Abstract
Growth of adipose tissue involves differentiation of preadipocytes into mature lipid-containing adipocytes. The matrix metalloproteinases (MMPs) are known regulators of adipose tissue biology, and previous studies suggested a key role for gelatinase B (MMP-9) in adipogenesis. In the present study we have evaluated a potential functional role of MMP-9 by performing gene silencing in 3T3-F442A preadipocytes. At the end of a 12-day differentiation period, no significant differences were observed between MMP-9 knockdown cells and the control cells with respect to intracytoplasmatic lipid content, or expression of the adipogenic markers aP2, PPARγ, Lpl, and adiponectin, or of the preadipocyte marker Pref-1. Thus, in vitro differentiation of 3T3-F442A preadipocytes into mature adipocytes is not significantly affected by the loss of MMP-9.
Keywords: preadipocyte, adipogenesis, adipose tissue, matrix metalloproteinase, gelatinase
Introduction
White adipose tissue responds dynamically to alterations in the nutritional status. Expansion of adipose tissue requires hypertrophy of existing adipocytes and/or hyperplasia (through adipogenesis), together with angiogenesis and intensive remodeling of the extracellular matrix (ECM). Matrix metalloproteinases (MMPs), and in particular the gelatinase subtypes, have been identified as key players in these processes.1 Gelatinase A (MMP-2) and gelatinase B (MMP-9) are secreted by adipocytes and their activity is modulated during adipose tissue expansion.2-4 Previous studies using murine 3T3-F442A or 3T3-L1 preadipocyte cell lines have suggested that MMP-2 and/or MMP-9 are positive regulators of adipocyte differentiation.2,5 Moreover, in a murine nutritionally-induced obesity model, administration of the relatively specific gelatinase inhibitors tolylsam and ABT-518 resulted in impaired adipose tissue development without, however, discriminating between MMP-2 and MMP-9.6-8 Surprisingly, the in vitro use of various synthetic MMP inhibitors has led to discrepant results. A study with human preadipocytes suggested that specifically MMP-9 might play a role in differentiation and in the regulation of adipose tissue development.9 However, this seems in contradiction with studies showing that MMP-2, but not MMP-9, gene deficiency in mice impairs nutritionally induced obesity.7,10 To elucidate whether MMP-9 indeed plays a key role in preadipocyte differentiation, we have performed selective MMP-9 gene silencing in 3T3-F442A cells.
Results
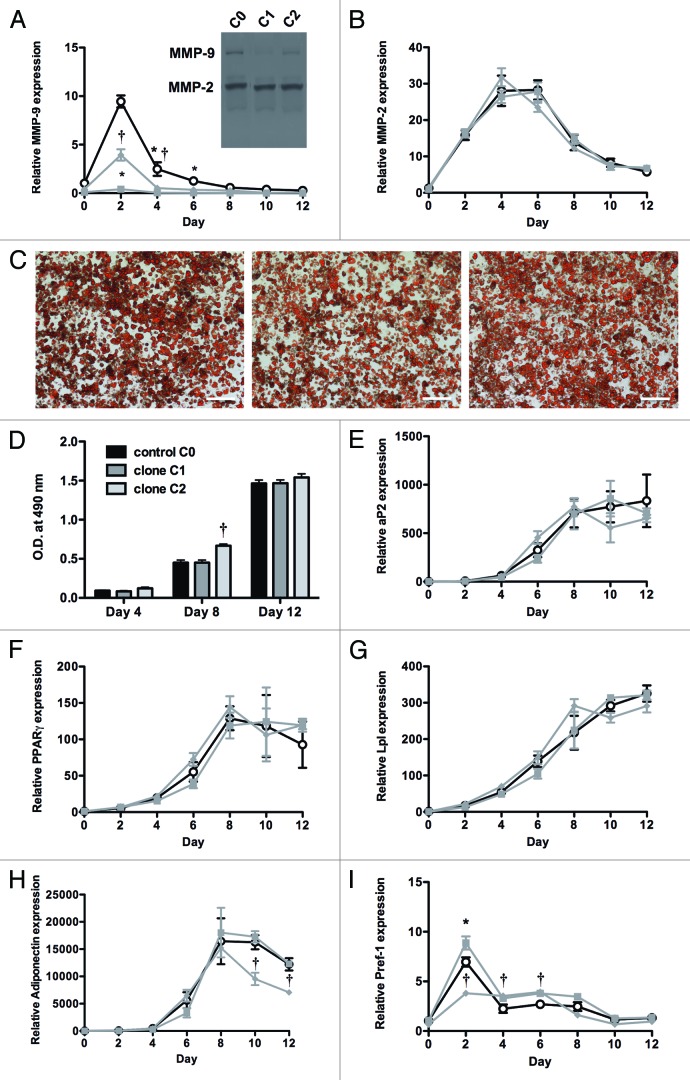
