Abstract
The ability of animals to avoid strongly alkaline pH is critical for survival. However, the means by which they sense high pH has not been determined. We have previously found that the nematode Caenorhabditis elegans (C. elegans) avoids environmental pH above 10.5. Detection involves ASH nociceptive neurons as the major sensors. Upon stimulation, transient receptor potential vanilloid-type (TRPV) ion channels encoded by osm-9 and ocr-2 play an essential role in Ca2+ entry into ASH. Here we report that C. elegans mutants deficient in a G-protein α subunit, GOA-1, failed to avoid strongly alkaline pH with normal Ca2+ influx into ASH. These results suggest that GOA-1 regulates signal transmission downstream of Ca2+ influx through OSM-9/OCR-2 TRPV channels in ASH.
Keywords: alkalinity sensing, chemotaxis, nociceptor, G protein, TRPV channels
Survival requires that animals monitor environmental pH. Mammalian trigeminal neurons respond to pH ranging from 7.8 to 10.0.1 Fish,2 shrimp,3 insects,4 and nematodes5 are also sensitive to environmental alkalinity. Secretion of the peptide hormone, gastrin, in the stomach is promoted by luminal alkalinization,6 and capsaicin-sensitive afferent neurons seem to be involved in this process.7 However, little is known about how sensory neurons detect it. C. elegans has proven extremely useful for dissecting the molecular bases of behavior. The nervous system of adult hermaphrodites comprises 302 neurons, including 12 pairs of amphid sensory neurons with ciliated dendrites.8 Among the sensory neurons, 3 pairs, ASH, ADL, and AWB, are responsible for sensing chemical repellents.9,10 Stimulation of these neurons triggers reverse locomotion. In particular, ASH is polymodal, and is required for nociception; mechanosensation, osmosensation, and chemosensation.11,12 TRPV ion channels, which consist of OSM-9 and OCR-2 subunits, are expressed in ASH, and are involved in sensation of strongly alkaline pH.13 Mutations in ocr-2 reduce all the 3 forms of nociception.14 Both osmosensation and mechanosensation require ODR-3, a G-protein α-subunit, in ASH.15 These observations suggest that OSM-9 and OCR-2 are not directly activated by mechanical stimuli,16 and that the signals detected by unknown sensor molecules may be transmitted to OSM-9/OCR-2 channels through ODR-3.
Our previous study on the aversion of C. elegans to strongly alkaline pH showed that the nematode senses pH higher than 10.5 as a noxious stimulus via ASH nociceptive sensory neurons, in which OSM-9/OCR-2 TRPV channels play an essential role.13 Furthermore, it is known that ODR-3 appears to be generally important for the cellular response to most, if not all, repellents sensed by ASH.17 To determine whether any upstream factors regulate OSM-9/OCR-2 channels in strongly alkaline-pH sensation, we analyzed behavior of C. elegans mutants deficient in G-protein α-subunits using a chemotaxis assay. Details of the experiments were described in our previous study13 on the aversion of C. elegans to strongly alkaline pH. ASH is known to express at least 10 G-protein α-subunits: EGL-30, GOA-1, GPA-1, GPA-3, GPA-11, GPA-13, GPA-14, GPA-15, GSA-1, and ODR-3.15,18,19 Among mutants defective in these genes, only goa-1 mutants were unable to avoid strongly alkaline pH (Fig. 1A). All the other mutants, including odr-3, retreated from the noxious stimulus. However, Ca2+ transients in ASH of goa-1 mutants were clearly observed at levels similar to those of wild-type N2 (Fig. 1B), indicating that GOA-1 functions downstream of OSM-9/OCR-2 channels in intracellular signaling, perhaps in synaptic exocytosis, or in downstream neurons of ASH. Indeed, it has previously been shown that GOA-1 does not affect neuronal depolarization in response to aversive stimuli such as high osmolality and quinine, but acts in ASH to modulate downstream transmission of intracellular signals.20
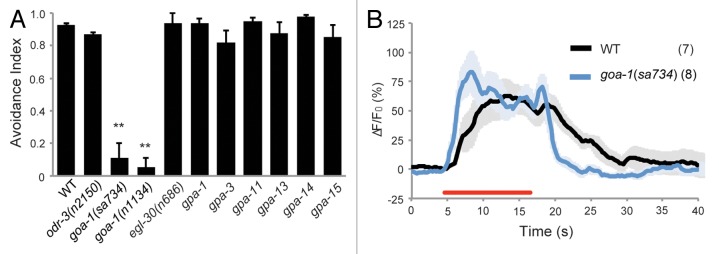
Figure 1. Behavior and imaging analyses of mutants deficient in a G protein α subunit. (A) Avoidance indices of wild-type and mutant animals. Assays were performed using petri dishes with 4 quadrants as described previously.13 Error bars indicate the SEM of 5 independent assays. **p < 0.01. (B) Ca2+ imaging of ASH in wild-type and goa-1 animals upon stimulation with pH 11.2. The red line represents the period of time during which animals were stimulated with pH 11.2 buffer. Numbers of recordings are shown in parentheses, and light color shading denotes the SEM.
