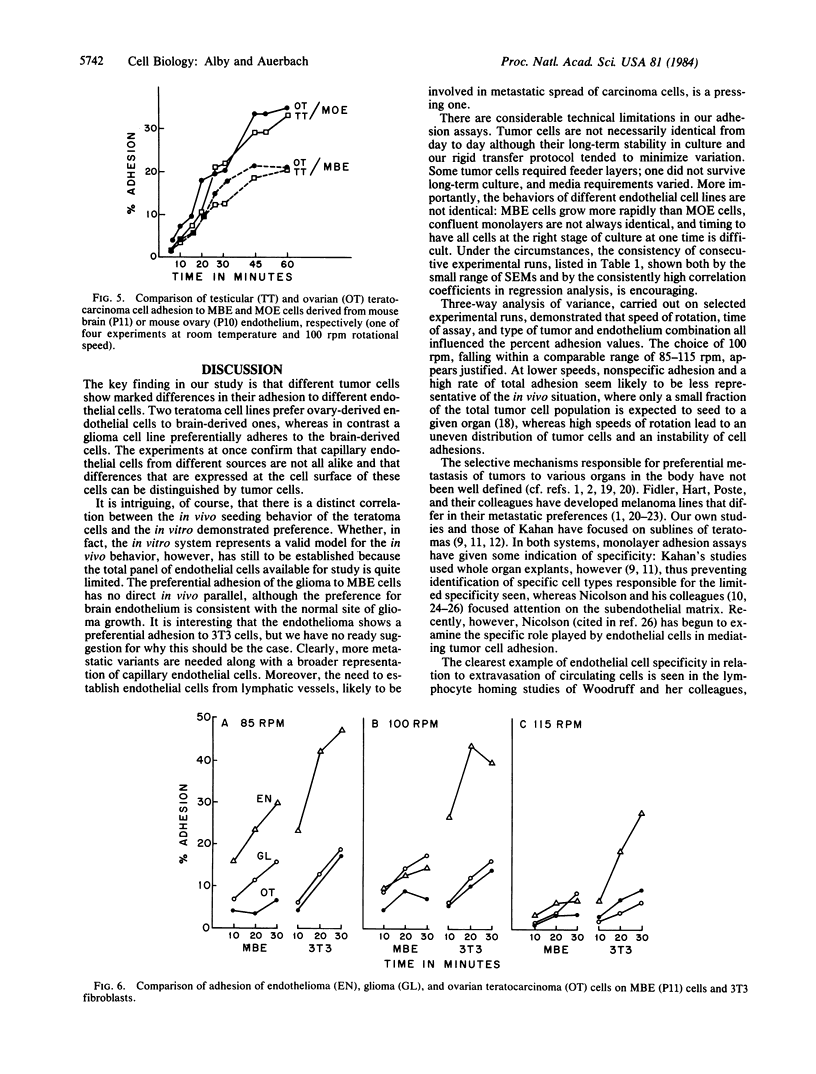
Adhesion studies were carried out to determine the relative ability of glioma cells and ovary-derived teratoma cells to adhere to endothelial cells obtained from mouse brain capillaries (designated MBE cell line) or mouse ovaries (designated MOE cell line). The teratoma cells showed preferential adhesion to MOE cells, whereas the glioma cells showed preferential adhesion to the MBE cell line. In contrast, the glioma and teratoma cells adhered equally to L929 and 3T3 fibroblasts. A testicular teratoma with ovary-seeking properties in vivo also adhered preferentially to MOE cells, while the preference for MBE cells was shared by glioma cells with an endothelioma and a bladder tumor line. The endothelioma, interestingly, showed a marked preferential adhesion to 3T3 cells, thus distinguishing it from the glioma. The experiments demonstrate that capillary endothelial cells derived from different sources are not alike and that differences expressed at the cell surface of these cells can be distinguished by tumor cells.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auerbach R., Alby L., Grieves J., Joseph J., Lindgren C., Morrissey L. W., Sidky Y. A., Tu M., Watt S. L. Monoclonal antibody against angiotensin-converting enzyme: its use as a marker for murine, bovine, and human endothelial cells. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7891–7895. doi: 10.1073/pnas.79.24.7891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher E. C., Scollay R. G., Weissman I. L. Organ specificity of lymphocyte migration: mediation by highly selective lymphocyte interaction with organ-specific determinants on high endothelial venules. Eur J Immunol. 1980 Jul;10(7):556–561. doi: 10.1002/eji.1830100713. [DOI] [PubMed] [Google Scholar]
- Butcher E., Scollay R., Weissman I. Evidence of continuous evolutionary change in structures mediating adherence of lymphocytes to specialised venules. Nature. 1979 Aug 9;280(5722):496–498. doi: 10.1038/280496a0. [DOI] [PubMed] [Google Scholar]
- Chin Y. H., Carey G. D., Woodruff J. J. Lymphocyte recognition of lymph node high endothelium. IV. Cell surface structures mediating entry into lymph nodes. J Immunol. 1982 Nov;129(5):1911–1915. [PubMed] [Google Scholar]
- DeBault L. E., Kahn L. E., Frommes S. P., Cancilla P. A. Cerebral microvessels and derived cells in tissue culture: isolation and preliminary characterization. In Vitro. 1979 Jul;15(7):473–487. doi: 10.1007/BF02618149. [DOI] [PubMed] [Google Scholar]
- Fidler I. J., Gersten D. M., Hart I. R. The biology of cancer invasion and metastasis. Adv Cancer Res. 1978;28:149–250. doi: 10.1016/s0065-230x(08)60648-x. [DOI] [PubMed] [Google Scholar]
- Fidler I. J., Hart I. R. Biological diversity in metastatic neoplasms: origins and implications. Science. 1982 Sep 10;217(4564):998–1003. doi: 10.1126/science.7112116. [DOI] [PubMed] [Google Scholar]
- Fidler I. J. Metastasis: quantitative analysis of distribution and fate of tumor emboli labeled with 125 I-5-iodo-2'-deoxyuridine. J Natl Cancer Inst. 1970 Oct;45(4):773–782. [PubMed] [Google Scholar]
