Abstract
Background
This study aimed to evaluate the effect of travoprost with sofZia® preservative system for lowering the intraocular pressure (IOP) of Japanese normal tension glaucoma (NTG) patients.
Methods
In this prospective, multicenter, open-label study, Japanese NTG patients with baseline IOPs <20 mmHg were enrolled after three consecutive time measurements taken at screening and baseline visits. Travoprost with sofZia® was instilled once daily. Lowering effect on IOP, conjunctival hyperemia, superficial punctate keratopathy, and adverse events were examined at week 4, 8, and 12 after drug instillation.
Results
One-hundred and three of the 107 enrolled patients (baseline IOP =15.2±2.0 mmHg [mean ± standard deviation]) completed the study. The mean IOP value as well as percent reduction was significantly reduced at each visit after travoprost with sofZia® initiation (P<0.0001). The conjunctival hyperemia score was 1 or less throughout the study, though it increased significantly over time. No significant change was observed in superficial punctate keratopathy. The cumulative incidence of side effects such as eyelash changes, eyelid pigmentation, and deepening of the upper lid was 47.6%, 27.2%, and 16.5%, respectively.
Conclusion
Travoprost preserved with sofZia® effectively lowered the IOP of Japanese NTG patients. It was well tolerated with few discontinuations due to adverse events.
Keywords: travoprost with sofZia® preservative system, normal tension glaucoma (NTG), prostaglandin analogue
Introduction
Travoprost is a prostaglandin (PG) F2α analogue which is known to lower intraocular pressure (IOP). Travoprost preserved with antiseptic benzalkonium chloride (BAC) (Travatan® 0.004%, Alcon Laboratories, Inc., Fort Worth, TX, USA) or sofZia® preservative system (Travatan Z® 0.004%, Alcon Laboratories, Inc.) have been prescribed for glaucoma patients, but currently only the latter is available in Japan.
Travoprost with BAC has demonstrated a similar IOP-lowering effect as 0.005% latanoprost in a Phase III comparative clinical trial on patients with primary open-angle glaucoma or ocular hypertension.1 Travoprost preserved with sofZia® has a similar IOP-lowering effect as seen in travoprost preserved with BAC.2
In Japan, the most common form of glaucoma is normal tension glaucoma (NTG), which accounts for about 90% of primary open-angle glaucoma.3 It also accounts for ~33% of primary open angle glaucoma in the United States and European Union.4,5
Lowering IOP is the only evidence-based therapy for glaucoma and a recommended treatment of NTG.6,7 PG-related eye drops, including travoprost with sofZia®, remain the drugs of first choice for glaucoma management. However, in the past, few studies have been conducted on patients with NTG.8–10
We studied the IOP-lowering effect of travoprost with sofZia® in Japanese patients with NTG with the baseline IOPs <20 mmHg. To the best of our knowledge, this is the first report on the effect and safety of travoprost with sofZia® in a large number of NTG patients.
Material and methods
Study design
This prospective, multicenter, open-label study was conducted from September 2010 to August 2011 in accordance with the principles of the Declaration of Helsinki. Of the potentially eligible patients, the only ones enrolled in the study were those who signed an informed consent form after having been given an explanation of its contents and having been informed of expected treatment for study participants. The protocol was reviewed and approved by the Ethics Review Committee of Asano Clinic and was approved separately by the following institutions’ respective internal ethics review committees: Tohoku University Hospital, Juntendo University Urayasu Hospital, Jikei University School of Medicine, Nakano General Hospital, Fussa Hospital, Fukui Saiseikai Hospital, Nissei Hospital, and Minami-Matsuyama Hospital. This study was published on the University Hospital Medical Information Network Clinical Trials Registry (UMIN-CTR) (Registration ID: UMIN000007026).
Patients
Newly diagnosed NTG patients or patients with a prior diagnosis of NTG but currently untreated were recruited from 22 institutions across Japan (Table S1) as potential patients.
Enrolled patients were 1) male or female at least 20 years of age, 2) had untreated IOPs (baseline IOPs <20 mmHg) consistently confirmed by at least three consecutive measurements taken at the screening and baseline visits, and 3) had glaucomatous optic disc damage and glaucomatous visual field defects.
Patients were excluded from the study if they had one or more of the following conditions: 1) any anterior eye segment abnormality preventing reliable Goldmann applanation tonometry, 2) mean deviation of less than −15 dB by a Humphrey field analyzer, 3) complications due to chronic/recurrent uveitis, scleritis, or corneal herpes, 4) history of hypersensitivity to PG-related drugs, 5) ocular injury, intraocular surgery, or ocular laser surgery within 3 months before the baseline examinations, and 6) use of a corticosteroid or any IOP-lowering drug other than travoprost during the study. Pregnant/breast-feeding women, patients with psychological problems, and those considered by the attending ophthalmologist to be inappropriate study participants were also excluded. Dry eye patients were not excluded in this study.