Stable MMP-9 knockdown in 3T3-F442A cells was obtained with all five shRNA constructs, as compared with the C0 control. The most efficient shRNA clones are referred to as clones C1 (TRCN0000031231) and C2 (TRCN0000031232). MMP-9 knockdown at day 0 was evidenced by (1) mRNA determination (3.9 ± 1.1 and 17 ± 2.9 copies/105 β-actin for clones C1 and C2 respectively, as compared with 43 ± 3.9 copies/105 β-actin for control C0; P = 0.002 and P = 0.004); (2) total antigen levels (0.25 ± 0.11 and 1.75 ± 0.36 ng/ml for clones C1 and C2 respectively, vs. 3.46 ± 0.48 ng/ml for control C0; P = 0.02 and P = 0.03) and (3) quantification of gelatinolytic activity (Fig. 1A insert), revealing a reduction of (pro)MMP-9 by 79% or 45% for clones C1 or C2, respectively. In contrast, MMP-2 expression was not significantly affected by MMP-9 knockdown, as indicated by comparable mRNA levels (91 ± 27 and 142 ± 29 copies/105 β-actin for clones C1 and C2 respectively, as compared with 123 ± 38 copies/105 β-actin for control C0). Quantification of gelatin zymography confirmed comparable residual levels of (pro)MMP-2 for clones C1 (84% of C0) and C2 (89% of C0) (Fig. 1A insert). In unconditioned medium, MMP-2 and MMP-9 antigens were not detectable.
Figure 1. Effect of MMP-9 gene silencing on in vitro differentiation of 3T3-F442A preadipocytes into mature adipocytes. (A and B) Expression of MMP-9 (A) and MMP-2 (B) during a 12 d differentiation period of preadipocytes with MMP-9 knockdown (■, clone C1; ◆, clone C2) or control C0 cells (O). The insert in panel A shows a gelatin zymography of conditioned medium of 3T3-F442A preadipocytes at day 0 of differentiation. (C) Representation of the Oil Red O staining at day 12 for C0 (left panel), C1 (middle panel), and C2 (right panel). The scale bar corresponds to 200 µm. (D), Quantification of Oil Red O uptake (O.D. at 490 nm) at experimental day 4, 8, and 12. (E–I) Time course of the expression of the markers aP2 (E), PPARγ (F), Lpl (G), adiponectin (H), and Pref-1 (I) during differentiation. mRNA levels are expressed relative to control cells (C0) on day 0, and normalized for the housekeeping gene β-actin. Data are means ± SEM of 3 independent experiments performed in duplicate. *P < 0.05 for C1; †P < 0.05 for C2, versus control C0.
During differentiation of 3T3-F442A preadipocytes into mature adipocytes, MMP-9 expression in C0 control cells sharply increased up to day 2 and then gradually declined. Stable MMP-9 knockdown was maintained throughout the 12-d differentiation period with C1, whereas with C2, moderately enhanced expression was only observed at day 2 (Fig. 1A). MMP-2 mRNA expression reached a plateau at day 4 to 6 and then decreased again (Fig. 1B). The time course was very similar for both constructs. At experimental day 12, MMP-9 antigen levels in cultured medium were markedly reduced as compared with day 0 for the control C0 (0.63 ± 0.06 ng/ml) as well as for clone C1 (undetectable) and C2 (0.14 ± 0.04 ng/ml). Thus, at the end of differentiation, MMP-9 levels remained significantly lower for the clones C1 and C2 as compared with C0.
