Abstract
Profilin-1 (pfn) is a small ubiquitous protein that can bind to: (1) G-actin, (2) phosphatidylinositol 4,5-bisphosphate, and (3) a heterogeneous group of proteins harboring poly-l-proline stretches. Through these interactions, pfn integrates signaling from a diverse array of extracellular cues with actin cytoskeleton dynamics. Cumulating evidence indicates that changes in pfn levels are associated and may play a pathogenic role in such inflammatory diseases as atherosclerosis and glomerulonephritis. We recently demonstrated that high fat diet (HFD) increases pfn expression in the white adipose tissue (WAT), but not in the liver or the muscle. Pfn heterozygote mice (PfnHet) were protected against HFD-induced glucose intolerance, and WAT and systemic inflammation, when compared to pfn wild-type mice. In addition to blunted accumulation of macrophages and reduced “pro-inflammatory” cytokines, the WAT of PfnHet exhibited preserved frequency of regulatory T cells. These findings suggest that pfn levels in WAT—both adipocytes and hematopoietic-derived cells—can modulate immune homeostasis within the WAT and glucose tolerance systemically. Here, we review the interaction of pfn with his diverse array of binding partners and discuss mechanisms that may underlie the effects of pfn dosage on insulin sensitivity and metabolic inflammation.
Keywords: profilin-1, insulin resistance, inflammation, adipose tissue macrophages, regulatory T cells
Introduction
It is estimated that obesity affects more than one-third of the US adult population1 with its prevalence steadily increasing also in children and adolescents.2 The worldwide social and economic burden of obesity has recently led the American Medical Association and other key medical societies to recognize obesity as an independent disease, rather than simply a risk factor for a growing list of ailments. These include, but are not limited to, type 2 diabetes mellitus (T2DM), non-alcoholic fatty liver disease, atherosclerosis, and obstructive sleep apnea.
The hypothesis has been set forth that the low-grade chronic inflammation observed in experimental models of obesity and in obese individuals may represent a common pathophysiological trait of obesity-associated abnormalities.3 As discussed below, several lines of investigations have helped define a dynamic cross-talk and possibly interdependence between the changes in the immune system and metabolic processes occurring in obesity and insulin resistance (IR), both systemically and at the tissue level (reviewed in ref. 4).
Tremendous progress has been attained in our understanding of the events fueling this “metabolic” inflammation, and the efficacy of therapies targeting candidate inflammatory pathways is being actively investigated in patients with obesity and T2DM.5,6
Yet, the molecular mechanisms responsible for immune cell recruitment in the adipose tissue and vascular wall upon high fat diet (HFD) remain largely elusive. In this context, we addressed the effect of profilin-1 in HFD-induced IR and adipose tissue inflammation.
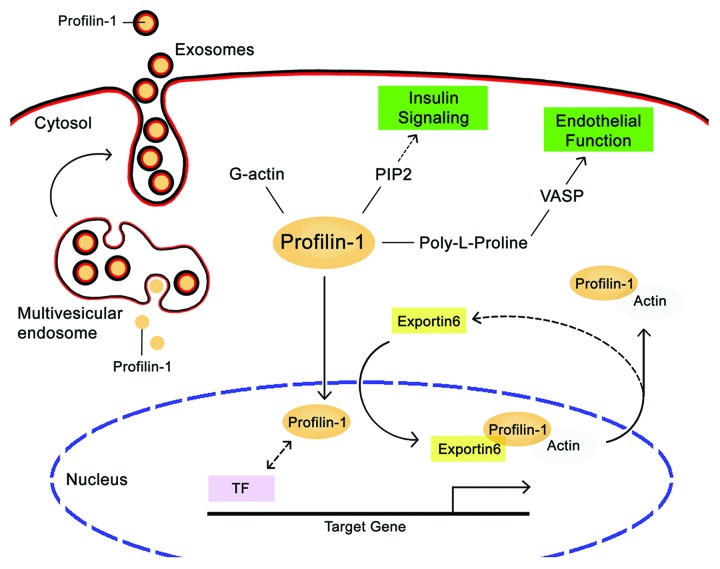