Procedures
One drop of travoprost with sofZia® preservative system was instilled either bilaterally or unilaterally into the conjunctival sac at the discretion of the attending ophthalmologist once daily, between 8 pm and 10 pm for 12 weeks.
IOP was measured in duplicate at 4, 8, and 12 weeks after the initiation of treatment with a Goldmann applanation tonometer by the same operator within 2 hours before or after the scheduled time. The mean values of IOPs in the eye having the higher IOP or the right eye (if the left and right eyes had the same IOP) were used for the analysis.
The degrees of superficial punctate keratopathy (SPK) and conjunctival hyperemia were assessed by slit-lamp observation at 4, 8, and 12 weeks after the initiation of treatment. SPK was graded 0 to 4 at each of five areas according to the corneal diagram of the National Eye Institute.11 Conjunctival hyperemia was scored according to four grades: 0, no vasodilatation; 1, vasodilation of mainly small blood vessels; 2, vasodilation of both large and small blood vessels; 3, marked vasodilation including both large and small blood vessels. The degrees of SPK and hyperemia were judged by each ophthalmologist using a prepared reference diagram.
Eyelash changes, eyelid pigmentation, and deepening of the upper lid were assessed visually by a doctor on duty in each institution. Presence or absence of changes was recorded.
Subjective symptoms such as ocular irritation, foreign body sensation, dryness, and itching were scored on a four-point scale: absent, few, moderate, and apparent. Few, moderate and apparent symptoms were counted as the cumulative incidence.
Blood pressure (mmHg) and pulse rate (beats per minute [bpm]) were measured at the beginning and at the end of the study. Visual fields were measured before and after the end of the study, and mean deviation (MD) values were compared (Humphrey field analyzer SITA standard Program 30-2 or Program 24-2; Carl Zeiss Meditec, Inc., Dublin, CA, USA).
Statistical analysis
All collected data from each institution were sent to Tajimi Iwase Eye Clinic in a manner that protected patient confidentiality.
An ophthalmologist not involved in the data collection process independently verified NTG diagnosis based on ocular fundus photographs and visual field tests of enrolled patients.
Baseline IOP was defined as the mean of three IOP measurements taken before treatment at the screening and baseline visits. IOP reduction and percent reduction of IOP from baseline was assessed in patients who were followed-up and treated for at least 4 weeks between visits, and compliance of treatment was confirmed at each visit. The results were analyzed by repeated measures analysis of variance (ANOVA) and multiple comparison using Tukey’s HSD (honestly significant difference) test.
Degree of SPK and conjunctival hyperemia were analyzed employing the Wilcoxon signed rank test. Cumulative incidence of eyelash change, eyelid pigmentation, deepening of the upper lid, and subjective symptoms were expressed as a percentage of patients.
The changes in blood pressure, pulse rate, and MD of the visual field before and after the study were analyzed using the paired t-test.
The statistical analysis software used was JMP version 9.0 (SAS, Cary, NC, USA). All tests were two-tailed with a significance level set at 5%.
Results
One-hundred and seven patients with a diagnosis of NTG were included in this study. Of the 107 patients, 103 patients (48 men and 55 women) with a mean age of 61.4±13.6 years (28–87 years) completed the study. Four patients discontinued the study: one (0.9%) was lost to follow-up, and three (2.8%) discontinued due to side effects. One of these three patients developed lacrimation, discharge, hyperemia, and a foreign body sensation immediately after instillation on day 1. Two patients developed hyperemia, lower lid swelling, and foreign body sensation at 1–2 months after instillation and wished to discontinue the study. The study compliance rate was 96.3%.
Travoprost with sofZia® preservative system (baseline IOP =15.2±2.0 mmHg) significantly reduced IOP at each test point (P<0.0001) (Figure 1).
Figure 1.
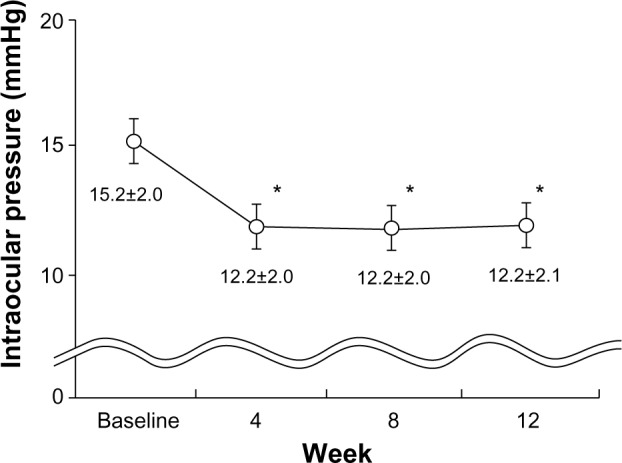
IOP before and after travoprost with sofZia® administration.
