Abstract
Plasmodium falciparum (Pf) malaria causes 200 million cases worldwide, 8 million being severe and complicated leading to ∼1 million deaths and ∼100,000 abortions annually. Plasmodium falciparum erythrocyte membrane protein 1 (PfEMP1) has been implicated in cytoadherence and infected erythrocyte rosette formation, associated with cerebral malaria; chondroitin sulphate-A attachment and infected erythrocyte sequestration related to pregnancy-associated malaria and other severe forms of disease. An endothelial cell high activity binding peptide is described in several of this ∼300 kDa hypervariable protein’s domains displaying a conserved motif (GACxPxRRxxLC); it established H-bonds with other binding peptides to mediate red blood cell group A and chondroitin sulphate attachment. This motif (when properly modified) induced PfEMP1-specific strain-transcending, fully-protective immunity for the first time in experimental challenge in Aotus monkeys, opening the way forward for a long sought-after vaccine against severe malaria.
Introduction
Malaria-infected children’s sera originally recognised PfEMP1 in infected erythrocyte (IE) agglutination tests [1], as a highly polymorphic very large (∼300 kDa molecular weight); protein encoded by >60 variable, genes (Pf var). PfEMP1 has an extracellular ectodomain consisting of 2 to 9 highly variable in amino acid sequence, length and composition domains; constituted by an N-terminal segment (NTS), a Duffy-binding-like (DBL) 1α domain and a cysteine interdomain region (CIDR) α1 (forming the head structure) and DBL2X, C2, DBL3X, DBL4ε, DBL5ε, DBL6ε and DBL7ε domains followed by a transmembrane region (TM), and an intracytoplasmic acidic terminal segment (ATS), inserted into IE membrane [2]–[4].
PfEMP1 can be classified into 5 groups (A–E) based on the nucleotide sequence similarity of the upstream promoter sequence (UPS) [5], having 6 major DBL domain classes (α, β, γ, δ, ε and X). Each DBL domain consist of hypervariable and conserved regions and contains 3 subdomains (S1, S2 and S3) having 10 semi-conserved homology blocks (HB 1–10 consisting of 7 to 21 residues) conserved in all domain classes, most frequently localised in subdomains S1 (HB4), S2 (HB3, HB5) and S3 (HB2, HB1) [5], [6].
PfEMP1 can also be grouped according to 23 domain cassettes (DC), the most frequent ones DC1 to 3, spanning the entire protein while the others include 2–4 domains [6].
The DBLα1 domain, binds blood group A and forms rosettes by adhering to uninfected erythrocytes (UE) [7] being associated with cerebral malaria (CM) [8]. DBL3X and DBL6ε bind to chondroitin sulphate proteoglycans (CSPG) whilst DBL2X, DBL3X, DBL5ε and DBL6ε bind to chondroitin sulphate-A (CSA) [9], [10], leading to IE sequestration in the placenta, thereby inducing pregnancy-associated malaria (PAM) and abortions, mainly in primigravidas.
A robust, highly specific, sensitive functional methodology has been thoroughly described for tailor-made vaccine development aimed at PfEMP1 (ipso facto severe malaria), recognising variable and conserved HABPs (cHABPs) in relevant invasion molecules by working with ∼15 to 20 mer-long peptides [11]. cHABPs are immunologically silent since they do not induce immune responses; however, when their critical binding residues have been properly modified [12]–[14] they become highly immunogenic and protection-inducing modified HABPs (mHABPs).
Materials and Methods
Ethics Statement
The present study was approved by the Fundación Instituto de Inmunología’s animal ethics committee. The capture of Aotus monkeys (International Union for Conservation of Nature and Natural Resources (IUCN) status: least concern), the pertinent maintenance, immunization challenge and research procedures have been authorized by the official Colombian environmental authority in the Amazonian region (CORPOAMAZONIA, resolutions 0066/Sep/2006, 0028/May/2010, 0632/Jun/2010 and 0042/Jan/2011 and previous authorizations beginning in 1982).
The US Committee on the Care and Use of Laboratory Animals’ guidelines were followed for all animal handling procedures, in turn complying with Colombian regulations for biomedical research (resolution 8430/1993 and law 84/1989). Monkeys at the station were numbered, sexed, weighed, given a physical-clinical exam and kept temporally in individual cages, prior to all experimental procedures. They were kept in controlled conditions regarding temperature (25°–30° centigrade) and relative humidity (83%), similar to those present in their natural environment. The monkeys’ diet was based on a supply of fruit typical of the amazon region (i.e. such primates’ natural diet), vegetables and a nutritional supplement including vitamins, minerals and proteins. Environmental enrichment included visual barriers to avoid social conflict, feeding devices, some branches and vegetation, perches and habitat. Any procedure requiring animal handling was practiced by trained veterinary personnel and animals were submitted to sedation and analgesia procedures to reduce stress when necessary [15]. The monkeys were cared for by expert veterinarians and biologists and supervised weekly by CORPOAMAZONIA veterinarians.
All individuals were released back into the Amazon jungle after the experimental procedures and 30–40 days of quarantine and clinical evaluation in optimal health conditions, as approved by CORPOAMAZONIA and in the presence of its officials.
Peptide Synthesis and Radiolabelling
All peptides were synthesised using standard t-Boc solid-phase peptide synthesis (SPPS) strategy [16]. A tyrosine residue was added to the C-terminus of peptides lacking it to allow radiolabelling, as widely described [14].
Polymeric peptides were obtained for immunisation purposes by adding CG to N- and -C termini, as previously described [14].
Binding Assays with PfEMP1 Peptides
PfEMP1 binding to endothelial cells (C32 cells) and RBC was performed according to previously described protocols [14]. Peptides having binding activity greater than or equal to 2% (0.02 ratio) were considered high-activity binding peptides (HABPs), according to previously-established criteria [11].
Animals and Immunisation
Groups of 4–10 Aotus monkeys proving IFA negative for P. falciparum blood stage, kept in our monkey colony in the Amazon jungle (Leticia, Colombia) according to National Institute of Health guidelines for animal handling and Colombia Ministry of Health laws (resolution 8430 of 1993 and law 84 of 1989) and directly supervised by CORPOAMAZONIA officials [17] and legal permits and authorization for capture and housing by the Colombian Ministry of the Environment have been in force for more than 30 years and there has been strong collaboration with the Colombian Association of Indian Authorities (ATICOYA, ASITAM and AZCAITA, representing ∼40 Indian communities) (pertinent documentation available on request), CORPOAMAZONIA 0266 (Dec/2010) being the most recent authorization.
