Abstract
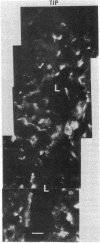
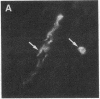
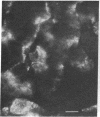
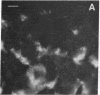
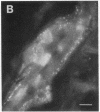
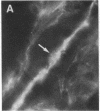
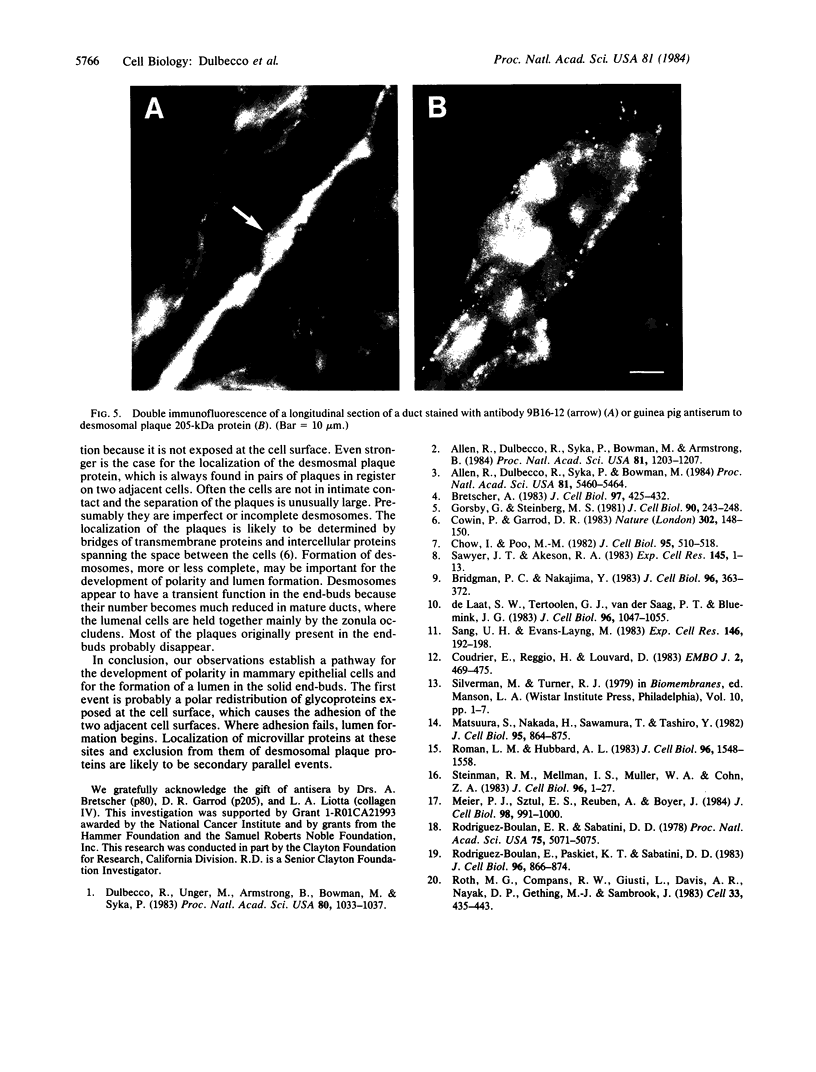
During the development of the rat mammary gland, ducts are formed from end-buds, which contain the stem cells. In this process a lumen is formed in the semisolid mass of the end-bud, and the cells acquire polarity. We have studied this process by following the localization of three inframembranous proteins present in the cells of both end-buds and ducts: microvillin, the microvillar protein p80, and the desmosomal plaque protein p205. We find that the development of ducts is accompanied by a redistribution of these proteins, which in immature parts of the end-buds are found together in the cell. Microvillin and p80 go together to the apical pole of the cells, in contact with the lumen, whereas p205 goes to the basal surface, in contact with cells of the myoepithelial lineage. The acquisition of polarity occurs at the same time as a lumen begins to form by local gaps between cells. It seems likely that the redistribution of the inframembraneous proteins is the consequence of the localization of surface glycoproteins that affect in opposite ways the adhesion between the cells.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R., Dulbecco R., Syka P., Bowman M., Armstrong B. Developmental regulation of cytokeratins in cells of the rat mammary gland studied with monoclonal antibodies. Proc Natl Acad Sci U S A. 1984 Feb;81(4):1203–1207. doi: 10.1073/pnas.81.4.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher A. Purification of an 80,000-dalton protein that is a component of the isolated microvillus cytoskeleton, and its localization in nonmuscle cells. J Cell Biol. 1983 Aug;97(2):425–432. doi: 10.1083/jcb.97.2.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridgman P. C., Nakajima Y. Distribution of filipin-sterol complexes on cultured muscle cells: cell-substratum contact areas associated with acetylcholine receptor clusters. J Cell Biol. 1983 Feb;96(2):363–372. doi: 10.1083/jcb.96.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow I., Poo M. M. Redistribution of cell surface receptors induced by cell-cell contact. J Cell Biol. 1982 Nov;95(2 Pt 1):510–518. doi: 10.1083/jcb.95.2.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coudrier E., Reggio H., Louvard D. Characterization of an integral membrane glycoprotein associated with the microfilaments of pig intestinal microvilli. EMBO J. 1983;2(3):469–475. doi: 10.1002/j.1460-2075.1983.tb01446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowin P., Garrod D. R. Antibodies to epithelial desmosomes show wide tissue and species cross-reactivity. Nature. 1983 Mar 10;302(5904):148–150. doi: 10.1038/302148a0. [DOI] [PubMed] [Google Scholar]