Notes: IOP is presented as the mean ± SD. *P<0.0001 for significant change of IOP compared with baseline by repeated measures analysis of variance and multiple comparison using Tukey’s HSD test. sofZia® (Travatan® 0.004%, Alcon Laboratories, Inc., Fort Worth, TX, USA).
Abbreviations: HSD, honestly significant difference; IOP, intraocular pressure; SD, standard deviation.
A more than 20% IOP reduction from baseline was observed in about 50% of eyes at each test point. Reductions of 10%–20% were observed in 35%–40% of patients, and reductions of less than 10% IOP were seen in only 11%–12% of treated eyes. The average reduction rate of IOP was 19.4%–19.8% at each visit, and these percent reductions were statistically significant (P<0.0001) (Figure 2).
Figure 2.
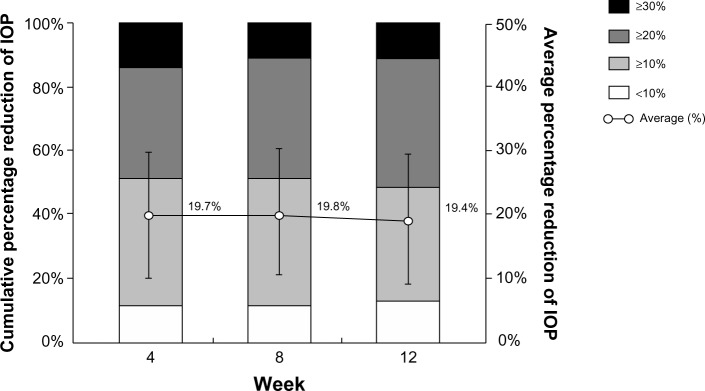
Percentage reduction of IOP with drug administration.
Notes: IOP reduction rates are classified into four categories represented by each column:
 ≥30%,
≥30%,
 ≥20%,
≥20%,
 ≥10%,
≥10%,
 <10%. Open circle (
<10%. Open circle (
 ) represents the mean ± SD. Vertical axes: Left and right axes represent cumulative and average percentage reduction of IOP, respectively.
) represents the mean ± SD. Vertical axes: Left and right axes represent cumulative and average percentage reduction of IOP, respectively.
Abbreviations: IOP, intraocular pressure; SD, standard deviation.
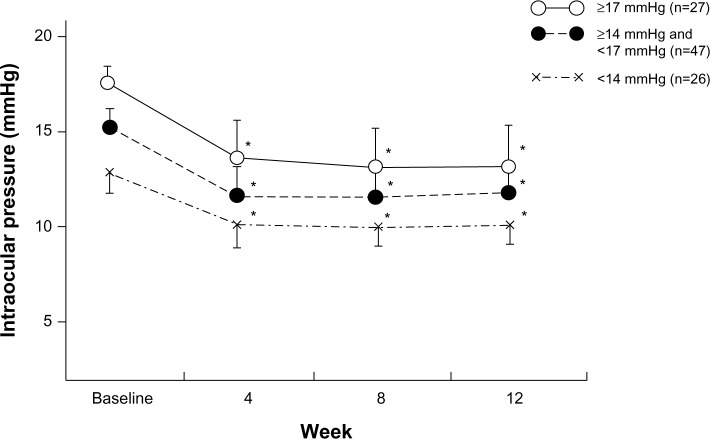
Study eyes were divided into three groups (<14 mmHg, ≥14 mmHg and <17 mmHg, and ≥17 mmHg) according to baseline IOP. The mean IOP reduction as well as IOP percent reductions in each group was decreased significantly at each test point (P<0.0001) (Figure 3).
Figure 3.
Change of IOP in three groups with drug administration.
Notes: Eyes were classified into three groups based on their baseline IOP: ○ ≥17 mmHg, ● ≥14 mmHg and 17 mmHg, × <14 mmHg. *P<0.0001 for significant change of IOP compared with each baseline by repeated measures analysis of variance and multiple comparison using Tukey’s HSD test.
Abbreviations: HSD, honestly significant difference; IOP, Intraocular pressure.
