Abstract
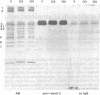
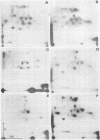
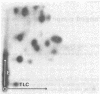
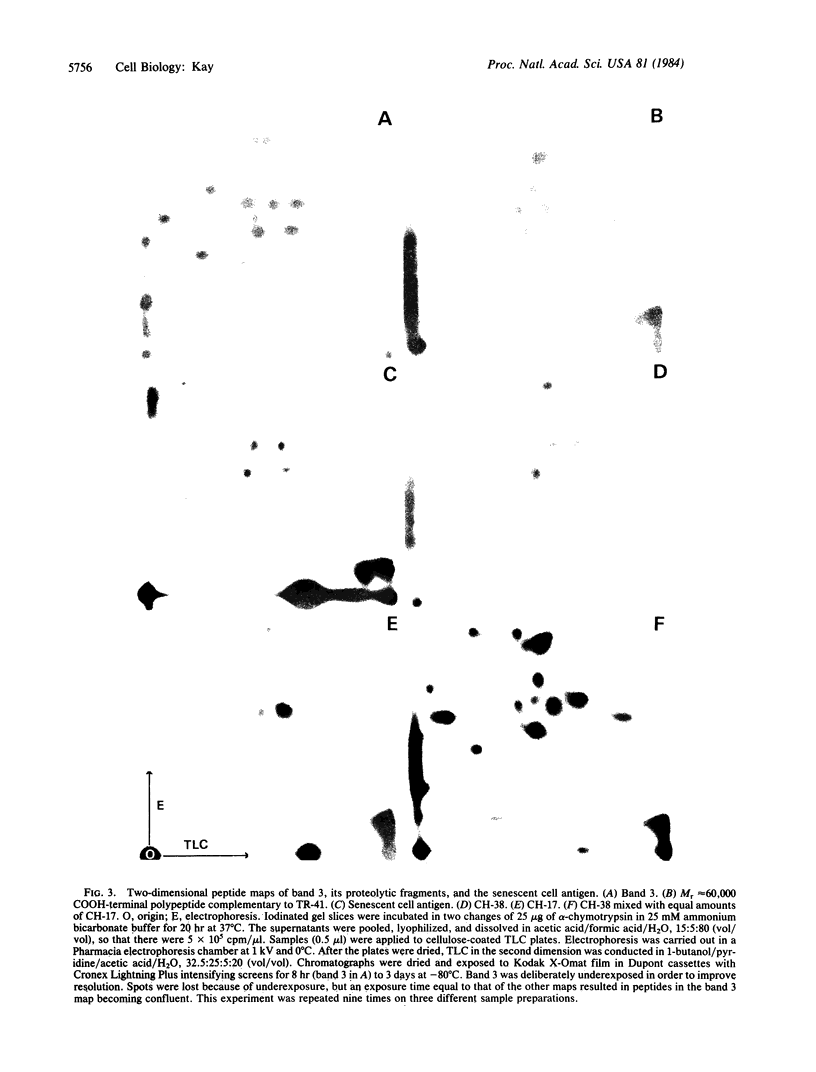
Senescent cell antigen is a glycosylated polypeptide, migrating in the band 4.5 region of NaDodSO4/polyacrylamide gels, that appears on the surface of senescent and damaged cells. Appearance of the senescent cell antigen initiates specific binding of IgG autoantibodies to it and the removal of erythrocytes (RBCs). Previous experiments suggested that the senescent cell antigen may be immunologically related to an integral membrane protein designated band 3 that is involved in anion transport across the RBC membrane. In the present studies, senescent cell antigen was mapped along the band 3 molecule by using topographically defined fragments of band 3. Both binding of IgG eluted from senescent RBCs ("senescent cell IgG") to defined proteolytic fragments of band 3 in immunoblots and two-dimensional peptide mapping of senescent cell antigen, band 3, and defined proteolytic fragments of band 3 were used to localize senescent cell antigen along the band 3 molecule. The data suggest that the antigenic determinants of the senescent cell antigen that are recognized by physiologic IgG autoantibodies reside on an external portion of a naturally occurring transmembrane fragment of band 3 that has lost a Mr approximately equal to 40,000 cytoplasmic (NH2-terminal) segment and part of the anion-transport region. A critical cell-age-specific cleavage of band 3 appears to occur in the transmembrane, anion-transport region of band 3.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appell K. C., Low P. S. Partial structural characterization of the cytoplasmic domain of the erythrocyte membrane protein, band 3. J Biol Chem. 1981 Nov 10;256(21):11104–11111. [PubMed] [Google Scholar]
- Bennett G. D., Kay M. M. Homeostatic removal of senescent murine erythrocytes by splenic macrophages. Exp Hematol. 1981 Mar;9(3):297–307. [PubMed] [Google Scholar]
- Elder J. H., Pickett R. A., 2nd, Hampton J., Lerner R. A. Radioiodination of proteins in single polyacrylamide gel slices. Tryptic peptide analysis of all the major members of complex multicomponent systems using microgram quantities of total protein. J Biol Chem. 1977 Sep 25;252(18):6510–6515. [PubMed] [Google Scholar]