The members of the profilin family (profilin-1, -2, and -3) are well conserved through lower and higher eukaryotes and were first characterized as small actin-binding proteins regulating the dynamics of actin polymerization.7 Profilin-1 (pfn) binds G-actin and stimulates the exchange of ADP to ATP on G-actin, thereby controlling the pool of ATP–actin in the cell. Also, the pfn•actin complex can promote actin filament elongation by directing actin monomers onto free barbed ends, following the dissociation of capping proteins (Fig. 1).8
Figure 1. Schematic overview of profilin-1 network of interactions. Profilin-1 can bind to G-actin, PIP2, and proteins with poly-l-proline (PLP) stretches, including VASP. Other characterized PLP-containing profilin1-binding partners are not reported for simplicity. While a small amount of profilin-1 diffuses into nucleus and possibly interacts with transcription factors (TF), the profilin-1•actin complex is exported back in the cytoplasm by exportin 6. Additionally, profilin-1 can be secreted extracellularly within exosomes, which originate from multivesicular endosome. Direct interactions between profilin-1 and ligands are indicated by solid lines, whereas presumable associations are indicated by dashed lines. Abbreviations: PIP2, phosphoinositides 4,5-bisphosphate; VASP, vasodilator-stimulated phosphoprotein.
In addition to G-actin, pfn can bind to phosphoinositides (PI), including PI(4,5)-bisphosphate (PtdIns[4,5]P2),9 and to poly-l-proline (PLP) stretches,10 which are harbored by a plethora of proteins with such diverse functions as membrane trafficking, GTPase signaling, and possibly transcriptional activity (reviewed in ref. 11). With regard to the latter, a small pool of pfn distributes to the nucleus, is actively shuttled back to the cytosol by exportin 6,12 and can regulate the activity of the transcription factor p42POP in cultured cells.13 Although its significance is poorly understood, pfn nuclear localization is an enticing finding because it could represent a link between cytoskeletal dynamics and transcriptional activity or chromatin remodeling. Another relevant point in pfn cell distribution is that, despite the lack of a signal peptide, pfn can be secreted in the extracellular space, possibly in the context of small vesicles that originate from the multivesicular endosome called exosomes.14
Effects of Pfn Dosage in Vascular and Adipose Tissue Inflammation
The extensive information regarding pfn’s function in cell physiology is not matched by a clear understanding of its role in disease.
Initially, we became interested in pfn in the setting of a peptide-phage display library screening aimed at identifying proteins that were differentially expressed on the luminal surface of the aorta of diabetic rats, as compared with nondiabetic age-matched animals. We found that pfn expression was increased in the aortic endothelium of streptozotocin-diabetic rats, in endothelial cells (EC) exposed to oxidized cholesterol or oxidized LDL—but not to high glucose concentrations— and in atherosclerotic lesions.15 Of note, the lack of regulation by high glucose concentration was in apparent contrast with prior findings in mesangial cells..16 Also, pfn heterozygote mice (PfnHet) showed a marked reduction in atherosclerotic burden and macrophage accumulation in the vascular wall, when compared with pfn wild-type mice (PfnWT), and macrophages isolated from PfnHet displayed blunting of inflammatory pathways.17 Finally, we demonstrated that oxidized cholesterol was able to upregulate pfn expression in a STAT3-dependent manner in cultured EC and possibly in the aorta of diabetic rats.18