The mean hyperemia scores were increased significantly (P<0.0001) at each time point, but 93 patients (90.3%) had the score of 1 or below throughout the study.
No significant differences in the mean SPK scores were observed in each time point (Table 1). Total SPK score of 87 patients was 0 prior to the treatment. SPK were worsened among 12 patients (13.8%). Sixteen patients had an SPK score 1 or higher prior to the instillation. Decrease in SPK scores was observed after 12 weeks in 12 patients (75.0%). For one patient, the total SPK score was 9 initially but decreased to 0 after 12 weeks.
Table 1.
SPK and hyperemia scores
| Baseline | 4 week | 8 week | 12 week | |
|---|---|---|---|---|
| Total SPK score | 0.35±1.12 | 0.30±0.70 | 0.40±0.86 | 0.32±0.81 |
| Superior | 0.01±0.10 | 0.00±0.00 | 0.00±0.00 | 0.00±0.00 |
| Nasal | 0.05±0.22 | 0.06±0.31 | 0.09±0.32 | 0.08±0.33 |
| Temporal | 0.06±0.42 | 0.01±0.10 | 0.05±0.22 | 0.02±0.14 |
| Central | 0.04±0.27 | 0.02±0.20 | 0.02±0.14 | 0.03±0.17 |
| Inferior | 0.17±0.51† | 0.21±0.48† | 0.24±0.49† | 0.19±0.47† |
| Hyperemia score | 0.14±0.40 | 0.54±0.61* | 0.53±0.61* | 0.51±0.63* |
Notes: Scores are indicated as mean ± SD.
P<0.0001 for significant change of hyperemia score compared with baseline by Wilcoxon signed rank test.
P<0.01 for significant difference of SPK score at each time point compared with other corneal area by Wilcoxon signed rank test.
Abbreviations: SD, standard deviation; SPK, superficial punctate keratopathy.
The most common side effects were eyelash growth (49 patients, 47.6%), followed by increase in eyelid pigmentation (28 patients, 27.2%) and deepening of the upper eyelid (17 patients, 16.5%).
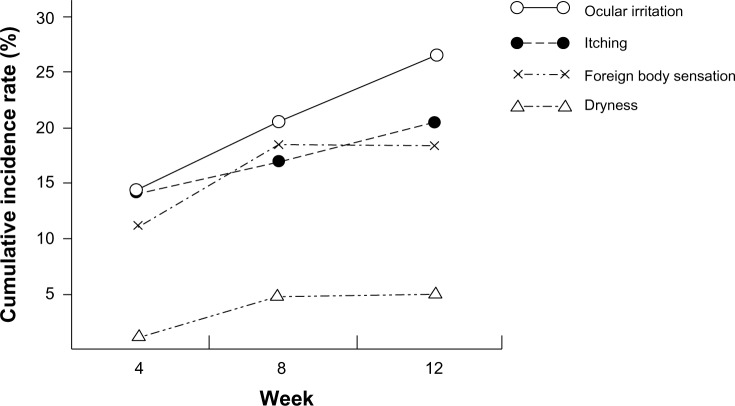
Cumulative incidence of subjective symptoms detected in 42 patients was 40.8% during 12 weeks. Ocular irritation (26.2%) was the most frequent symptom, followed by itching (18.5%), foreign body sensation (17.5%), and dryness (4.9%). Ocular irritation and itching were noted throughout the observation period, while foreign body sensation and dryness were seen for 8 weeks (Figure 4).
Figure 4.
Cumulative incidence of subjective symptoms.
Notes: The cumulative incidence of adverse events in this study was 40.8% (by week 12). The most frequently observed event was ocular irritation [○: 26.2%, n=27], itching (●: 18.5%, n=19), foreign body sensation (×: 17.5%, n=18) and dryness (∆: 4.9%, n=5).
MD value of visual field between baseline (−4.61±4.95 dB) and the 12 weeks time point (−4.36±4.89 dB) was not significantly different (P=0.1069).
No significant differences in a systolic and diastolic blood pressure and pulse rate were observed in 101/103 patients (systolic blood pressure: 130.2±15.0/128.0±17.0 mmHg, P=0.0835; diastolic blood pressure: 79.7±10.6/78.6±11.3 mmHg, P=0.2994; pulse rate: 71.9±10.5/72.0±10.6 bpm, P=0.9539).
Discussion
Travoprost with sofZia® preservative system significantly reduced IOP in Japanese NTG patients with minimal local side effects.
Though PGs are the first remedy for glaucoma with high IOP, the effectiveness and safety has not adequately been studied in patients with NTG. Travoprost with sofZia® use has been reported in limited numbers of NTG patients, with a potent IOP lowering effect and minimal corneal damage.12,13 We, therefore, expanded on these findings and conducted a study with a large number of Japanese NTG patients.