Oil Red O staining at days 4, 8 and 12 (Fig. 1C) of differentiation did not indicate major differences in intracytoplasmatic lipid content of 3T3-F442A cells transduced with either clone C1 or C2 as compared with control C0. Extraction and quantification of Oil Red O uptake confirmed this observation (Fig. 1D). Only at experimental day 8 was Oil Red O staining in cells transduced with C2, but not C1, significantly higher than control C0. Quantitation of the expression of adipogenic markers aP2 (Fig. 1E), PPARγ (Fig. 1F), and Lpl (Fig. 1G) confirmed that the differentiation of 3T3-F442A preadipocytes was not significantly affected by MMP-9 gene silencing using either clone C1 or C2. The expression pattern of adiponectin was similar for C0 and C1, with an increase up to day 8 and thereafter expression levels declined (Fig. 1H). This decrease starting from day 8 to 12 was significantly more pronounced for clone C2. These findings were further supported by the comparable time course of mRNA expression of the preadipocyte marker Pref-1 (Fig. 1I).
Discussion
It has been shown previously that MMPs and their tissue inhibitors (TIMPs) are differentially expressed in adipose tissues during obesity and modulated during adipocyte differentiation.11,12 Several studies have suggested a functional role for the gelatinases (MMP-2 and -9) class of MMPs in preadipocyte differentiation, adipogenesis, and adipose tissue development.2-9 It has previously been suggested that MMP-9 might be a key regulator of adipose tissue development.9 Moreover, a polymorphism in the MMP-9 promoter (rs3918242) is strongly associated with obesity.13 Studies with gene deficient mice, however, indicated a functional role for MMP-2, but not MMP-9, in development of nutritionally induced obesity.7,10 Studies with MMP inhibitors have, however, not been able to discriminate between MMP-2 and MMP-9 (and other MMPs) due to lack of specificity. It has been reported that the hydroxamate-based MMP inhibitors ilomastat (galardin, GM6001), batimastat (BB-94), and CT1746 reduced adipose conversion in vitro.2,5,11,12 In contrast, Alexander et al. noticed that ilomastat promotes differentiation, rather than inhibiting it.14 Enhanced differentiation was also observed when 3T3-F442A cells were treated with the gelatinase inhibitors tolylsam or ABT-518.6,15 It should be noted that broad-spectrum inhibitors can also exert collateral inhibition of members of the zinc-metalloproteinase superfamily, such as a disintegrin and metalloproteinase (ADAM) and ADAM with thrombospondin motif (ADAMTS) families. For instance, ilomastat targets MMP-2, -3, -7, -8, -9, -12, -14, -15, and -26, and ADAM-9, -17, and -19, thus affecting a wide range of physiological processes.
To evaluate a potential role of MMP-9 in preadipocyte differentiation, we have in the present study performed gene silencing of MMP-9 in 3T3-F442A preadipocytes, an established model of in vitro adipogenesis.16 Using shRNA lentiviral transduction particles (2 different clones), we have obtained efficient MMP-9 knockdown, as shown by up to 91% reduction of MMP-9 mRNA expression and up to 93% reduction of MMP-9 antigen in conditioned culture medium. These data were confirmed by reduced activity on gelatin zymography. Furthermore, knockdown of MMP-9 was stable throughout the 12-d differentiation period.
Using this approach, we did not observe a significant effect of MMP-9 gene silencing on differentiation of 3T3-F442A preadipocytes into mature adipocytes. This was supported by comparable levels of intracytoplasmatic lipids, as monitored by Oil Red O staining at several time points throughout the differentiation process. In addition, we observed comparable expression levels of the adipocyte markers aP2, PPARγ, Lpl, adiponectin, and the preadipocyte marker Pref-1. These observations could not be explained by a compensatory effect of MMP-2, as MMP-2 mRNA, protein and activity levels were not significantly affected by MMP-9 knockdown. MMP-2 expression reached a plateau at day 4 to 6 of differentiation and thereafter also declined. Interestingly, we observed a sharp increase in MMP-9 expression at day 2 of differentiation of the control cells, followed by a rapid decline toward the end of the differentiation. MMP-9 is known to activate several other MMPs and can hydrolyse several ECM components, such as gelatins, several collagen types, aggrecan, elastin, vitronectin, etc. Furthermore, in vivo gene regulation is considerably more complex than in an in vitro setting.17 In addition, the complexity of the ECM of adipose tissue cannot be fully mimicked in an experimental in vitro set-up.18 Although it cannot be excluded that low residual expression of MMP-9 is sufficient to support differentiation, it is more likely that MMP-9 does not play a key role in preadipocyte differentiation, and that other factors drive differentiation.