Aotus monkeys were subcutaneously immunised twice or three times with 250 µg polymerised peptide (on days 1, 20 and 40) which had been previously homogenised with Freund’s complete adjuvant for the 1st dose and Freund’s incomplete adjuvant for the 2nd and 3rd doses. Controls received only Freund’s adjuvant and saline solution on the same days. Blood samples were taken on day 1 before (P0) the first immunisation and 20 days after the 2nd (II20) and 3rd (III20) immunisations for immunological analysis [17].
PfEMP1 Detection by Immunofluorescence
Modifications of Staalsoe’s method (Cytrometry 35∶329) were used. Late trophozoite- and schizont-enriched FCB-2 P. falciparum cultures (5–10% parasitaemia) or highly infected Aotus-adapted P. falciparum FVO strain-enriched schizonts or late trophozoites were spun for 5 min at 1,800 rpm and left for a further 20 min to form sediment, washed three to four times with Tris-buffered Hanks’ solution (TBH) (10 ml 0.15M Tris buffer, pH 7.2, 90 ml 0.9% NaCl, and 100 ml Hanks’ solution) and then diluted to give 1% suspension.
Samples were sequentially treated for 15 min with 200 µl of the appropriate immune serum dilution followed by an anti-goat anti-Aotus IgG F (ab) 2 fragment conjugated with fluorescein isothiocyanate. Slides were washed with TBH supplemented with 50 ll Tween 20 per 100 ml between each sequential incubation. All incubations were performed at room temperature in a humidified chamber. Monolayers were counterstained by adding one drop of ethidium bromide per well to enable parasitised erythrocytes to be visualised. After a few seconds, slides were washed with distilled water, mounted and read at 100x in oil immersion.
Western Blot Analysis
FVO strain culture Pf-schizont lysate was electrophoretically separated and transferred to nitrocellulose membranes. Each nitrocellulose strip was individually incubated with Aotus monkey sera diluted 1∶200 in blocking solution, washed several times and incubated with goat anti-Aotus IgG, F(ab) 2 fragment alkaline phosphatase (AP) conjugated at 1∶1,000 dilution and developed with NBT/BCIP [18].
Challenge and Parasitaemia Assessment
Immunised and control Aotus monkeys were intravenously infected 20 days after the last immunisation with 100,000 P. falciparum FVO-strain infected RBC, a dose known to be 100% infective for these monkeys [17].
Protection was defined as the complete absence of parasites in blood during the 15 days of the experiment. Non-protected monkeys developed patent parasitaemia on day 5 or 6, reaching >5% levels between days 8 and 10. They then received treatment with antimalarial drugs and were kept in quarantine until ensuring complete cure, to be returned into the jungle later on [17].
Parasitaemia was measured daily for each monkey, starting on day 5 after challenge, using immunofluorescence for reading parasitised RBC percentage on Acridine Orange-stained slides [17].
CD Analysis
Peptide structures in solution were acquired by circular dichroism measurement in water and 30% TFE mix. The spectra were obtained on a JASCO J-810 spectrometer at room temperature. Data was assessed at 190 to 260 nm wavelength using 20 nm/min scan rate and 1 nm band with. The data was collected using Spectra Manager Software and analysed using SELCOM3, CONTILL and CDSSTR database [19].
NMR Spectroscopy
8 or 10 milligrams of each peptide (6583, 6584 and 6622) were dissolved in 500 µl TFE-d3/H20 (30/70 v/v). The basic NMR structure determination protocol [20] was as follows: proton spectra were assigned by DQF-COSY, TOCSY and NOESY; TOCSY and DQF-COSY spectra were then used to identify individual spin systems (amino acids) and NOESY (400 ms mixing time) was used for determining peptide primary and secondary structure. TOCSY spectra recorded at different temperatures (285–315 K) were used to obtain amide temperature coefficients for predicting hydrogen bonds (-ΔδHN/ΔTppb/K), as thoroughly described beforehand [14], [21].
Structural Calculation
Peptide structure was determined by Accelrys software. NOE peaks, selected from 400 ms NOESY data sets, were integrated and converted into distance restraints. These restraints were grouped as strong, medium and weak (1.8–2.5 Å, 2.5–3.5 Å, and 3.5–5.0 Å distance restraints, respectively). Hydrogen bond constraints were introduced for slow exchange rate peptide NH, distance ranges involving likely NH–O hydrogen bonds were set at 1.8–2.5 Å. A family of 50 structures was obtained using Distance Geometry (DGII) software and then refined using simulated annealing protocol (DISCOVER software) to select those having reasonable geometry and fewer violations.
HAPB Superimposition on Crystallised DBL Protein Fragments
The 3D structure of DBL domains from PDB 2XU0 [22], 3CML [23] and 2WAU databases [9] was used for selecting peptide regions presenting high activity binding peptides (HABP) based on aminoacid sequence alignment between strains. InsightII biopolymer molecular software (Accelrys Inc.) was used for such superimposition using backbone superimposition based on RMSD criteria as well as H-bond measurement between HABPs forming the niche which is important for binding site receptors.