In the present study, more than 100 patients were dosed with travoprost with sofZia®, and their IOPs were reduced significantly from baseline at each time point. A significant average percent reduction of IOP (20% or more) was also seen in the majority of patients at each test point. Although the target IOP level in the treatment of NTG has yet to be established, a 20% IOP reduction from baseline is a reasonable target in Japan.7,14
Approximately 10% of the patients did not reach a 10% IOP reduction rate from their baseline. Nonresponders to PGs account for approximately 20% of glaucoma patients with high IOP;15 therefore, the rate of “nonresponders” in the present study was fewer than in other studies. Eight patients were “late responders,” whose initial IOP-lowering rate was less than 10%, but their IOP decreased further at 2–3 months in this study. These results suggest that at least a 3-month observation period was needed to determine the effectiveness of travoprost with sofZia® and other PG-related eye drops.
Significant reduction in IOP was observed in all three groups according to baseline IOP. In Japan, about 20% of NTG eyes have baseline IOPs of 14 mmHg or less. Even for those patients, travoprost with sofZia® was able to further reduce their IOPs. Therefore, it is a suitable option to reach the therapeutic goals in patients with NTG in Japan.
In this study, SPK and conjunctival hyperemia, the most frequent side effects of PG instillation therapy, were scored using the National Eye Institute classification and reference pictures for the objective evaluation. Conjunctival hyperemia showed significant worsening after dosing. Hyperemia might affect patient adherence; however, the overall scores remained low throughout the study period. Hence no patient discontinued the study due to hyperemia, and the overall scores were even slightly lower than in a previous report.16 Therefore, we concluded that the conjunctival hyperemia observed in this study is not particularly remarkable and is consistent with those seen in other PG-related eye drops.17,18 One patient was discontinued due to allergic symptoms such as hyperemia and lacrimation on day 1. PGs are not suitable in rare cases,19 so assessing the patient’s medical history prior to the application of the drug is important.
There was no significant increase in SPK scores in each area of the cornea, although baseline SPK score was significantly higher in the lower corneal area of dry eye patients. Some suggest travoprost with sofZia® is safest for the cornea;20–22 however, we could not directly compare it with travoprost preserved with BAC in NTG patients because the latter is not available in Japan. The result in this study suggests that travoprost with sofZia® is safe on the cornea.
Compared with the previous reports on travoprost with sofZia®,23,24 the incidence of eyelash change, eyelid pigmentation, and deepening of the upper lid was not high. But we did not assess the results with photography, therefore the incidence could be underestimated. It would be ideal to have a third person evaluate these changes; however, this study was a clinical trial across Japan, therefore, it was difficult to take standardized photos using the same photographic instruments. The cumulative incidence of subjective symptoms was similar to a rate in the previous report.25 The MD values of the visual field showed no significant change. The mean blood pressure and pulse rate also showed no significant change after treatment. Although adverse events were observed, we conclude that their frequency was not high, and they are clinically tolerable.
Conclusion
Our study indicates that travoprost with sofZia® preservative system has a significant IOP-lowering effect in Japanese patients with NTG and is well tolerated.
Supplementary materials
Table S1.
IOP CHecked and Assessed in Normal tension Glaucoma by Exceptional Glaucomatologists (CHANGE) Study Group
| Institution | Researcher |
|---|---|
| 1. Tohoku University, Department of Ophthalmology, Miyagi, Japan | Nobuo Fuse |
| 2. Yaoeda Eye Clinic, Niigata, Japan | Kiyoshi Yaoeda |
| 3. Hara Eye Hospital, Tochigi, Japan | Takeshi Hara |
| 4. Juntendo University Urayasu Hospital, Chiba, Japan | Itaru Kimura |
| 5. The Jikei University School of Medicine Department of Ophthalmology, Tokyo, Japan | Tadashi Nakano |
| 6. Yoshikawa Eye Clinic, Tokyo, Japan | Keiji Yoshikawa |
| 7. Ueno Eye Clinic, Tokyo, Japan | Tairo Kimura |
| 8. Nakano General Hospital, Tokyo, Japan | Hirotaka Suzumura |
| 9. Fussa Hospital, Department of Ophthalmology, Tokyo, Japan | Toyoaki Tsumura |
| 10. Nihonmatsu Eye Hospital, Tokyo, Japan | Mami Nanno |
| 11. Matsukura Eye Clinic, Kanagawa, Japan | Shuji Matsukura |
| 12. Nishikamakura Tanino Eye Clinic, Kanagawa, Japan | Tomihiko Tanino |
| 13. Fukui-ken Saiseikai Hospital, Department of Ophthalmology, Fukui, Japan | Koji Nitta |
| 14. Tajimi Iwase Eye Clinic, Gifu, Japan | Aiko Iwase |
| 15. Yamabayashi Eye Clinic, Aichi, Japan | Shigeki Yamabayashi |
| 16. Nissei Hospital, Department of Ophthalmology, Osaka, Japan | Reiko Sugimoto |
| 17. H irakata Yamagishi Eye Clinic, Osaka, Japan | Kazuya Yamagishi |
| 18. Kozaki Eye Clinic, Osaka, Japan | Jun Kozaki |
| 19. Minami-Matsuyama Hospital, Department of Ophthalmology, Ehime, Japan | Shiro Mizoue |
| 20. Mizoguchi Eye Clinic, Nagasaki, Japan | Takanori Mizoguchi |
| 21. Ozaki Eye Clinic, Miyazaki, Japan | Mineo Ozaki |
| 22. Unoki Eye Clinic, Kagoshima, Japan | Kazuhiko Unoki |
Abbreviation: IOP, intraocular pressure.