- Folkman J., Haudenschild C. C., Zetter B. R. Long-term culture of capillary endothelial cells. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5217–5221. doi: 10.1073/pnas.76.10.5217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkman J., Haudenschild C. Angiogenesis in vitro. Nature. 1980 Dec 11;288(5791):551–556. doi: 10.1038/288551a0. [DOI] [PubMed] [Google Scholar]
- Gallatin W. M., Weissman I. L., Butcher E. C. A cell-surface molecule involved in organ-specific homing of lymphocytes. Nature. 1983 Jul 7;304(5921):30–34. doi: 10.1038/304030a0. [DOI] [PubMed] [Google Scholar]
- Grumet M., Hoffman S., Edelman G. M. Two antigenically related neuronal cell adhesion molecules of different specificities mediate neuron-neuron and neuron-glia adhesion. Proc Natl Acad Sci U S A. 1984 Jan;81(1):267–271. doi: 10.1073/pnas.81.1.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunwald G. B., Pratt R. S., Lilien J. Enzymic dissection of embryonic cell adhesive mechanisms. III. Immunological identification of a component of the calcium-dependent adhesive system of embryonic chick neural retina cells. J Cell Sci. 1982 Jun;55:69–83. doi: 10.1242/jcs.55.1.69. [DOI] [PubMed] [Google Scholar]
- Hart I. R., Fidler I. J. Cancer invasion and metastasis. Q Rev Biol. 1980 Jun;55(2):121–142. doi: 10.1086/411730. [DOI] [PubMed] [Google Scholar]
- Hart I. R. The selection and characterization of an invasive variant of the B16 melanoma. Am J Pathol. 1979 Dec;97(3):587–600. [PMC free article] [PubMed] [Google Scholar]
- Hoffman S., Sorkin B. C., White P. C., Brackenbury R., Mailhammer R., Rutishauser U., Cunningham B. A., Edelman G. M. Chemical characterization of a neural cell adhesion molecule purified from embryonic brain membranes. J Biol Chem. 1982 Jul 10;257(13):7720–7729. [PubMed] [Google Scholar]
- Kahan B. Ovarian localization by embryonal teratocarcinoma cells derived from female germ gells. Somatic Cell Genet. 1979 Nov;5(6):763–780. doi: 10.1007/BF01542640. [DOI] [PubMed] [Google Scholar]
- Kahan B. The stability of X-chromosome inactivation: studies with mouse-human cell hybrids and mouse teratocarcinomas. Basic Life Sci. 1978;12:297–328. doi: 10.1007/978-1-4684-3390-6_22. [DOI] [PubMed] [Google Scholar]
- Kraal G., Weissman I. L., Butcher E. C. Differences in in vivo distribution and homing of T cell subsets to mucosal vs nonmucosal lymphoid organs. J Immunol. 1983 Mar;130(3):1097–1102. [PubMed] [Google Scholar]
- Kramer R. H., Nicolson G. L. Interactions of tumor cells with vascular endothelial cell monolayers: a model for metastatic invasion. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5704–5708. doi: 10.1073/pnas.76.11.5704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOSCONA A. Rotation-mediated histogenetic aggregation of dissociated cells. A quantifiable approach to cell interactions in vitro. Exp Cell Res. 1961 Jan;22:455–475. doi: 10.1016/0014-4827(61)90122-7. [DOI] [PubMed] [Google Scholar]
- Nicolson G. L. Metastatic tumor cell attachment and invasion assay utilizing vascular endothelial cell monolayers. J Histochem Cytochem. 1982 Mar;30(3):214–220. doi: 10.1177/30.3.7061823. [DOI] [PubMed] [Google Scholar]