Results and Discussion
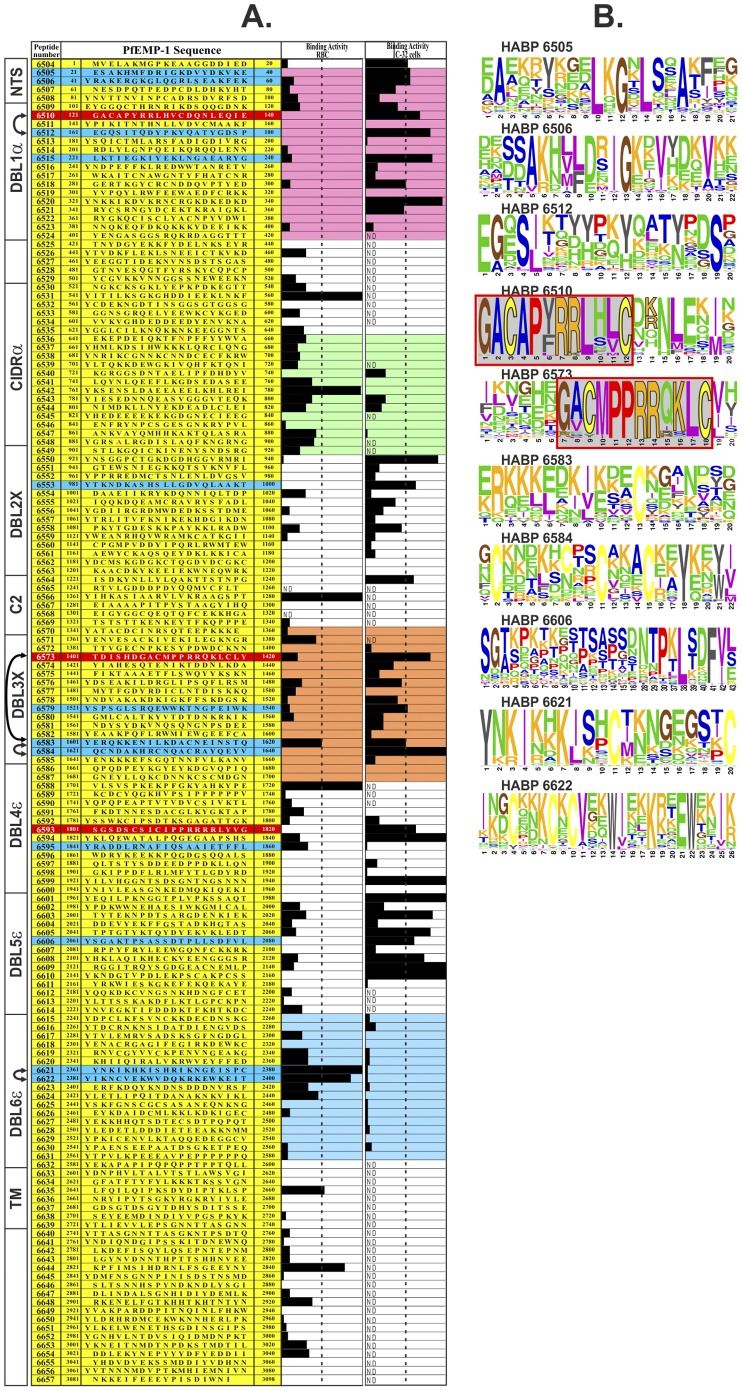
One hundred and fifty 20-mer long peptides were synthesised using the Dd2var1 clone PfEMP1 amino acid sequence, finding 25 HABPs able to bind specifically to C32 endothelial cells (amelanotic melanoma-derived) and 10 O+ red blood cell (RBC) binding HABPs (Figure 1A). Twelve C32 HABPs and two RBC HABPs were randomly selected for being modified as mHABPs [12]–[14].
Figure 1. Identification of PfEMP1 HABPs and variability sequence between Plasmodium falciparum strains.
(A) Dd2 PfEMP1-based amino-acid sequence synthetic peptides’ RBC and C32 cell binding activity (black bars represent specific binding activity slope); above 2% (dotted line) were considered HABPs [11]–[14]. Blue shows HABPs chosen for immunization and red those containing canonical or homologous (GACxPxRRxxLC) binding motif. Left, schematic representation of PfEMP1 domains showing H-bonds between HABPs (arrows); head structure recombinant fragments containing NTS and DBL1α (fuchsia), CDR1α (green), DBL3X (orange) and DBL6ε (blue), 3D structure determined by X-ray crystallography. (B) Sequence logos for amino acid conservation in corresponding HABPs according to their frequency in >100 strains; each amino acid height reflects their relative frequency (%) and thus their contribution to conservation.
Ninety-two mHABPs (synthesised using Dd2 sequence, Indochina) were used for immunising groups of four to ten Aotus monkey groups per mHABP, since Aotus immune system is similar to that of humans (90%–100% identity) [24]. Immunogenicity was determined by immunofluorescence antibody test (IFA) using the FCB-2 strain (Colombia) and reactivity by Western blot (WB) using FVO strain (Vietnam) IE lysate. mHABP protection-inducing ability was determined following 2nd or 3rd immunisation by intravenously inoculating 100,000 fresh IE from other Aotus previously infected with the heterologous Aotus-adapted FVO strain [12]–[14].
Around ∼30% mHABPs induced high IFA titres (1∶160–1∶640) (Figure 2 and 3A), thus demonstrating transcontinental strain-transcending antibody (Ab) induction, even though most did not induce any protection against experimental challenge (Figure 2).
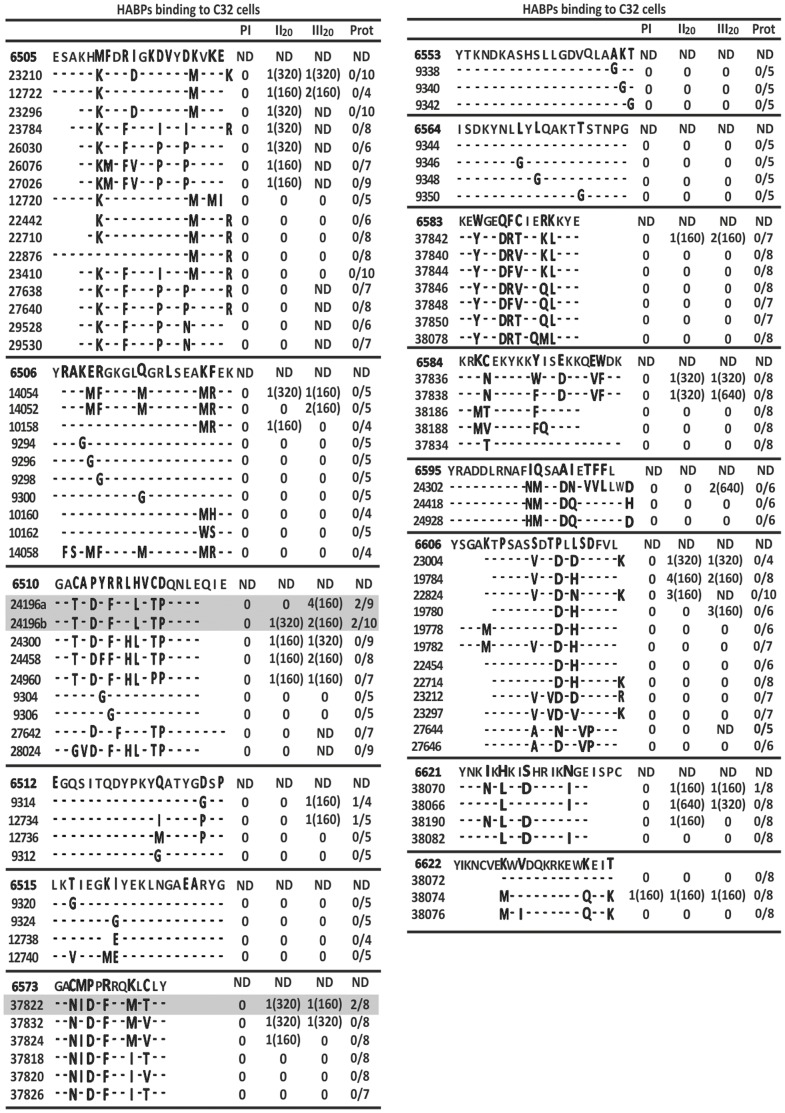
Figure 2. Humoral immune response and protective efficacy induced by PfEMP1 HABPs derived peptides in Aotus monkeys.