Acknowledgments
This study was conducted with funding and support from the Japan Association of Health Service and Alcon Japan Ltd. The authors thank Kozaburo Hayashi for reviewing the manuscript.
Footnotes
Disclosure
Dr Mizoue has received compensation for the manuscript from Alcon Japan Ltd, and lecture fees from Alcon Japan Ltd, Pfizer Japan Inc., MSD K K, NIDEK Co, Ltd, Senju Pharmaceutical Co, Ltd, Santen Pharmaceutical Co, Ltd, and Kaken Pharmaceutical Co, Ltd. Dr Nakano has received lecture fees from Alcon Japan Ltd, Santen Pharmaceutical Co, Ltd, Otsuka Pharmaceutical Co, Ltd, R-Tech Ueno Ltd, Senju Pharmaceutical Co, Ltd, Carl Zeiss Co, Ltd, Kaken Pharmaceutical Co, Ltd, MSD K K, and Pfizer Japan Inc., Dr Fuse has received lecture fees from Alcon Japan Ltd, Santen Pharmaceutical Co, Ltd, Pfizer Japan Inc., and MSD KK. Dr Iwase has received consultant fees from Topcon Corporation and fees for expert testimony from KOWA Pharmaceutical Co, Ltd, and lecture fees from Alcon Japan Ltd, Santen Pharmaceutical Co, Ltd, Carl Zeiss Co, Ltd, and Pfizer Japan Inc., Dr Matsumoto has received lecture fees from Alcon Japan Ltd, Santen Pharmaceutical Co, Ltd, and Kaken Pharmaceutical Co, Ltd. Dr Yoshikawa has received fees for expert testimony from Santen Pharmaceutical Co, Ltd, and lecture fees from Alcon Japan Ltd, Santen Pharmaceutical Co, Ltd, Pfizer Japan Inc., Kaken Pharmaceutical Co, Ltd, Senju Pharmaceutical Co, Ltd, and MSD KK. The authors report no other conflicts of interest in this work.
References
- 1.Netland PA, Landry T, Sullivan EK, et al. Travoprost Study Group Travoprost compared with latanoprost and timolol in patients with open-angle glaucoma or ocular hypertension. Am J Ophthalmol. 2001;132(4):472–484. doi: 10.1016/s0002-9394(01)01177-1. [DOI] [PubMed] [Google Scholar]
- 2.Lewis RA, Katz G, Weiss MJ, et al. Travoprost BAC-free Group Travoprost 0.004% with and without benzalkonium chloride: a comparison of safety and efficacy. J Glaucoma. 2007;16(1):98–103. doi: 10.1097/01.ijg.0000212274.50229.c6. [DOI] [PubMed] [Google Scholar]
- 3.Iwase A, Suzuki Y, Araie M, et al. Tajimi Study Group, Japan Glaucoma Society The prevalence of primary open-angle glaucoma in Japanese: the Tajimi Study. Ophthalmology. 2004;111(9):1641–1648. doi: 10.1016/j.ophtha.2004.03.029. [DOI] [PubMed] [Google Scholar]
- 4.Klein BE, Klein R, Sponsel WE, et al. Prevalence of glaucoma: The Beaver Dam Eye Study. Ophthalmology. 1992;99(10):1499–1504. doi: 10.1016/s0161-6420(92)31774-9. [DOI] [PubMed] [Google Scholar]
- 5.Bonomi L, Marchini G, Marraffa M, et al. Prevalence of glaucoma and intraocular pressure distribution in a defined population. The Egna-Neumarkt Study. Ophthalmology. 1998;105(2):209–215. doi: 10.1016/s0161-6420(98)92665-3. [DOI] [PubMed] [Google Scholar]
- 6.The effectiveness of intraocular pressure reduction in the treatment of normal-tension glaucoma. Collaborative Normal-Tension Glaucoma Study-Group. Am J Ophthalmol. 1998;126(4):498–505. doi: 10.1016/s0002-9394(98)00272-4. [DOI] [PubMed] [Google Scholar]
- 7.The Japan glaucoma society guidelines for glaucoma (3rd edition) Nihon Ganka Gakkai Zasshi. 2012;116(1):3–46. Japanese. [PubMed] [Google Scholar]