The upregulation of pfn in response to oxidized lipids and the decreased accumulation of macrophages in PfnHet suggested that pfn haploinsufficiency exerted its anti-atherogenic phenotype, at least in part, by reducing vascular inflammation, which is triggered by macrophage recruitment into the nascent plaque due to unregulated lipid accumulation.19
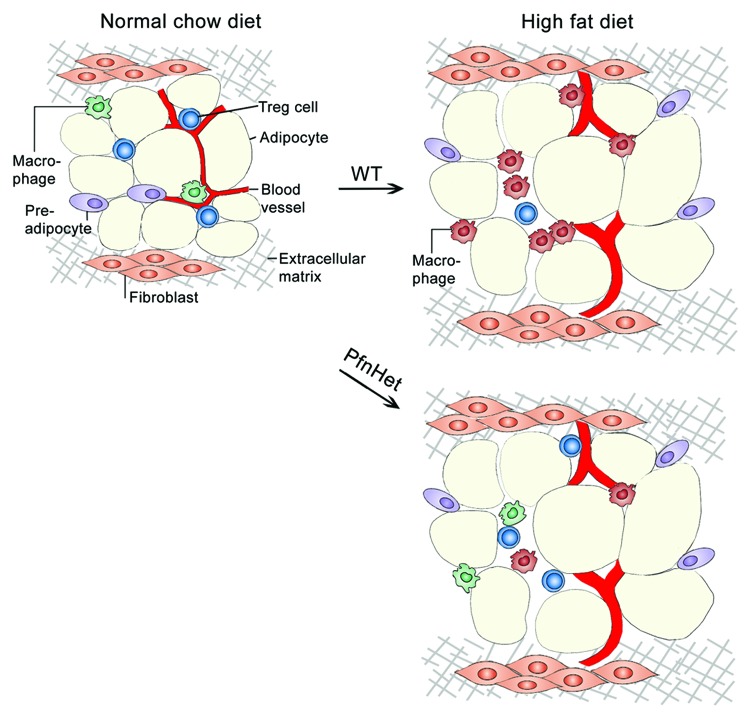
This low-grade inflammation in the vascular wall exhibits similarities with the chronic inflammation occurring in the expanding white adipose tissue (WAT)20 that may contribute to the pathogenesis of IR and T2DM.21 In addition to mature adipocytes, the WAT comprises other cell types, including preadipocytes, fibroblasts, vascular EC, and smooth muscle cells, and immune cells such as adipose tissue macrophages (ATMs) and T cells, which together constitute the stromal vascular fraction (SVF). Specifically, WAT expansion is accompanied by accumulation of ATMs22-24 with tremendous phenotypic heterogeneity and often displaying mixed M1/M2 features (reviewed in ref. 20). With regard to T cells, increased ratio of CD8+ to CD4+ T cells25 and decreased frequency of regulatory T cells (Treg)26 were observed in HFD-fed mice. Mounting evidence indicates that WAT-specific Treg provide cues that influence immune homeostasis in WAT, possibly by keeping inflammation in check upon HFD or in genetic models of obesity.27
As mentioned above, PfnHet mice were protected against atherosclerosis.17 Because of the common underpinnings between vascular wall and WAT inflammation, we asked whether pfn could play a role in obesity-induced WAT inflammation. We found that HFD increased pfn expression in both adipocytes and SVF of epididymal WAT, but not in the liver or the muscle, and that pfn expression in WAT correlated with F4/80, an established marker for mature macrophages. PfnHet mice fed HFD were protected from glucose intolerance, and exhibited reduced ATM accumulation and serum proinflammatory cytokines, when compared with PfnWT mice. Of note, ATMs from PfnHet mice were biased toward a M2-like phenotype, which correlated with preserved Treg frequency (Fig. 2). No significant change in adipocyte size or number was noted in PfnHet mice.28 In humans, proteomic analysis of the subcutaneous adipose tissue of “old” (52.2 ± 4.7, mean age ± SD) vs. BMI-matched “young” (26.2 ± 4.3) obese individuals revealed that pfn was more abundant in adipocytes isolated from the older group.29
Figure 2. Profilin-1 heterozygote mice are protected against high fat diet-induced IR and inflammation. Under normal chow diet, adipose tissue stromal vascular fraction (SVF) contains regulatory T cells (Treg) and macrophages with a M2-like phenotype (green macrophages). Upon high fat diet, the SVF of wild-type mice exhibits increased accumulation of macrophages with a M1-like phenotype (red macrophages) and reduced frequency of Treg. Conversely, PfnHet mice showed a more heterogenous macrophage population (both red and green macrophages) and preserved Treg population. This is an oversimplified representation of the diverse array of macrophage subsets observed upon high fat diet.