Aotus monkeys’ humoral immune responses and protective immunity induced by PfEMP1-derived peptides, according to our serial numbering system with corresponding amino acid sequence (modifications in bold). Reciprocal IFA antibody titres in bleeding 20 days after second (II20) and third (III20) immunisation and number of protected monkeys in experimental challenge [12], [14].
Figure 3. Immunological assessment in animal model trials using modified HABPs.
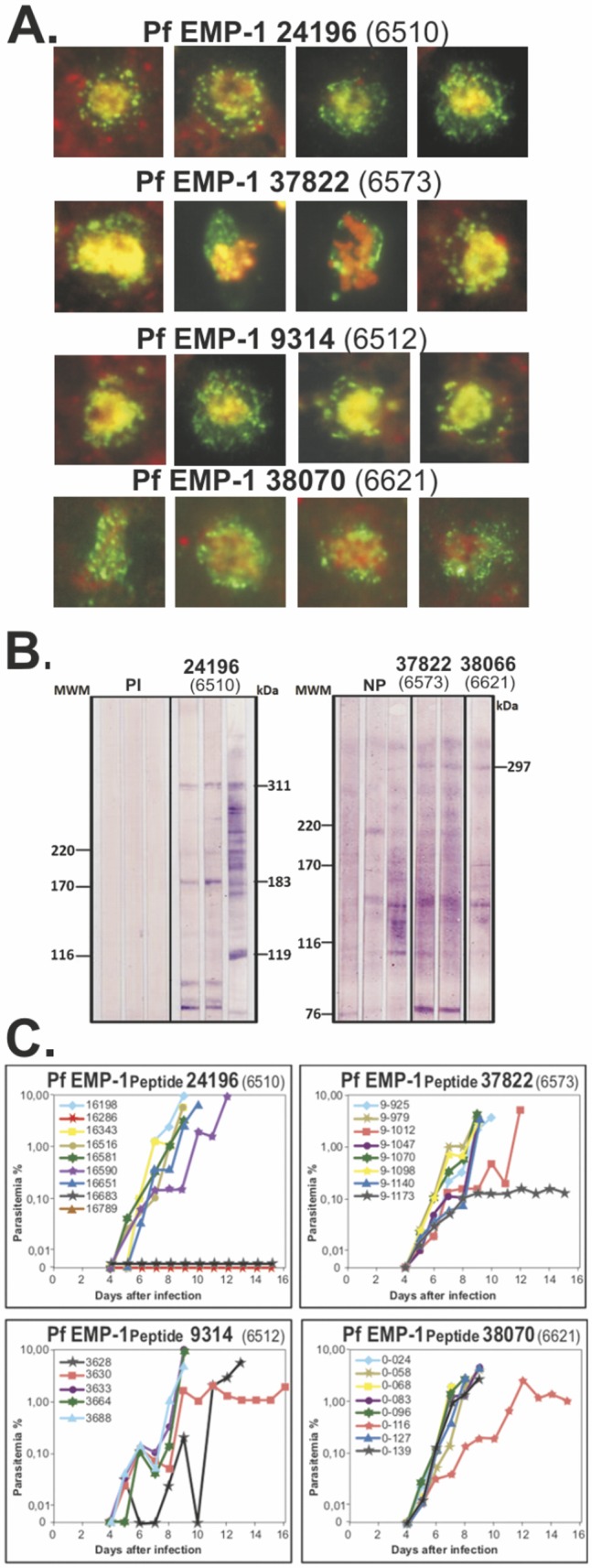
(A) IFA assay showing characteristic PfEMP1 dotted pattern on IE membrane, using sera from immunised Aotus monkeys, corresponding mHABP number on top. (B) WB recognition of ∼300 kDa protein in IE lysate from mHABP-immunised Aotus sera. PI: preimmune sera; NP: non-protected. (C) Comparative course of parasitaemia in Aotus immunised with mHABPs. Note the complete absence of parasites (full protective immunity) induced by 24196 (6510) in the first trial; the second with 10 monkeys gave similar results.
Strikingly, mHABP 24196 (GATADYFRLLVTPQNLE) derived from 6510 (GACAPYRRLHVCDQNLEQIE) (mHABP numbers and their modifications shown hereafter in bold), containing the GACxPxRRxxLC motif (Figure 1B), induced high Ab titres (≥1∶160) in 4/9 Aotus as assessed by IFA (Figure 2); WB recognised a 310 kDa protein similar to PfEMP1 molecular weight (Figure 3B). Two Aotus became fully protected against experimental challenge (0 parasites in blood) throughout the whole experiment (Figure 3C) and 2/10 from a new group of Aotus monkeys became totally protected (the same ones displaying high IFA titres and reacting with the ∼310 kDa band (by WB), demonstrating that 24196 induced strain-transcending fully protective immunity in 4/19 (∼21%) monkeys. 24196 (GATADYFRLLVTPQNLE) displayed a characteristic HLADRβ1*0405 binding motif and binding register (highlighted in grey), an allele found with similar frequency in humans and Aotus (∼21%) [24].