- Dulbecco R., Unger M., Armstrong B., Bowman M., Syka P. Epithelial cell types and their evolution in the rat mammary gland determined by immunological markers. Proc Natl Acad Sci U S A. 1983 Feb;80(4):1033–1037. doi: 10.1073/pnas.80.4.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbsky G., Steinberg M. S. Isolation of the intercellular glycoproteins of desmosomes. J Cell Biol. 1981 Jul;90(1):243–248. doi: 10.1083/jcb.90.1.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuura S., Nakada H., Sawamura T., Tashiro Y. Distribution of an asialoglycoprotein receptor on rat hepatocyte cell surface. J Cell Biol. 1982 Dec;95(3):864–875. doi: 10.1083/jcb.95.3.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier P. J., Sztul E. S., Reuben A., Boyer J. L. Structural and functional polarity of canalicular and basolateral plasma membrane vesicles isolated in high yield from rat liver. J Cell Biol. 1984 Mar;98(3):991–1000. doi: 10.1083/jcb.98.3.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez Boulan E., Sabatini D. D. Asymmetric budding of viruses in epithelial monlayers: a model system for study of epithelial polarity. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5071–5075. doi: 10.1073/pnas.75.10.5071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Boulan E., Paskiet K. T., Sabatini D. D. Assembly of enveloped viruses in Madin-Darby canine kidney cells: polarized budding from single attached cells and from clusters of cells in suspension. J Cell Biol. 1983 Mar;96(3):866–874. doi: 10.1083/jcb.96.3.866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman L. M., Hubbard A. L. A domain-specific marker for the hepatocyte plasma membrane: localization of leucine aminopeptidase to the bile canalicular domain. J Cell Biol. 1983 Jun;96(6):1548–1558. doi: 10.1083/jcb.96.6.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth M. G., Compans R. W., Giusti L., Davis A. R., Nayak D. P., Gething M. J., Sambrook J. Influenza virus hemagglutinin expression is polarized in cells infected with recombinant SV40 viruses carrying cloned hemagglutinin DNA. Cell. 1983 Jun;33(2):435–443. doi: 10.1016/0092-8674(83)90425-7. [DOI] [PubMed] [Google Scholar]
- Sawyer J. T., Akeson R. A. Clonal myoblasts and myotubes show differences in lectin-binding patterns. Exp Cell Res. 1983 Apr 15;145(1):1–13. doi: 10.1016/s0014-4827(83)80003-2. [DOI] [PubMed] [Google Scholar]
- Steinman R. M., Mellman I. S., Muller W. A., Cohn Z. A. Endocytosis and the recycling of plasma membrane. J Cell Biol. 1983 Jan;96(1):1–27. doi: 10.1083/jcb.96.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U H. S., Evans-Layng M. Polar redistribution of Na+K+ATPase in aggregating MDCK cells. Exp Cell Res. 1983 Jun;146(1):192–198. doi: 10.1016/0014-4827(83)90337-3. [DOI] [PubMed] [Google Scholar]
- de Laat S. W., Tertoolen L. G., van der Saag P. T., Bluemink J. G. Quantitative analysis of modulations in numerical and lateral distribution of intramembrane particles during the cell cycle of neuroblastoma cells. J Cell Biol. 1983 Apr;96(4):1047–1055. doi: 10.1083/jcb.96.4.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]