So, how do pfn levels affect glucose tolerance and HFD-induced inflammation? Our findings suggest that pfn haploinsufficiency maintains WAT immune homeostasis by “skewing” ATM toward an anti-inflammatory M2-like phenotype, possibly as a result of an intact pool of Treg in the face of HFD. However, we are cognizant of the limitations of such interpretation in the setting of this model of whole-body pfn haploinsufficiency. Here, we discuss hypotheses that expand the results presented in our recent paper28 and that may contribute to the protective effects observed in PfnHet mice.
Pfn and WAT endothelial function
It has been proposed that IR contributes to the reduced production of nitric oxide (NO) in EC and to the endothelial dysfunction observed in patients with metabolic syndrome and T2DM.30,31 Reduced NO signaling, and overexpression of adhesion molecules and chemotactic factors are hallmarks of endothelial dysfunction that may accelerate inflammatory processes within the vascular wall. Pfn overexpression in cultured EC upregulated ICAM-1 expression and decreased NO signaling15 and increased EC adhesion to fibronectin by increasing the recruitment of fibronectin receptor to the plasma membrane.32 Also, the pro-atherogenic factor homocysteine increased pfn expression in human umbilical vein EC treated under laminar flow conditions.33 On the other hand, PfnHet mice (backcrossed on the atherosclerosis prone LDL-receptor deficient background) were protected against atherosclerosis and the decrease in NO signaling observed in PfnWT mice.17 In the vasculature, pfn can control NO signaling via at least 2 mechanisms: (1) the activation of eNOS and NO production in EC, and (2) the phosphorylation of VASP, which is a well-established downstream target of NO signaling in vascular smooth muscle cells15,34,35 but also a binding partner of pfn by virtue of its PLP stretches.36
The consequences of HFD or obesity on WAT capillary EC are poorly understood. Immune cell recruitment through extravasation at sites of inflammation requires a fine-tuned rearrangement of EC cytoskeleton.37 The role of excess pfn in the pathophysiology of endothelial dysfunction in vitro or in the aorta of atherosclerosis-prone mice suggests that pfn may exert similar effects in the WAT vasculature upon HFD. However, isolation and ex vivo analysis of WAT capillary EC free of contaminating adhering blood elements has proven a daunting task (unpublished observation). Alternatively, studies in EC-specific pfn knockout would be informative but would not allow distinguish the effects of pfn in WAT capillaries vs. other vasculature beds.
In summary, the role of pfn in WAT endothelial dysfunction remains unproven; more broadly, the development of novel approaches to study freshly isolated WAT capillary EC is a priority in the field as it would provide insight in the mechanisms for immune cell recruitment and possibly new therapeutic targets for IR and T2DM.
Pfn and PI3K/AKT signaling
Phosphatidylinositol 3-kinase (PI3K), which is essential for many cellular responses to insulin (reviewed in ref. 38), phosphorylates membrane bound PtdIns(4,5)P2 to generate phosphatidylinositol 3,4,5-triphosphate (PtdIns[3,4,5]P3), which propagates the metabolic signals of insulin in part through the Akt pathway. The downstream targets of Akt include glucose transporters (GLUT), glycogen synthase kinase-3, the forkhead transcription factors, cAMP response element-binding protein, and under certain conditions eNOS. Of interest, pfn interacts with PtdIns(4,5)P2, the substrate for PI3K. Thus, one could speculate that pfn overexpression in cells (e.g., adipocytes) would impede insulin signaling by sequestering PtdIns(4,5)P2 and that, conversely, the protective phenotype of PfnHet could be due to enhanced insulin action in insulin-dependent tissues including the WAT. In this view, pfn overexpression in breast cancer cells reduced PtdIns(3,4,5)P3 generation and suppressed Akt activation through PTEN upon EGF stimulation.39 It remains unsettled if changes in pfn levels affect insulin-mediated PI3K/Akt activation in insulin-dependent tissues.
GLUT4 translocation is another downstream target of insulin signaling that may be affected by pfn levels as the actin cytoskeleton is known to be crucial for insulin-simulated GLUT4 translocation (reviewed in ref. 40). However, a modest pfn overexpression in 3T3-L1, as a model for the 2- to 3-fold HFD-induced upregulation, did not modify GLUT4 quantity or cell distribution under both basal and insulin-stimulated conditions (unpublished observations, G.R.R.). These studies do not support the hypothesis that pfn overexpression would per se impair glucose tolerance by affecting GLUT4 translocation, although they do not definitively rule out a more indirect role for pfn in insulin signaling.