Methods and Procedures
Murine 3T3-F442A preadipocytes16 were grown in basal medium (Dulbecco’s modified Eagle’s medium [DMEM; Invitrogen] supplemented with 10% bovine calf serum [BCS, iron supplemented; Hyclone] and 1% PenStrep [Invitrogen]) and passaged when preconfluent. To obtain stable gene silencing of MMP-9 (NM_013599), the “MISSION shRNA lentiviral transduction particles” system (Sigma-Aldrich) was used. Five target clones were provided (TRCN0000031229-31233) and non-target shRNA transduction particles (SHC002V, hereafter “C0”) were used as negative control. Transduction was performed as previously described.19 Knockdown was verified on gelatin zymography7 by comparing conditioned medium of cells transfected with the different constructs with that of the control cells. To induce differentiation, cells were seeded at a density of 30 × 103 cells cm−2 and when reached confluency (designated as experimental day 0), further differentiated as previously described.19 At regular time points, cell lysates were taken for RNA extraction. On experimental day 4, 8, and 12, the extent of differentiation was assessed by quantification of Oil Red O uptake by lipid-containing cells, as described elsewhere.6
Commercially available ELISAs were used to determine antigen levels of MMP-2 (Abcam) and MMP-9 (R&D Systems). DNA-free total RNA extraction from 3T3-F442A cells and subsequent preparation of cDNA were performed as previously described.6 The expression levels of adipocyte fatty acid-binding protein (aP2), peroxisome proliferator-activated receptor-γ (PPARγ), and preadipocyte factor 1 (Pref-1) were determined with real-time PCR using primers and 6-carboxy-fluorescein-labeled probes, as reported elsewhere.6 TaqMan gene expression assays (Applied Biosystems) were used to follow the expression of MMP-2 (Mm00439498_m1), MMP-9 (Mm00442991_m1), lipoprotein lipase (Lpl; Mm00434764_m1), and adiponectin (Mm00456425_m1). Fold differences in gene expression were calculated from cycle threshold (Ct) values with the ΔΔCt method, using β-actin as housekeeping gene. β-actin expression did not markedly change (≤10% variation) during the 12-d differentiation period. Data are expressed relative to C0 control cells on day 0 of the differentiation experiments, or as copy numbers of target-mRNA relative to 105 copies of β-actin for the gene knockdown.
Data are expressed as means ± SEM. Differences between two groups were analyzed with the non-parametric Mann–Whitney U test. Comparison of progress curves was performed by two-way ANOVA. Analysis was done with Prism 5 (GraphPad Software Inc.). Two-sided P < 0.05 was considered statistically significant. Bonferroni correction was applied for multiple testing.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
Skillful technical assistance by I Vorsters and C Vranckx is gratefully acknowledged. This study was supported financially by a grant from the “Fonds voor Wetenschappelijk Onderzoek-Vlaanderen” (FWO; G.02.240.07). The Center for Molecular and Vascular Biology is supported by the “Programmafinanciering KU Leuven” (PF/10/014).
Footnotes
Previously published online: www.landesbioscience.com/journals/adipocyte/article/26966
References
- 1.Christiaens V, Scroyen I, Lijnen HR. Role of proteolysis in development of murine adipose tissue. Thromb Haemost. 2008;99:290–4. doi: 10.1160/TH07-10-0589. [DOI] [PubMed] [Google Scholar]
- 2.Bouloumié A, Sengenès C, Portolan G, Galitzky J, Lafontan M. Adipocyte produces matrix metalloproteinases 2 and 9: involvement in adipose differentiation. Diabetes. 2001;50:2080–6. doi: 10.2337/diabetes.50.9.2080. [DOI] [PubMed] [Google Scholar]
- 3.Lijnen HR, Maquoi E, Holvoet P, Mertens A, Lupu F, Morange P, Alessi MC, Juhan-Vague I. Adipose tissue expression of gelatinases in mouse models of obesity. Thromb Haemost. 2001;85:1111–6. [PubMed] [Google Scholar]