- 8.Tomita G, Araie M, Kitazawa Y, Tsukahara S. A three-year prospective, randomized and open comparison between latanoprost and timolol in Japanese normal-tension glaucoma patients. Eye (Lond) 2004;18(10):984–989. doi: 10.1038/sj.eye.6701373. [DOI] [PubMed] [Google Scholar]
- 9.Kondo N, Sawada A, Yamamoto T, Taniguchi T. Correlation between individual differences in intraocular pressure reduction and outflow facility due to latanoprost in normal-tension glaucoma patients. Jpn J Ophthalmol. 2006;50(1):20–24. doi: 10.1007/s10384-005-0267-z. [DOI] [PubMed] [Google Scholar]
- 10.Ang GS, Kersey JP, Shepstone L, Broadway DC. The effect of travoprost on daytime intraocular pressure in normal tension glaucoma: a randomised controlled trial. Br J Ophthalmol. 2008;92(8):1129–1133. doi: 10.1136/bjo.2007.135269. [DOI] [PubMed] [Google Scholar]
- 11.Lemp MA. Report of the National Eye Institute/Industry workshop on clinical trials in dry eyes. CLAO J. 1995;21(4):221–232. [PubMed] [Google Scholar]
- 12.Nomura Y, Nakakura S, Moriwaki M, Takahashi Y, Shiraki K. Effect of travoprost on 24-hour intraocular pressure in normal tension glaucoma. Clin Ophthalmol. 2010;4:643–647. doi: 10.2147/opth.s10521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Inoue K, Iwasa M, Wakakura M, Tomita G. Effects of BAK-free travoprost treatment for 3 years in patients with normal tension glaucoma. Clin Ophthalmol. 2012;6:1315–1319. doi: 10.2147/OPTH.S33816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aoyama A, Ishida K, Sawada A, Yamamoto T. Target intraocular pressure for stability of visual field loss progression in normal-tension glaucoma. Jpn J Ophthalmol. 2010;54(2):117–123. doi: 10.1007/s10384-009-0779-z. [DOI] [PubMed] [Google Scholar]
- 15.Ikeda Y, Mori K, Ishibashi T, Naruse S, Nakajima N, Kinoshita S. Latanoprost nonresponders with open-angle glaucoma in the Japanese population. Jpn J Ophthalmol. 2006;50(2):153–157. doi: 10.1007/s10384-005-0293-x. [DOI] [PubMed] [Google Scholar]
- 16.Aihara M, Oshima H, Araie M, EXTraKT study group Effects of SofZia-preserved travoprost and benzalkonium chloride-preserved latanoprost on the ocular surface – a multicentre randomized single-masked study. Acta Ophthalmol. 2013;91(1):e7–e14. doi: 10.1111/j.1755-3768.2012.02565.x. [DOI] [PubMed] [Google Scholar]
- 17.Parrish RK, Palmberg P, Sheu WP, XLT Study Group A comparison of latanoprost, bimatoprost, and travoprost in patients with elevated intraocular pressure: a 12-week, randomized, masked-evaluator multicenter study. Am J Ophthalmol. 2003;135(5):688–703. doi: 10.1016/s0002-9394(03)00098-9. [DOI] [PubMed] [Google Scholar]
- 18.Nakano T, Yoshikawa K, Kimura T, Suzumura H, Nanno M, Noro T. Efficacy and safety of tafluprost in normal-tension glaucoma with intraocular pressure of 16 mmHg or less. Jpn J Ophthalmol. 2011;55(6):605–613. doi: 10.1007/s10384-011-0082-7. [DOI] [PubMed] [Google Scholar]
- 19.Lai CH, Lai IC, Chi CC. Allergic contact dermatitis caused by latanoprost ophthalmic solution. Eur J Ophthalmol. 2006;16(4):627–629. doi: 10.1177/112067210601600423. [DOI] [PubMed] [Google Scholar]