Pfn and transcription factors
In addition to pfn nuclear localization, the interaction between pfn and nuclear proteins supports a possible role of pfn in transcriptional regulation. For example, pfn binds to the survival of motor neuron SMN protein,41 which is a nuclear factor that is essential for splicing.42 Additionally, pfn can regulate the activity of the transcription factor p42POP in cultured cells.13 These interactions appear to be mediated by the PLP binding domain of pfn. Our laboratory is currently investigating the hypothesis that pfn overexpression could enhance or suppress the transcriptional activity of nuclear factors with key functions in inflammation.
Extracellular pfn
Pfn is commonly recognized as an intracellular protein since it primarily resides inside the cell and lacks a signal peptide. However, there is emerging evidence that pfn may have extracellular functions. Pfn can be detected in the human serum under normal conditions; also, pfn serum levels positively correlate with the degree of aortic atherosclerosis in patients with coronary artery disease.43 In the same study, we demonstrated that extracellular pfn could induce such pro-atherogenic events as cell proliferation and chemotaxis.43 Extracellular pfn was also detected in an experimental model of glomerulonephritis.44 What are the stimuli and mechanisms for pfn secretion, and is there a specific receptor/ligand on the cell membrane? Pfn can be secreted in the extracellular space via exosomes,14 which are small (50–100 nm) membrane vesicles of endocytic origin secreted by most cells including T cells, B cells, and dendritic cells. Exosomes are signaling payloads found to contain adhesion molecules, “danger signals” originating from apoptotic cells, mRNA, and microRNA (reviewed in refs. 45 and 46) that can exert various effects on target immune cells (reviewed in ref. 47).
To date, there is no evidence that pfn specifically recognizes any cell membrane receptor(s). This scientific gap has limited our understanding of the putative functions of extracellular pfn and its role in disease.
In summary, it is now recognized that pfn function extends beyond its key role in the actin cytoskeleton. Studies from many laboratories highlighted the heterogeneity of pfn binding partners and implicated pfn in basic cellular processes. Our recent findings demonstrated that PfnHet are protected against HFD-induced glucose intolerance and WAT and systemic inflammation, possibly through maintaining AT homeostasis. These findings are likely the integrated “net outcome” of pfn effects on distinct pathways, including activation of yet-to-be characterized nuclear factors, binding to putative extracellular partners, and modulation of insulin signaling.
Sorting which of these platform(s) mediate metabolic effects of pfn will likely provide meaningful information in the pathogenesis of IR and T2DM, and will pave the way for novel targeted therapeutic approaches to these conditions.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported by the following grants to G.R.R.: JDRF Career Development Award 2-2004-609; Pilot and Feasibility Program-Joslin Diabetes Center; and Pilot and Feasibility Program-Boston Obesity Nutrition Research Center.
