Abstract
The discovery of irisin as a novel and promising peptidic hormone has raised hopes regarding the hypothesis that irisin may provide additional benefits, not only for obesity and diabetes, but also for a wide range of pathological conditions since this hormone may prove to be therapeutically and clinically beneficial. In addition, a new hormone, betatrophin, has recently been identified by Yi and coworkers. Both hormones are connected by a new pathway clearly involved in insulin resistance. We hypothesize here how these hormones may be linked and their possible implications in both aged-reduced restricted regenerative capacity and dedifferentiated β cells of diabetic patients.
Keywords: diabetes, metabolism, obesity, oxidative stress, β cells
It has recently been suggested that the best treatment, and a potential cure, for both forms of diabetes, i.e., type 1 (T1D) and type 2 diabetes (T2D), is to replace or regenerate the pancreatic β-cell mass.1 Interestingly, the recently indentified hormone, betatrophin, could be involved in this.2,3 This hormone, discovered by Yi and collaborators, specifically increases β-cell mass in mice,4 thereby raising hopes for regenerative β-cell therapy in humans. In addition, the quite recent discovery of another novel and promising peptidic hormone called irisin has also been reported by Spiegelman’s group.5 Irisin basically acts on the cells of white adipose tissue and its concentrations increase after endurance exercise training in both mice and humans, thus increasing total energy expenditure and mitigating diet-induced insulin resistance in certain animal models.5 All these biological effects ultimately contribute in reducing obesity and insulin resistance. In addition, age and skeletal muscle mass are clearly predictors of circulating irisin levels, such levels of young male athletes being several times higher than those of middle-aged, obese women.6,7 Circulating irisin is also significantly lower in individuals with T2D compared with non-diabetic controls, whereas recombinant irisin administration to fat mice significantly improves glucose tolerance.8
Irisin acts on white adipose cells in culture and in vivo to stimulate uncoupling protein 1 (UCP1) expression and a broad program of brown-fat-like development.5 The expression of UCP1 is regulated by several transcriptional factors, including peroxisome proliferator-activated receptor gamma (PPAR-γ) coactivator-1 α (PGC-1α), which can be induced by exposure to cold temperatures. Likewise, the precursor of irisin, the fibronectin type III domain-containing protein 5 (FNDC5) and a type I transmembrane protein of skeletal muscle, is a PGC-1α-dependent myokine that is cleaved and secreted from muscle during exercise.5 More interestingly, we have recently found an age-associated lack of expression of PGC-1α in response to exercise training and to other stimuli, such as cold induction or thyroid hormone treatment. In addition, aged rodents behave as PGC-1α knockout (KO) animals.9
Clarifying the action mechanism of irisin, Zhang and coworkers recently reported that the irisin-induced browning effect marked by enhanced expression of browning gene UCP1 was mediated via activation of p38 mitogen-activated protein kinase (p38 MAPK) and extracellular regulated protein kinase (ERK), in this fashion playing a central role in mediating the irisin browning function.8 PGC-1α is regulated by activation of cAMP/PKA/p38 MAPK signaling pathways. It is also well known that p38 MAPK plays an important role in the coordination of cellular responses to many stress stimuli, mediating oxidative stress-sensitive cell signaling pathways. In fact, reactive oxygen species (ROS) overproduction plays a role in p38 activation. More importantly, irisin also promotes the expression of betatrophin.8
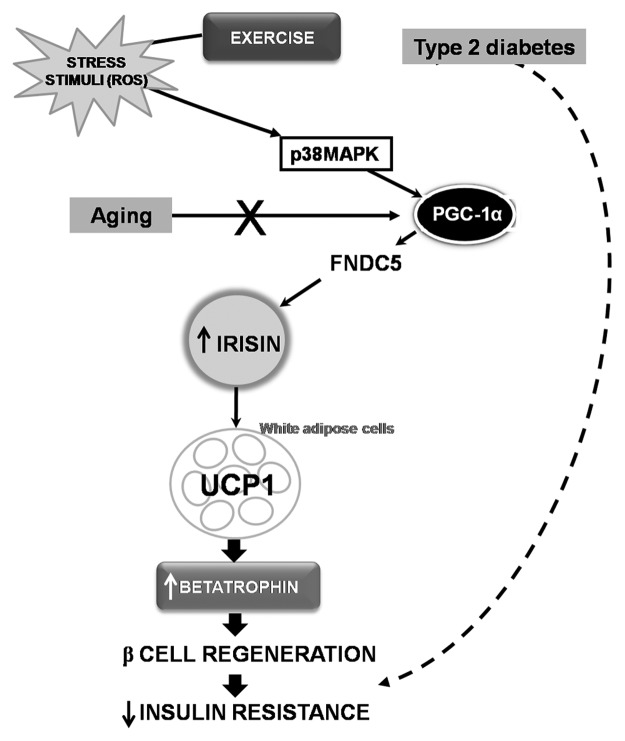
Therefore, PGC1-α expression in muscle stimulates an increase in the expression of FNDC5 and secreted irisin and the latter acts on white adipose cells to stimulate UCP1expression, mediated via activation of p38 MAPK, and promotes the expression of betatrophin. On the other hand, lack of expression of PGC-1α is associated with age, which concurs with the fact that the aged population has lower levels of irisin and that adaptive β-cell regeneration declines with age in mammals. In accordance with this, it is worth noting that aged people usually suffer from insulin resistance. Similarly, T2D patients have lower concentrations of this hormone whereas exercise, and therefore a rise in ROS levels, increases circulating irisin concentrations, which certainly improves glucose tolerance and insulin resistance. To conclude, the following pathway, ROS → p38 MAPK → PGC-1α → irisin → betatrophin → β-cell regeneration (Fig. 1), may be involved in the production mechanism of insulin resistance, which would provide new possibilities for exploring defective pathways involved in aged-reduced restricted regenerative capacity and the dedifferentiated β cells of diabetic patients.