6510 did not induce antibodies in animal immunizations [25], [26] and antibodies raised against recombinant proteins containing this sequence have not recognised 6510, nor African human immune sera [27], confirming 6510’s immunological silence [12]–[14].
Two of the eight Aotus immunised with 37822 (GANIDPFRQMLTLY) derived from 6573 (GACMPPRRQKLCLY), also containing the GACxPxRRxxLC motif (Figure 1B) (underlined) localised in DBL3X, developed high Ab titres (≥1∶160) (Figure 2) and was partially protected, parasitaemia being maintained at around ∼0.1% throughout the experiment (Figure 3C). 37822 displayed HLADRβ1*1501 binding motifs and registers (grey) having similar frequency in Aotus (∼15%) and humans.
Such striking data showed that the canonical GACxPxRRxxLC motif localised in HB4 (≡HBb) [4]–[6], in the “head structure”, is the critical sequence inducing strain-transcending full protective immunity when appropriately modified, as in 24196 (6510), whilst 37822 (modified from homologous 6573) localized in DBL3X induced partial protective immunity. A homologous sequence (PxRRxxxC) present in DBL4ε domain is contained in 6593 (not used for immunisations) (Figure 1A) and a shorter PxRRxxLx sequence was found in DBL6ε N-terminus in some strains [28], confirming this motif’s presence in nearly all DBL domains [5].
Interestingly, highly-immunogenic mHABPS, like 9314 (EGQSITQDYPKYQATYGGSP) derived from 6512 (EGQSITQDYPKYQATYGDSP) and 38070 (YNKNKLKIDHRIKIGE) derived from 6621 (YNKIKHKISHRIKNGEISPC), also induced high antibody titres and partial protective immunity (parasitaemia being maintained at ∼1.5% in 1/8 monkeys each mHABP throughout the experiment, Figure 3C), similar to semi-immune African-adults, suggesting partial strain-transcending immunity, surpassed by tremendous polymorphism. 9314 and 38070 had perfectly classical HLADRβ1*0101 and HLADRβ1*0301 binding motifs and registers, respectively (grey).
IE usually express only one PfEMP1 at a time but the parasite switches var gene expression, by a mechanism involving a var intron re-localization regulated by an 18 bp nuclear binding element that regulates actin polymerization [29] and leads to the change in host-cell receptor specificity and serotype [30], [31], evading the immune response [32]. Such polymorphism could partly explain the partial protective immunity obtained, despite mHABPs being properly modified [12] and high antibody titres being induced (Figure 2) but it has been also demonstrated that PfEMP1 specifically induces a large panel of immune suppression mechanisms among these the early production of human γ interferon [33], but the domain (s) involved in such scape mechanisms remains to be identified.
Strain-transcending Protection-inducing mHABP 3D Structure
The 3D structure of the rosette-forming blood group A-binding Palo Alto VarO strain (Uganda) “head structure” containing the NTS-DBL1α-CIDR1γ region [34], the A4 strain (Brazil) chondroitin-sulphate-proteoglycan (CSPG) binding DBL3X domain [23], [35] and the 3D7 strain (unknown origin) DBL6ε domain binding to CSA has been determined by X-ray crystallography [9].
(reminder: our HABPs are based on Indochina Dd2 sequence). Our group determined that native HABP 6505 displayed a perfect α-helix structure by 1H-NMR (Figure 4A, yellow) [14]; when superimposed onto the NTS-DBL1α 3D structure (Figure 4A, green) it gave a 0.16 rmsd and 6583 and 6584 (Figure 4B, fuchsia and dark blue) had α-helix conformation (Figure S1 and Table S1), giving 0.43 and 0.52 rmsd, respectively, when superimposed onto the DBL3X sequence [23], [35]. It has thus been thoroughly demonstrated that chemically-synthesised HABPs display the same 3D structure as biologically-derived recombinant proteins [11], [12].
Figure 4. Structural characterization of HABPs present in crystallized Duffy binding like domains (DBL).
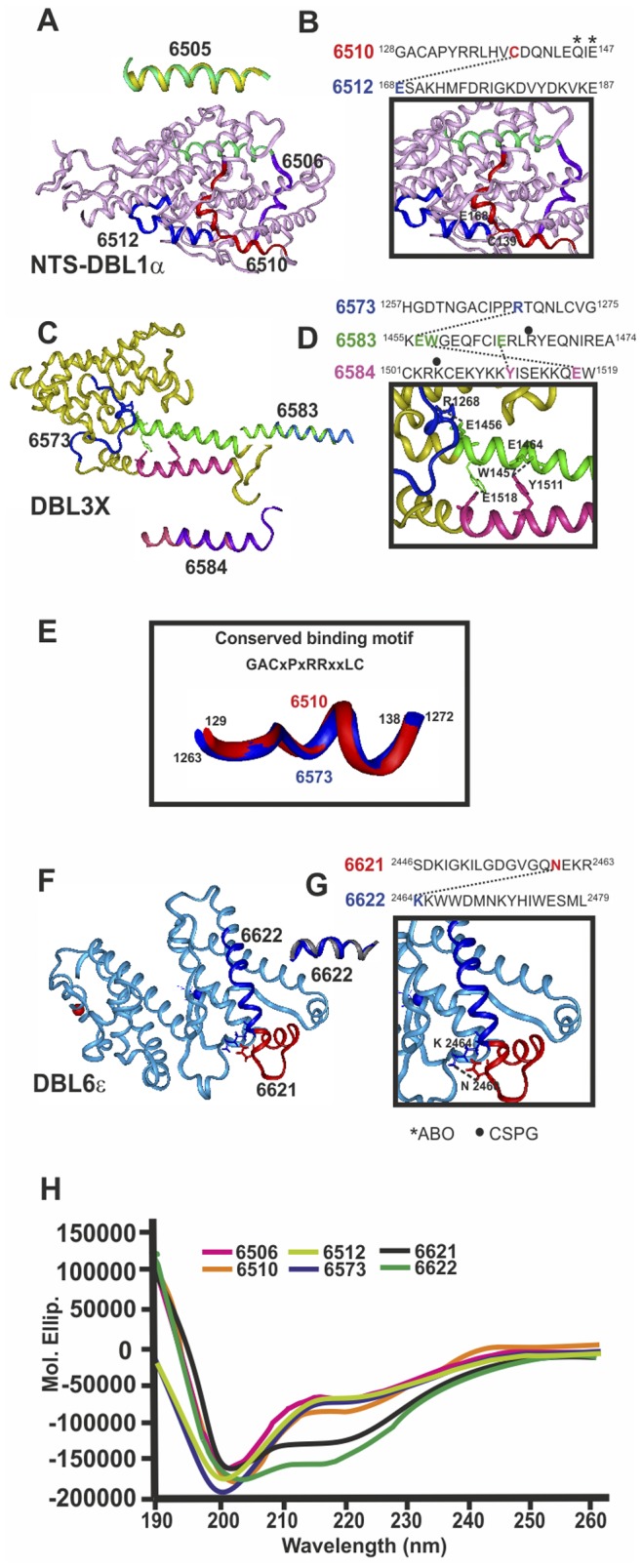
DBL domain 3D structure determined by X-ray crystallography A) Head structure: DBL1α (PDB 2XU0) (pink), C) DBL3X (PDB 3CML) (yellow), F) DBL6ε (PDB 2WAU) (pale blue). 1H-NMR-determined structure localisation, displaying the perfect fit of HABP 6505 (yellow) superimposed onto DBL1α, 6583 (dark blue) and 6584 (purple) onto DBL3X and 6622 (grey) onto DBL6ε. B, D, G). H-bonds between HABP residues and their corresponding sequence on top, displaying relevant residues in binding to A blood group trisaccharides and CSPG (asterisk and black dot, respectively). E) Superimposed conserved binding motif fragments from 6510 and 6573. H) CD spectra for corresponding HABPs.