Glossary
Abbreviations:
- ATM
adipose tissue macrophage
- eNOS
endothelial nitric oxide synthase
- PIP2
phosphatidylinositol 4,5-bisphosphate
- PI3K
phosphatidylinositol 3-kinase
- Pfn
profilin-1
- Treg
regulatory T cells
- VASP
vasodilator-stimulated phosphoprotein
- STAT
signal transducer and activator of transcription
Footnotes
Previously published online: www.landesbioscience.com/journals/adipocyte/article/26965
References
- 1.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999-2010. JAMA. 2012;307:491–7. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 2.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999-2010. JAMA. 2012;307:483–90. doi: 10.1001/jama.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Odegaard JI, Chawla A. Pleiotropic actions of insulin resistance and inflammation in metabolic homeostasis. Science. 2013;339:172–7. doi: 10.1126/science.1230721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mathis D, Shoelson SE. Immunometabolism: an emerging frontier. Nat Rev Immunol. 2011;11:81. doi: 10.1038/nri2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldfine AB, Fonseca V, Jablonski KA, Chen YD, Tipton L, Staten MA, Shoelson SE, Targeting Inflammation Using Salsalate in Type 2 Diabetes Study Team* Salicylate (salsalate) in patients with type 2 diabetes: a randomized trial. Ann Intern Med. 2013;159:1–12. doi: 10.7326/0003-4819-159-1-201307020-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Larsen CM, Faulenbach M, Vaag A, Ehses JA, Donath MY, Mandrup-Poulsen T. Sustained effects of interleukin-1 receptor antagonist treatment in type 2 diabetes. Diabetes Care. 2009;32:1663–8. doi: 10.2337/dc09-0533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carlsson L, Nyström LE, Sundkvist I, Markey F, Lindberg U. Actin polymerizability is influenced by profilin, a low molecular weight protein in non-muscle cells. J Mol Biol. 1977;115:465–83. doi: 10.1016/0022-2836(77)90166-8. [DOI] [PubMed] [Google Scholar]
- 8.Kang F, Purich DL, Southwick FS. Profilin promotes barbed-end actin filament assembly without lowering the critical concentration. J Biol Chem. 1999;274:36963–72. doi: 10.1074/jbc.274.52.36963. [DOI] [PubMed] [Google Scholar]
- 9.Lassing I, Lindberg U. Specific interaction between phosphatidylinositol 4,5-bisphosphate and profilactin. Nature. 1985;314:472–4. doi: 10.1038/314472a0. [DOI] [PubMed] [Google Scholar]
- 10.Lambrechts A, Verschelde JL, Jonckheere V, Goethals M, Vandekerckhove J, Ampe C. The mammalian profilin isoforms display complementary affinities for PIP2 and proline-rich sequences. EMBO J. 1997;16:484–94. doi: 10.1093/emboj/16.3.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Witke W. The role of profilin complexes in cell motility and other cellular processes. Trends Cell Biol. 2004;14:461–9. doi: 10.1016/j.tcb.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 12.Stüven T, Hartmann E, Görlich D. Exportin 6: a novel nuclear export receptor that is specific for profilin.actin complexes. EMBO J. 2003;22:5928–40. doi: 10.1093/emboj/cdg565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lederer M, Jockusch BM, Rothkegel M. Profilin regulates the activity of p42POP, a novel Myb-related transcription factor. J Cell Sci. 2005;118:331–41. doi: 10.1242/jcs.01618. [DOI] [PubMed] [Google Scholar]
- 14.Théry C, Boussac M, Véron P, Ricciardi-Castagnoli P, Raposo G, Garin J, Amigorena S. Proteomic analysis of dendritic cell-derived exosomes: a secreted subcellular compartment distinct from apoptotic vesicles. J Immunol. 2001;166:7309–18. doi: 10.4049/jimmunol.166.12.7309. [DOI] [PubMed] [Google Scholar]
- 15.Romeo G, Frangioni JV, Kazlauskas A. Profilin acts downstream of LDL to mediate diabetic endothelial cell dysfunction. FASEB J. 2004;18:725–7. doi: 10.1096/fj.03-0841fje. [DOI] [PubMed] [Google Scholar]