- 20.Uematsu M, Kumagami T, Shimoda K, et al. Polyoxyethylene hydrogenated castor oil modulates benzalkonium chloride toxicity: comparison of acute corneal barrier dysfunction induced by travoprost Z and travoprost. J Ocul Pharmacol Ther. 2011;27(5):437–444. doi: 10.1089/jop.2010.0175. [DOI] [PubMed] [Google Scholar]
- 21.Aihara M, Otani S, Kozaki J, et al. Long-term effect of BAK-free travoprost on ocular surface and intraocular pressure in glaucoma patients after transition from latanoprost. J Glaucoma. 2012;21(1):60–64. doi: 10.1097/IJG.0b013e3181fc8129. [DOI] [PubMed] [Google Scholar]
- 22.Ammar DA, Noecker RJ, Kahook MY. Effects of benzalkonium chloride-preserved, polyquad-preserved, and sofZia-preserved topical glaucoma medications on human ocular epithelial cells. Adv Ther. 2010;27(11):837–845. doi: 10.1007/s12325-010-0070-1. [DOI] [PubMed] [Google Scholar]
- 23.Inoue K, Shiokawa M, Higa R, et al. Adverse periocular reactions to five types of prostaglandin analogs. Eye (Lond) 2012;26(11):1465–1472. doi: 10.1038/eye.2012.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maruyama K, Shirato S, Tsuchisaka A. Incidence of deepening of the upper eyelid sulcus after topical use of travoprost ophthalmic solution in Japanese. J Glaucoma. 2014 doi: 10.1097/IJG.0b013e31826a7e09. [DOI] [PubMed] [Google Scholar]
- 25.Yamazaki S, Nanno M, Kimura T, Suzumura H, Yoshikawa K. Effects of switching to SofZia-preserved travoprost in patients who presented with superficial punctate keratopathy while under treatment with latanoprost. Jpn J Ophthalmol. 2010;54(1):7–14. doi: 10.1007/s10384-009-0754-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.
IOP CHecked and Assessed in Normal tension Glaucoma by Exceptional Glaucomatologists (CHANGE) Study Group
| Institution | Researcher |
|---|---|
| 1. Tohoku University, Department of Ophthalmology, Miyagi, Japan | Nobuo Fuse |
| 2. Yaoeda Eye Clinic, Niigata, Japan | Kiyoshi Yaoeda |
| 3. Hara Eye Hospital, Tochigi, Japan | Takeshi Hara |
| 4. Juntendo University Urayasu Hospital, Chiba, Japan | Itaru Kimura |
| 5. The Jikei University School of Medicine Department of Ophthalmology, Tokyo, Japan | Tadashi Nakano |
| 6. Yoshikawa Eye Clinic, Tokyo, Japan | Keiji Yoshikawa |
| 7. Ueno Eye Clinic, Tokyo, Japan | Tairo Kimura |
| 8. Nakano General Hospital, Tokyo, Japan | Hirotaka Suzumura |
| 9. Fussa Hospital, Department of Ophthalmology, Tokyo, Japan | Toyoaki Tsumura |
| 10. Nihonmatsu Eye Hospital, Tokyo, Japan | Mami Nanno |
| 11. Matsukura Eye Clinic, Kanagawa, Japan | Shuji Matsukura |
| 12. Nishikamakura Tanino Eye Clinic, Kanagawa, Japan | Tomihiko Tanino |
| 13. Fukui-ken Saiseikai Hospital, Department of Ophthalmology, Fukui, Japan | Koji Nitta |
| 14. Tajimi Iwase Eye Clinic, Gifu, Japan | Aiko Iwase |
| 15. Yamabayashi Eye Clinic, Aichi, Japan | Shigeki Yamabayashi |
| 16. Nissei Hospital, Department of Ophthalmology, Osaka, Japan | Reiko Sugimoto |
| 17. H irakata Yamagishi Eye Clinic, Osaka, Japan | Kazuya Yamagishi |
| 18. Kozaki Eye Clinic, Osaka, Japan | Jun Kozaki |
| 19. Minami-Matsuyama Hospital, Department of Ophthalmology, Ehime, Japan | Shiro Mizoue |
| 20. Mizoguchi Eye Clinic, Nagasaki, Japan | Takanori Mizoguchi |
| 21. Ozaki Eye Clinic, Miyazaki, Japan | Mineo Ozaki |
| 22. Unoki Eye Clinic, Kagoshima, Japan | Kazuhiko Unoki |
Abbreviation: IOP, intraocular pressure.