- 4.Van Hul M, Lijnen HR. Effect of weight loss on gelatinase levels in obese mice. Clin Exp Pharmacol Physiol. 2011;38:647–9. doi: 10.1111/j.1440-1681.2011.05552.x. [DOI] [PubMed] [Google Scholar]
- 5.Croissandeau G, Chrétien M, Mbikay M. Involvement of matrix metalloproteinases in the adipose conversion of 3T3-L1 preadipocytes. Biochem J. 2002;364:739–46. doi: 10.1042/BJ20011158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van Hul M, Bauters D, Himmelreich U, Kindt N, Noppen B, Vanhove M, Lijnen HR. Effect of gelatinase inhibition on adipogenesis and adipose tissue development. Clin Exp Pharmacol Physiol. 2012;39:49–56. doi: 10.1111/j.1440-1681.2011.05635.x. [DOI] [PubMed] [Google Scholar]
- 7.Van Hul M, Lijnen HR. A functional role of gelatinase A in the development of nutritionally induced obesity in mice. J Thromb Haemost. 2008;6:1198–206. doi: 10.1111/j.1538-7836.2008.02988.x. [DOI] [PubMed] [Google Scholar]
- 8.Van Hul M, Lijnen HR. Matrix metalloproteinase inhibition impairs murine adipose tissue development independently of leptin. Endocr J. 2011;58:101–7. doi: 10.1507/endocrj.K10E-267. [DOI] [PubMed] [Google Scholar]
- 9.Bourlier V, Zakaroff-Girard A, De Barros S, Pizzacalla C, de Saint Front VD, Lafontan M, Bouloumié A, Galitzky J. Protease inhibitor treatments reveal specific involvement of matrix metalloproteinase-9 in human adipocyte differentiation. J Pharmacol Exp Ther. 2005;312:1272–9. doi: 10.1124/jpet.104.077263. [DOI] [PubMed] [Google Scholar]
- 10.Van Hul M, Piccard H, Lijnen HR. Gelatinase B (MMP-9) deficiency does not affect murine adipose tissue development. Thromb Haemost. 2010;104:165–71. doi: 10.1160/TH09-10-0739. [DOI] [PubMed] [Google Scholar]
- 11.Maquoi E, Munaut C, Colige A, Collen D, Lijnen HR. Modulation of adipose tissue expression of murine matrix metalloproteinases and their tissue inhibitors with obesity. Diabetes. 2002;51:1093–101. doi: 10.2337/diabetes.51.4.1093. [DOI] [PubMed] [Google Scholar]
- 12.Chavey C, Mari B, Monthouel MN, Bonnafous S, Anglard P, Van Obberghen E, Tartare-Deckert S. Matrix metalloproteinases are differentially expressed in adipose tissue during obesity and modulate adipocyte differentiation. J Biol Chem. 2003;278:11888–96. doi: 10.1074/jbc.M209196200. [DOI] [PubMed] [Google Scholar]
- 13.Andrade VL, Fernandes KS, Bosco AA, Tanus-Santos JE, Sandrim VC. Functional polymorphism located in MMP-9 gene promoter is strongly associated with obesity. DNA Cell Biol. 2012;31:1054–7. doi: 10.1089/dna.2011.1526. [DOI] [PubMed] [Google Scholar]
- 14.Alexander CM, Selvarajan S, Mudgett J, Werb Z. Stromelysin-1 regulates adipogenesis during mammary gland involution. J Cell Biol. 2001;152:693–703. doi: 10.1083/jcb.152.4.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van Hul M, Bauters D, Lijnen RH. Differential effects of a gelatinase inhibitor on adipocyte differentiation and adipose tissue development. Clin Exp Pharmacol Physiol. 2013;40:689–97. doi: 10.1111/1440-1681.12154. [DOI] [PubMed] [Google Scholar]
- 16.Green H, Kehinde O. Spontaneous heritable changes leading to increased adipose conversion in 3T3 cells. Cell. 1976;7:105–13. doi: 10.1016/0092-8674(76)90260-9. [DOI] [PubMed] [Google Scholar]
- 17.Soukas A, Socci ND, Saatkamp BD, Novelli S, Friedman JM. Distinct transcriptional profiles of adipogenesis in vivo and in vitro. J Biol Chem. 2001;276:34167–74. doi: 10.1074/jbc.M104421200. [DOI] [PubMed] [Google Scholar]
- 18.Mariman EC, Wang P. Adipocyte extracellular matrix composition, dynamics and role in obesity. Cell Mol Life Sci. 2010;67:1277–92. doi: 10.1007/s00018-010-0263-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Christiaens V, Van Hul M, Lijnen HR, Scroyen I. CD36 promotes adipocyte differentiation and adipogenesis. Biochim Biophys Acta. 2012;1820:949–56. doi: 10.1016/j.bbagen.2012.04.001. [DOI] [PubMed] [Google Scholar]