- 16.Clarkson MR, Murphy M, Gupta S, Lambe T, Mackenzie HS, Godson C, Martin F, Brady HR. High glucose-altered gene expression in mesangial cells. Actin-regulatory protein gene expression is triggered by oxidative stress and cytoskeletal disassembly. J Biol Chem. 2002;277:9707–12. doi: 10.1074/jbc.M109172200. [DOI] [PubMed] [Google Scholar]
- 17.Romeo GR, Moulton KS, Kazlauskas A. Attenuated expression of profilin-1 confers protection from atherosclerosis in the LDL receptor null mouse. Circ Res. 2007;101:357–67. doi: 10.1161/CIRCRESAHA.107.151399. [DOI] [PubMed] [Google Scholar]
- 18.Romeo GR, Kazlauskas A. Oxysterol and diabetes activate STAT3 and control endothelial expression of profilin-1 via OSBP1. J Biol Chem. 2008;283:9595–605. doi: 10.1074/jbc.M710092200. [DOI] [PubMed] [Google Scholar]
- 19.Hansson GK, Libby P. The immune response in atherosclerosis: a double-edged sword. Nat Rev Immunol. 2006;6:508–19. doi: 10.1038/nri1882. [DOI] [PubMed] [Google Scholar]
- 20.Romeo GR, Lee J, Shoelson SE. Metabolic syndrome, insulin resistance, and roles of inflammation--mechanisms and therapeutic targets. Arterioscler Thromb Vasc Biol. 2012;32:1771–6. doi: 10.1161/ATVBAHA.111.241869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol. 2011;11:98–107. doi: 10.1038/nri2925. [DOI] [PubMed] [Google Scholar]
- 22.Patsouris D, Li PP, Thapar D, Chapman J, Olefsky JM, Neels JG. Ablation of CD11c-positive cells normalizes insulin sensitivity in obese insulin resistant animals. Cell Metab. 2008;8:301–9. doi: 10.1016/j.cmet.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lumeng CN, Deyoung SM, Bodzin JL, Saltiel AR. Increased inflammatory properties of adipose tissue macrophages recruited during diet-induced obesity. Diabetes. 2007;56:16–23. doi: 10.2337/db06-1076. [DOI] [PubMed] [Google Scholar]
- 24.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175–84. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nishimura S, Manabe I, Nagasaki M, Eto K, Yamashita H, Ohsugi M, Otsu M, Hara K, Ueki K, Sugiura S, et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med. 2009;15:914–20. doi: 10.1038/nm.1964. [DOI] [PubMed] [Google Scholar]
- 26.Feuerer M, Herrero L, Cipolletta D, Naaz A, Wong J, Nayer A, Lee J, Goldfine AB, Benoist C, Shoelson S, et al. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med. 2009;15:930–9. doi: 10.1038/nm.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lumeng CN, Maillard I, Saltiel AR. T-ing up inflammation in fat. Nat Med. 2009;15:846–7. doi: 10.1038/nm0809-846. [DOI] [PubMed] [Google Scholar]
- 28.Romeo GR, Pae M, Eberlé D, Lee J, Shoelson SE. Profilin-1 haploinsufficiency protects against obesity-associated glucose intolerance and preserves adipose tissue immune homeostasis. Diabetes. 2013;62:3718–26. doi: 10.2337/db13-0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alfadda AA, Benabdelkamel H, Masood A, Moustafa A, Sallam R, Bassas A, Duncan M. Proteomic analysis of mature adipo cytes from obese patients in relation to aging. Exp Gerontol. 2013;48:1196–203. doi: 10.1016/j.exger.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 30.Hsueh WA, Quiñones MJ. Role of endothelial dysfunction in insulin resistance. Am J Cardiol. 2003;92(4A):10J–7J. doi: 10.1016/S0002-9149(03)00611-8. [DOI] [PubMed] [Google Scholar]
- 31.Dandona P, Aljada A, Chaudhuri A, Bandyopadhyay A. The potential influence of inflammation and insulin resistance on the pathogenesis and treatment of atherosclerosis-related complications in type 2 diabetes. J Clin Endocrinol Metab. 2003;88:2422–9. doi: 10.1210/jc.2003-030178. [DOI] [PubMed] [Google Scholar]
- 32.Moldovan NI, Milliken EE, Irani K, Chen J, Sohn RH, Finkel T, Goldschmidt-Clermont PJ. Regulation of endothelial cell adhesion by profilin. Curr Biol. 1997;7:24–30. doi: 10.1016/S0960-9822(06)00024-8. [DOI] [PubMed] [Google Scholar]