Circular dichroism (CD) revealed that 6621 and 6622 had a helical structure (Figure 4H), while SELCON3 deconvolution analysis revealed 0.785, 0.836 and 0.898 turn and unordered structure composition for 6510, 6512 and 6573.
6510 (located in the NTS-DBL1α fragment subdomain S1 between cysteine 3 and 4) contained a short αH1 helix and short 310 H1 helix; partially unordered HABP 6573 (DBL3X subdomain S1) only displayed a short α-helix [23], the rest being unordered.
Thus native 6510 and 6573, parents of strain-transcending protective immunity inducing 24196 and 37822, respectively, containing the GACxPxRRxxLC motif, displayed an almost completely unordered and similar structure in DBL1α and DBL3X since 6510 superimposition onto 6573 gives 0.65 rmsd (Figure 4E) explaining in part the cross protective immunity; in sharp contrast with strain-transcending non-protective antibody-inducing HABPs having helix structures (6505, 6506, 6583,6584 and 6622) (Figure 4A, C, F) and partially protection inducing 6512 (unordered) and 6621 (α helical and partially unordered, by CD and X-ray crystallography), suggesting an association between structure and immunogenicity and protection [12]–[14].
Modifying H-bond-establishing Residues among cHABPs Induced Strain-transcending Immunity
3D analysis of 6510 (128GACAPYRRLHVC 139DQNLEQ*IE*147) showed that C139 HN established an H-bond with Oε1 from E168 present in 6512 HABP N-terminus (168 EGQSITQDYPKYQATYGDSP187) forming the niche where the A1 blood group terminal α-1,3 linked N-acetylgalactosamine (GalNAc) [34] bound through residues Q145 and E147 (asterisk in Figure 4B) [34], suggesting that modifying these H-bond-establishing residues among cHABPs via T139C replacement in 24196 was fundamental [12] for inducing fully-protective, strain-transcending antibody immunity (Figure 2 and 3C). Antibodies against these mHABPs might thus have been blocking IE to UE for rosette formation, thereby impeding IE agglutination and microvascular obstruction, associated with CM, making 24196 essential for severe malaria control in some individuals, as will be discussed later on.
By the same token, 6573 (1257HGDTNGACIPPR 1268QTQNLCVG1275), containing also conserved binding motif GACxPxRRxxLC (Figure 1A), established an H-bond between R1268 HNε and E1456 Oε2 present in 6583 (1455KE 1456 W 1457GEQFCIE 1464RLR•YEQNIRE1474); 6583 established another H-bond between E1464 Oε1 with OH in 6584 Y1511 (1501CKRK•CEKYKKY 1511ISEKKQE 1518W1519) to form a tripartite binding site for CSPG (Figure 4D, dot on top). Replacing 6573 R1268 by F in 37822 (1262GANIDPF 1268RQMLTLY1275) induced strain-transcending immunity, controlling parasitaemia at <1% throughout the experiment, due to these cHABPs’ tremendous genetic variability means that blocking this highly polymorphic CSPG binding site could be relevant for PAM control and other severe malaria-associated problems where CSPG is involved.
Two HABPs were localised in DBL6ε, consisting of 7 variable blocks (VB) having limited polymorphism. Completely 310-helix structure 6622 (2464 KKWWDMNKYHIWESML2479) determined by 1H-NMR (Figure 4F, grey ribbon) is localised in VB4; partially α-helical 6621 (2446SDKIGKILGDGVGQN 2460EKR2463) (Figure 4F, red ribbon) localised in one of the elbows of DBL6ε domains, in VB4 [28], [36], established a H-bond between N2460 O (6621) and K2464 HN (6622), forming a niche for a non-identified RBC receptor binding (Figure 4G).
D2456S replacement in 38070 (6621) induced high Ab titres and partial protective immunity (∼1.5% parasitaemia), again confirming inter-HABP H-bond breaking’s relevance in immune induction. 38070 displayed binding motifs and registers (grey) characteristic of HLADRβ1*0301 allele (YNKNKLKIDHRIKIGE), an allele found ∼15% frequency in monkeys and humans.
6622-derived 38074 (MWVDQKRKEWQEIK), inducing non-protective antibodies, displayed the HLADRβ1*0802 binding motif and register (grey); this HABP is in highly polymorphic region (Figure 1B).