- Peyriéras N., Hyafil F., Louvard D., Ploegh H. L., Jacob F. Uvomorulin: a nonintegral membrane protein of early mouse embryo. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6274–6277. doi: 10.1073/pnas.80.20.6274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponder B. A., Wilkinson M. M. Organ-related differences in binding of Dolichos biflorus agglutinin to vascular endothelium. Dev Biol. 1983 Apr;96(2):535–541. doi: 10.1016/0012-1606(83)90191-4. [DOI] [PubMed] [Google Scholar]
- Poste G., Fidler I. J. The pathogenesis of cancer metastasis. Nature. 1980 Jan 10;283(5743):139–146. doi: 10.1038/283139a0. [DOI] [PubMed] [Google Scholar]
- Potter V. R. Cancer as a problem in intercellular communication: regulation by growth-inhibiting factors (Chalones). Prog Nucleic Acid Res Mol Biol. 1983;29:161–173. doi: 10.1016/s0079-6603(08)60445-6. [DOI] [PubMed] [Google Scholar]
- Potter V. R. Phenotypic diversity in experimental hepatomas: the concept of partially blocked ontogeny. The 10th Walter Hubert Lecture. Br J Cancer. 1978 Jul;38(1):1–23. doi: 10.1038/bjc.1978.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth S. Studies on intercellular adhesive selectivity. Dev Biol. 1968 Dec;18(6):602–631. doi: 10.1016/0012-1606(68)90029-8. [DOI] [PubMed] [Google Scholar]
- Stamper H. B., Jr, Woodruff J. J. Lymphocyte homing into lymph nodes: in vitro demonstration of the selective affinity of recirculating lymphocytes for high-endothelial venules. J Exp Med. 1976 Sep 1;144(3):828–833. doi: 10.1084/jem.144.3.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens S. K., Weissman I. L., Butcher E. C. Differences in the migration of B and T lymphocytes: organ-selective localization in vivo and the role of lymphocyte-endothelial cell recognition. J Immunol. 1982 Feb;128(2):844–851. [PubMed] [Google Scholar]
- Vollmers H. P., Birchmeier W. Monoclonal antibodies that prevent adhesion of B 16 melanoma cells and reduce metastases in mice: crossreaction with human tumor cells. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6863–6867. doi: 10.1073/pnas.80.22.6863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther B. T., Ohman R., Roseman S. A quantitative assay for intercellular adhesion. Proc Natl Acad Sci U S A. 1973 May;70(5):1569–1573. doi: 10.1073/pnas.70.5.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss L., Haydock K., Pickren J. W., Lane W. W. Organ vascularity and metastatic frequency. Am J Pathol. 1980 Oct;101(1):101–113. [PMC free article] [PubMed] [Google Scholar]
- Winkelhake J. L., Nicolson G. L. Determination of adhesive properties of variant metastatic melanoma cells to BALB/3T3 cells and their virus-transformed derivatives by a monolayer attachment assay. J Natl Cancer Inst. 1976 Feb;56(2):285–291. doi: 10.1093/jnci/56.2.285. [DOI] [PubMed] [Google Scholar]
- Woodruff J. J., Kuttner B. J. Adherence of lymphocytes to the high endothelium of lymph nodes in vitro. Ciba Found Symp. 1980;71:243–263. doi: 10.1002/9780470720547.ch13. [DOI] [PubMed] [Google Scholar]
- Yoshida C., Takeichi M. Teratocarcinoma cell adhesion: identification of a cell-surface protein involved in calcium-dependent cell aggregation. Cell. 1982 Feb;28(2):217–224. doi: 10.1016/0092-8674(82)90339-7. [DOI] [PubMed] [Google Scholar]