- 33.Dardik R, Savion N, Gal N, Varon D. Flow conditions modulate homocysteine induced changes in the expression of endothelial cell genes associated with cell-cell interaction and cytoskeletal rearrangement. Thromb Haemost. 2002;88:1047–53. [PubMed] [Google Scholar]
- 34.Smolenski A, Bachmann C, Reinhard K, Hönig-Liedl P, Jarchau T, Hoschuetzky H, Walter U. Analysis and regulation of vasodilator-stimulated phosphoprotein serine 239 phosphorylation in vitro and in intact cells using a phosphospecific monoclonal antibody. J Biol Chem. 1998;273:20029–35. doi: 10.1074/jbc.273.32.20029. [DOI] [PubMed] [Google Scholar]
- 35.Oelze M, Mollnau H, Hoffmann N, Warnholtz A, Bodenschatz M, Smolenski A, Walter U, Skatchkov M, Meinertz T, Münzel T. Vasodilator-stimulated phosphoprotein serine 239 phosphorylation as a sensitive monitor of defective nitric oxide/cGMP signaling and endothelial dysfunction. Circ Res. 2000;87:999–1005. doi: 10.1161/01.RES.87.11.999. [DOI] [PubMed] [Google Scholar]
- 36.Reinhard M, Giehl K, Abel K, Haffner C, Jarchau T, Hoppe V, Jockusch BM, Walter U. The proline-rich focal adhesion and microfilament protein VASP is a ligand for profilins. EMBO J. 1995;14:1583–9. doi: 10.1002/j.1460-2075.1995.tb07146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carman CV, Sage PT, Sciuto TE, de la Fuente MA, Geha RS, Ochs HD, Dvorak HF, Dvorak AM, Springer TA. Transcellular diapedesis is initiated by invasive podosomes. Immunity. 2007;26:784–97. doi: 10.1016/j.immuni.2007.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature. 2001;414:799–806. doi: 10.1038/414799a. [DOI] [PubMed] [Google Scholar]
- 39.Das T, Bae YH, Wells A, Roy P. Profilin-1 overexpression upregulates PTEN and suppresses AKT activation in breast cancer cells. J Cell Physiol. 2009;218:436–43. doi: 10.1002/jcp.21618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brozinick JT, Jr., Berkemeier BA, Elmendorf JS. “Actin”g on GLUT4: membrane & cytoskeletal components of insulin action. Curr Diabetes Rev. 2007;3:111–22. doi: 10.2174/157339907780598199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Giesemann T, Rathke-Hartlieb S, Rothkegel M, Bartsch JW, Buchmeier S, Jockusch BM, Jockusch H. A role for polyproline motifs in the spinal muscular atrophy protein SMN. Profilins bind to and colocalize with smn in nuclear gems. J Biol Chem. 1999;274:37908–14. doi: 10.1074/jbc.274.53.37908. [DOI] [PubMed] [Google Scholar]
- 42.Pellizzoni L, Kataoka N, Charroux B, Dreyfuss G. A novel function for SMN, the spinal muscular atrophy disease gene product, in pre-mRNA splicing. Cell. 1998;95:615–24. doi: 10.1016/S0092-8674(00)81632-3. [DOI] [PubMed] [Google Scholar]
- 43.Caglayan E, Romeo GR, Kappert K, Odenthal M, Südkamp M, Body SC, Shernan SK, Hackbusch D, Vantler M, Kazlauskas A, et al. Profilin-1 is expressed in human atherosclerotic plaques and induces atherogenic effects on vascular smooth muscle cells. PLoS One. 2010;5:e13608. doi: 10.1371/journal.pone.0013608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tamura M, Tanaka H, Yashiro A, Osajima A, Okazaki M, Kudo H, Doi Y, Fujimoto S, Higashi K, Nakashima Y, et al. Expression of profilin, an actin-binding protein, in rat experimental glomerulonephritis and its upregulation by basic fibroblast growth factor in cultured rat mesangial cells. J Am Soc Nephrol. 2000;11:423–33. doi: 10.1681/ASN.V113423. [DOI] [PubMed] [Google Scholar]
- 45.Théry C. Exosomes: secreted vesicles and intercellular communications. F1000 Biol Rep. 2011;3:15. doi: 10.3410/B3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Théry C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol. 2009;9:581–93. doi: 10.1038/nri2567. [DOI] [PubMed] [Google Scholar]
- 47.Chaput N, Théry C. Exosomes: immune properties and potential clinical implementations. Semin Immunopathol. 2011;33:419–40. doi: 10.1007/s00281-010-0233-9. [DOI] [PubMed] [Google Scholar]