Recent studies have found predominant transcription of domain cassette DC8 (UPSB promoter followed by NTSβ-DBLα2-CIDRα1-DBLβ12-DBLγ4) and DC13 (encoding DBLα1.7-CIRDα1.4) (both containing the GACxPxRRxxLC motif) in blood samples from 70% of 88 Tanzanian children suffering severe malaria. This stresses the importance of 24196 (containing this motif) in inducing strain-transcending complete protective immunity against severe malaria in HLADRβ1*04 individuals, suggesting that more HABPs from other IE membrane-expressed molecules (like histidine-rich protein-II-derived 24230 (6800) under HLADRβ1*07 control [11]–[14], [37], STEVOR [38], [39]; RIFIN etc.) are needed to obtain definitive full protection against severe malaria.
These large functional-structural and immunological studies show that strain-transcending complete protective immunity against severe malaria can be fulfilled through previously defined principles [11]–[14] modifying the GACxPxRRxxLC conserved motif (canonical in the PfEMP1 “head structure”) binding to endothelial cells. This, in turn, leads towards a fully-protective, multi-epitope, multi-stage, minimal subunit-based, chemically-synthesised definitive antimalarial vaccine [11]–[14].
Supporting Information
Summary of sequential and medium range NOEs of 6583, 6584 and 6622. Summary of sequential and medium range NOEs determined in H2O/TFE-d3 (70%/30%). NOE intensity is indicated by bar height. The numbers inside the diagram are the 3J coupling constants. Δ represents residues involved in an H-bond.
(TIF)
Summary of 6583, 6584 and 6622 structure calculation.
(DOCX)
Acknowledgments
We would like to thank Mr. Jason Garry for reviewing the manuscript and making appropriate corrections.
Funding Statement
The authors have no support or funding to report.
References
- 1. Marsh K, Howard RJ (1986) Antigens induced on erythrocytes by P. falciparum: expression of diverse and conserved determinants. Science 231: 150–153. [DOI] [PubMed] [Google Scholar]
- 2. Su XZ, Heatwole VM, Wertheimer SP, Guinet F, Herrfeldt JA, et al. (1995) The large diverse gene family var encodes proteins involved in cytoadherence and antigenic variation of Plasmodium falciparum-infected erythrocytes. Cell 82: 89–100. [DOI] [PubMed] [Google Scholar]
- 3. Smith JD, Chitnis CE, Craig AG, Roberts DJ, Hudson-Taylor DE, et al. (1995) Switches in expression of Plasmodium falciparum var genes correlate with changes in antigenic and cytoadherent phenotypes of infected erythrocytes. Cell 82: 101–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Smith JD, Subramanian G, Gamain B, Baruch DI, Miller LH (2000) Classification of adhesive domains in the Plasmodium falciparum erythrocyte membrane protein 1 family. Mol Biochem Parasitol 110: 293–310. [DOI] [PubMed] [Google Scholar]
- 5. Lavstsen T, Turner L, Saguti F, Magistrado P, Rask TS, et al. (2012) Plasmodium falciparum erythrocyte membrane protein 1 domain cassettes 8 and 13 are associated with severe malaria in children. Proc Natl Acad Sci U S A 109: E1791–1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rask TS, Hansen DA, Theander TG, Gorm Pedersen A, Lavstsen T (2010) Plasmodium falciparum erythrocyte membrane protein 1 diversity in seven genomes–divide and conquer. PLoS Comput Biol 6. [DOI] [PMC free article] [PubMed]
- 7. Carlson J, Wahlgren M (1992) Plasmodium falciparum erythrocyte rosetting is mediated by promiscuous lectin-like interactions. J Exp Med 176: 1311–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Carlson J, Helmby H, Hill AV, Brewster D, Greenwood BM, et al. (1990) Human cerebral malaria: association with erythrocyte rosetting and lack of anti-rosetting antibodies. Lancet 336: 1457–1460. [DOI] [PubMed] [Google Scholar]
- 9. Khunrae P, Philip JM, Bull DR, Higgins MK (2009) Structural comparison of two CSPG-binding DBL domains from the VAR2CSA protein important in malaria during pregnancy. J Mol Biol 393: 202–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gamain B, Trimnell AR, Scheidig C, Scherf A, Miller LH, et al. (2005) Identification of multiple chondroitin sulfate A (CSA)-binding domains in the var2CSA gene transcribed in CSA-binding parasites. J Infect Dis 191: 1010–1013. [DOI] [PubMed] [Google Scholar]
- 11. Rodriguez LE, Curtidor H, Urquiza M, Cifuentes G, Reyes C, et al. (2008) Intimate molecular interactions of P. falciparum merozoite proteins involved in invasion of red blood cells and their implications for vaccine design. Chem Rev 108: 3656–3705. [DOI] [PubMed] [Google Scholar]
- 12. Patarroyo ME, Bermudez A, Patarroyo MA (2011) Structural and immunological principles leading to chemically synthesized, multiantigenic, multistage, minimal subunit-based vaccine development. Chem Rev 111: 3459–3507. [DOI] [PubMed] [Google Scholar]
- 13. Curtidor H, Vanegas M, Alba MP, Patarroyo ME (2011) Functional, immunological and three-dimensional analysis of chemically synthesised sporozoite peptides as components of a fully-effective antimalarial vaccine. Curr Med Chem 18: 4470–4502. [DOI] [PubMed] [Google Scholar]
- 14. Curtidor H, Torres MH, Alba MP, Patarroyo ME (2007) Structural modifications to a high-activity binding peptide located within the PfEMP1 NTS domain induce protection against P. falciparum malaria in Aotus monkeys. Biol Chem 388: 25–36. [DOI] [PubMed] [Google Scholar]
- 15.Committee for the update of the guide for the care and use of laboratory animals. (2011) Guide for the care and use of laboratory animals. National Research Council (U.S.). National Academies Press (US).
- 16. Houghten RA (1985) General method for the rapid solid-phase synthesis of large numbers of peptides: specificity of antigen-antibody interaction at the level of individual amino acids. Proc Natl Acad Sci U S A 82: 5131–5135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rodriguez R, Moreno A, Guzman F, Calvo M, Patarroyo ME (1990) Studies in owl monkeys leading to the development of a synthetic vaccine against the asexual blood stages of Plasmodium falciparum. Am J Trop Med Hyg 43: 339–354. [DOI] [PubMed] [Google Scholar]
- 18. Blake MS, Johnston KH, Russell-Jones GJ, Gotschlich EC (1984) A rapid, sensitive method for detection of alkaline phosphatase-conjugated anti-antibody on Western blots. Anal Biochem 136: 175–179. [DOI] [PubMed] [Google Scholar]
- 19. Chen YH, Yang JT, Martinez HM (1972) Determination of the secondary structures of proteins by circular dichroism and optical rotatory dispersion. Biochemistry 11: 4120–4131. [DOI] [PubMed] [Google Scholar]
- 20.Wuthrich K (1986) NMR of protein and nucleic acids. In: Wiley, editor. New York.