Figure 1. Schematic diagram hypothesizing the role of exercise and age in the p38–PGC-1α–irisin–betatrophin pathway and its possible implications in insulin resistance. Exercise raises ROS levels activating p38MAPK, whereas PGC-1α is regulated by activation of p38MAPK. PGC-1α, through an increase in expression of FNDC5, secretes irisin, which acts on white adipose cells to stimulate UCP1 expression. This promotes the expression of betatrophin and β-cell regeneration and decreases insulin resistence. In addition, lack of expression of PGC-1α is associated with age (X). Thus, irisin and betatrophin levels may decrease in both aged people and type 2 diabetes (T2D) patients. Consequently, exercise is an important tool to activate this pathway, thereby decreasing insulin resistance and improving glucoregulation in T2D patients. However, the activation of this pathway may fail during the aging process due to the lack of expression of PGC-1α, gradually increasing insulin resistance. p38 MAPK, p38 mitogen-activated protein kinase; PGC-1α, peroxisome proliferator-activated receptor gamma (PPAR-γ) coactivator-1 α; FNDC5, fibronectin type III domain-containing protein 5; UCP1, uncoupling protein 1.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/adipocyte/article/27370
References
- 1.Lickert H. Betatrophin fuels β cell proliferation: first step toward regenerative therapy? Cell Metab. 2013;18:5–6. doi: 10.1016/j.cmet.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 2.Crunkhorn S. Metabolic disorders: Betatrophin boosts β-cells. Nat Rev Drug Discov. 2013;12:504. doi: 10.1038/nrd4058. [DOI] [PubMed] [Google Scholar]
- 3.Kugelberg E. Diabetes: Betatrophin--inducing β-cell expansion to treat diabetes mellitus? Nat Rev Endocrinol. 2013;9:379. doi: 10.1038/nrendo.2013.98. [DOI] [PubMed] [Google Scholar]
- 4.Yi P, Park JS, Melton DA. Betatrophin: a hormone that controls pancreatic β cell proliferation. Cell. 2013;153:747–58. doi: 10.1016/j.cell.2013.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 5.Boström P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, Rasbach KA, Boström EA, Choi JH, Long JZ, et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481:463–8. doi: 10.1038/nature10777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gouni-Berthold I, Berthold HK, Huh JY, Berman R, Spenrath N, Krone W, Mantzoros CS. Effects of lipid-lowering drugs on irisin in human subjects in vivo and in human skeletal muscle cells ex vivo. PLoS One. 2013;8:e72858. doi: 10.1371/journal.pone.0072858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huh JY, Panagiotou G, Mougios V, Brinkoetter M, Vamvini MT, Schneider BE, Mantzoros CS. FNDC5 and irisin in humans: I. Predictors of circulating concentrations in serum and plasma and II. mRNA expression and circulating concentrations in response to weight loss and exercise. Metabolism. 2012;61:1725–38. doi: 10.1016/j.metabol.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang Y, Li R, Meng Y, Li S, Donelan W, Zhao Y, Qi L, Zhang M, Wang X, Cui T, et al. Irisin Stimulates Browning of White Adipocytes through Mitogen-Activated Protein Kinase p38 MAP Kinase and ERK MAP Kinase Signaling. Diabetes 2013. [DOI] [PubMed] [Google Scholar]
- 9.Derbré F, Gomez-Cabrera MC, Nascimento AL, Sanchis-Gomar F, Martinez-Bello VE, Tresguerres JA, Fuentes T, Gratas-Delamarche A, Monsalve M, Viña J. Age associated low mitochondrial biogenesis may be explained by lack of response of PGC-1α to exercise training. Age (Dordr) 2012;34:669–79. doi: 10.1007/s11357-011-9264-y. [DOI] [PMC free article] [PubMed] [Google Scholar]