- Kay M. M., Bennett G. D. Isolation and characterization of a senescent cell antigen. Blood. 1982 May;59(5):1111–1112. [PubMed] [Google Scholar]
- Kay M. M., Goodman S. R., Sorensen K., Whitfield C. F., Wong P., Zaki L., Rudloff V. Senescent cell antigen is immunologically related to band 3. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1631–1635. doi: 10.1073/pnas.80.6.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay M. M. Isolation of the phagocytosis-inducing IgG-binding antigen on senescent somatic cells. Nature. 1981 Feb 5;289(5797):491–494. doi: 10.1038/289491a0. [DOI] [PubMed] [Google Scholar]
- Kay M. M. Mechanism of removal of senescent cells by human macrophages in situ. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3521–3525. doi: 10.1073/pnas.72.9.3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay M. M. Role of physiologic autoantibody in the removal of senescent human red cells. J Supramol Struct. 1978;9(4):555–567. doi: 10.1002/jss.400090409. [DOI] [PubMed] [Google Scholar]
- Kay M. M., Sorensen K., Wong P., Bolton P. Antigenicity, storage, and aging: physiologic autoantibodies to cell membrane and serum proteins and the senescent cell antigen. Mol Cell Biochem. 1982 Nov 26;49(2):65–85. doi: 10.1007/BF00242486. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Markowitz S., Marchesi V. T. The carboxyl-terminal domain of human erythrocyte band 3. Description, isolation, and location in the bilayer. J Biol Chem. 1981 Jun 25;256(12):6463–6468. [PubMed] [Google Scholar]
- Ramjeesingh M., Rothstein A. The location of a chymotrypsin cleavage site and of other sites in the primary structure of the 17,000-dalton transmembrane segment of band 3, the anion transport protein of red cell. Membr Biochem. 1982;4(4):259–269. doi: 10.3109/09687688209065435. [DOI] [PubMed] [Google Scholar]
- Steck T. L., Koziarz J. J., Singh M. K., Reddy G., Köhler H. Preparation and analysis of seven major, topographically defined fragments of band 3, the predominant transmembrane polypeptide of human erythrocyte membranes. Biochemistry. 1978 Apr 4;17(7):1216–1222. doi: 10.1021/bi00600a013. [DOI] [PubMed] [Google Scholar]
- Steck T. L., Ramos B., Strapazon E. Proteolytic dissection of band 3, the predominant transmembrane polypeptide of the human erythrocyte membrane. Biochemistry. 1976 Mar 9;15(5):1153–1161. doi: 10.1021/bi00650a030. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]