- 21. Espejo F, Cubillos M, Salazar LM, Guzman F, Urquiza M, et al. (2001) Structure, Immunogenicity, and Protectivity Relationship for the 1585 Malarial Peptide and Its Substitution Analogues. Angew Chem Int Ed Engl 40: 4654–4657. [DOI] [PubMed] [Google Scholar]
- 22. Juillerat A, Lewit-Bentley A, Guillotte M, Gangnard S, Hessel A, et al. (2011) Structure of a Plasmodium falciparum PfEMP1 rosetting domain reveals a role for the N-terminal segment in heparin-mediated rosette inhibition. Proc Natl Acad Sci U S A 108: 5243–5248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Singh K, Gittis AG, Nguyen P, Gowda DC, Miller LH, et al. (2008) Structure of the DBL3x domain of pregnancy-associated malaria protein VAR2CSA complexed with chondroitin sulfate A. Nat Struct Mol Biol. 15: 932–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Suarez CF, Patarroyo ME, Trujillo E, Estupinan M, Baquero JE, et al. (2006) Owl monkey MHC-DRB exon 2 reveals high similarity with several HLA-DRB lineages. Immunogenetics 58: 542–558. [DOI] [PubMed] [Google Scholar]
- 25. Angeletti D, Albrecht L, Wahlgren M, Moll K (2013) Analysis of antibody induction upon immunization with distinct NTS-DBL1alpha-domains of PfEMP1 from rosetting Plasmodium falciparum parasites. Malar J 12: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Oguariri RM, Mattei D, Tena-Tomas C, Uhlemann AC, Kremsner PG, et al. (2003) Recombinant Duffy binding-like-alpha domains of Plasmodium falciparum erythrocyte membrane protein 1 elicit antibodies in rats that recognise conserved epitopes. Parasitol Res 90: 467–472. [DOI] [PubMed] [Google Scholar]
- 27. Oguariri RM, Borrmann S, Klinkert MQ, Kremsner PG, Kun JF (2001) High prevalence of human antibodies to recombinant Duffy binding-like alpha domains of the Plasmodium falciparum-infected erythrocyte membrane protein 1 in semi-immune adults compared to that in nonimmune children. Infect Immun 69: 7603–7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gangnard S, Badaut C, Ramboarina S, Baron B, Ramdani T, et al. (2013) Structural and Immunological Correlations between the Variable Blocks of the VAR2CSA Domain DBL6epsilon from Two Plasmodium falciparum Parasite Lines. J Mol Biol 425: 1697–1711. [DOI] [PubMed] [Google Scholar]
- 29. Zhang Q, Huang Y, Zhang Y, Fang X, Claes A, et al. (2011) A critical role of perinuclear filamentous actin in spatial repositioning and mutually exclusive expression of virulence genes in malaria parasites. Cell Host Microbe 10: 451–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Clausen TM, Christoffersen S, Dahlback M, Langkilde AE, Jensen KE, et al. (2012) Structural and functional insight into how the Plasmodium falciparum VAR2CSA protein mediates binding to chondroitin sulfate A in placental malaria. J Biol Chem 287: 23332–23345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Joergensen L, Bengtsson DC, Bengtsson A, Ronander E, Berger SS, et al. (2010) Surface co-expression of two different PfEMP1 antigens on single plasmodium falciparum-infected erythrocytes facilitates binding to ICAM1 and PECAM1. PLoS Pathog 6: e1001083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ghumra A, Semblat JP, Ataide R, Kifude C, Adams Y, et al. (2012) Induction of strain-transcending antibodies against Group A PfEMP1 surface antigens from virulent malaria parasites. PLoS Pathog 8: e1002665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. D’Ombrain MC, Voss TS, Maier AG, Pearce JA, Hansen DS, et al. (2007) Plasmodium falciparum erythrocyte membrane protein-1 specifically suppresses early production of host interferon-gamma. Cell Host Microbe 2: 130–138. [DOI] [PubMed] [Google Scholar]
- 34. Vigan-Womas I, Guillotte M, Juillerat A, Hessel A, Raynal B, et al. (2012) Structural basis for the ABO blood-group dependence of Plasmodium falciparum rosetting. PLoS Pathog 8: e1002781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Higgins MK (2008) The structure of a chondroitin sulfate-binding domain important in placental malaria. J Biol Chem 283: 21842–21846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Badaut C, Bertin G, Rustico T, Fievet N, Massougbodji A, et al. (2010) Towards the rational design of a candidate vaccine against pregnancy associated malaria: conserved sequences of the DBL6epsilon domain of VAR2CSA. PLoS One 5: e11276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cifuentes G, Patarroyo ME, Reyes C, Cortes J, Patarroyo MA (2007) A pre-PEXEL histidine-rich protein II erythrocyte binding peptide as a new way for anti-malarial vaccine development. Biochem Biophys Res Commun 360: 149–155. [DOI] [PubMed] [Google Scholar]
- 38. Niang M, Yan Yam X, Preiser PR (2009) The Plasmodium falciparum STEVOR multigene family mediates antigenic variation of the infected erythrocyte. PLoS Pathog 5: e1000307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Garcia JE, Puentes A, Curtidor H, Vera R, Rodriguez L, et al. (2005) Peptides from the Plasmodium falciparum STEVOR putative protein bind with high affinity to normal human red blood cells. Peptides 26: 1133–1143. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Summary of sequential and medium range NOEs of 6583, 6584 and 6622. Summary of sequential and medium range NOEs determined in H2O/TFE-d3 (70%/30%). NOE intensity is indicated by bar height. The numbers inside the diagram are the 3J coupling constants. Δ represents residues involved in an H-bond.
(TIF)
Summary of 6583, 6584 and 6622 structure calculation.
(DOCX)