As described above, ODR-3 appears to be generally important for the cellular response to most, if not all, repellents sensed by ASH,17 and the OSM-9/OCR-2 TRPV channel appears to be the signal generation channel downstream of G-protein coupled receptors (GPCRs) and ODR-3/GPA-3 G-protein signaling.21 As shown in the present study, however, mutant animals deficient in odr-3 or gpa-3 showed similar avoidance indices to those of wild-type animals. This suggests that the OSM-9/OCR-2 channel may be a direct sensor molecule for strongly alkaline pH, and that the channel may not be regulated by upstream GPCRs. Indeed, ammonia and intracellular alkalinization directly activate TRPV1 in cultured cells, via a mechanism that involves a cytoplasmic histidine residue of the channel.22 Thus, the present study suggests that OSM-9/OCR-2 TRPV channels may serve as sensor molecules for strongly alkaline pH, and that GOA-1 may act to modulate downstream signaling of TRPV channels in ASH. However, these results do not rule out possibilities that other molecules than GPCRs may act as a sensor, or that the G-proteins may act redundantly as sensors for strongly alkaline pH.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/cib/article/26668
References
- 1.Bryant BP. Mechanisms of somatosensory neuronal sensitivity to alkaline pH. Chem Senses. 2005;30:i196–7. doi: 10.1093/chemse/bjh182. [DOI] [PubMed] [Google Scholar]
- 2.Hartwell SI, Jin HJ, Cherry DS, Cairns J., Jr. Evaluation of statistical methods for avoidance data of schooling fish. Hydrobiologia. 1986;131:63–76. doi: 10.1007/BF00008325. [DOI] [Google Scholar]
- 3.West DW, Boubee JAT, Barrier RFG. Responses to pH of nine fishes and one shrimp native to New Zealand freshwaters. N Z J Mar Freshw Res. 1997;31:461–8. doi: 10.1080/00288330.1997.9516779. [DOI] [Google Scholar]
- 4.Merivee E, Ploomi A, Milius M, Luik A, Heideman M. Electrophysiological identification of antennal pH receptors in the ground beetle Pterostichus oblongopunctatus. Physiol Entomol. 2005;30:122–33. doi: 10.1111/j.1365-3032.2005.00435.x. [DOI] [Google Scholar]
- 5.Ward S. Chemotaxis by the nematode Caenorhabditis elegans: identification of attractants and analysis of the response by use of mutants. Proc Natl Acad Sci U S A. 1973;70:817–21. doi: 10.1073/pnas.70.3.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Konturek SJ, Rayford PL, Thompson JC. Effect of pH of gastric and intestinal meals on gastric acid and plasma gastrin and secretin responses in the dog. Am J Physiol. 1977;233:E537–43. doi: 10.1152/ajpendo.1977.233.6.E537. [DOI] [PubMed] [Google Scholar]
- 7.Nojima K, Sumii K, Sumii M, Okahara S, Haruma K, Yoshihara M, Kajiyama G. Acid-sensitive and alkaline-sensitive sensory neurons regulate pH dependent gastrin secretion in rat. Dig Dis Sci. 2000;45:1217–26. doi: 10.1023/A:1005570507166. [DOI] [PubMed] [Google Scholar]
- 8.Ward S, Thomson N, White JG, Brenner S. Electron microscopical reconstruction of the anterior sensory anatomy of the nematode Caenorhabditis elegans. J Comp Neurol. 1975;160:313–37. doi: 10.1002/cne.901600305. [DOI] [PubMed] [Google Scholar]
- 9.Troemel ER, Chou JH, Dwyer ND, Colbert HA, Bargmann CI. Divergent seven transmembrane receptors are candidate chemosensory receptors in C. elegans. Cell. 1995;83:207–18. doi: 10.1016/0092-8674(95)90162-0. [DOI] [PubMed] [Google Scholar]
- 10.Troemel ER, Kimmel BE, Bargmann CI. Reprogramming chemotaxis responses: sensory neurons define olfactory preferences in C. elegans. Cell. 1997;91:161–9. doi: 10.1016/S0092-8674(00)80399-2. [DOI] [PubMed] [Google Scholar]
- 11.Colbert HA, Smith TL, Bargmann CI. OSM-9, a novel protein with structural similarity to channels, is required for olfaction, mechanosensation, and olfactory adaptation in Caenorhabditis elegans. J Neurosci. 1997;17:8259–69. doi: 10.1523/JNEUROSCI.17-21-08259.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hart AC, Kass J, Shapiro JE, Kaplan JM. Distinct signaling pathways mediate touch and osmosensory responses in a polymodal sensory neuron. J Neurosci. 1999;19:1952–8. doi: 10.1523/JNEUROSCI.19-06-01952.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sassa T, Murayama T, Maruyama IN. Strongly alkaline pH avoidance mediated by ASH sensory neurons in C. elegans. Neurosci Lett. 2013 doi: 10.1016/j.neulet.2013.06.001. In press. [DOI] [PubMed] [Google Scholar]
- 14.Tobin DM, Madsen DM, Kahn-Kirby A, Peckol EL, Moulder G, Barstead R, Maricq AV, Bargmann CI. Combinatorial expression of TRPV channel proteins defines their sensory functions and subcellular localization in C. elegans neurons. Neuron. 2002;35:307–18. doi: 10.1016/S0896-6273(02)00757-2. [DOI] [PubMed] [Google Scholar]
- 15.Roayaie K, Crump JG, Sagasti A, Bargmann CI. The G alpha protein ODR-3 mediates olfactory and nociceptive function and controls cilium morphogenesis in C. elegans olfactory neurons. Neuron. 1998;20:55–67. doi: 10.1016/S0896-6273(00)80434-1. [DOI] [PubMed] [Google Scholar]
- 16.Xiao R, Xu XZ. Function and regulation of TRP family channels in C. elegans. Pflugers Arch. 2009;458:851–60. doi: 10.1007/s00424-009-0678-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hilliard MA, Apicella AJ, Kerr R, Suzuki H, Bazzicalupo P, Schafer WR. In vivo imaging of C. elegans ASH neurons: cellular response and adaptation to chemical repellents. EMBO J. 2005;24:63–72. doi: 10.1038/sj.emboj.7600493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jansen G, Thijssen KL, Werner P, van der Horst M, Hazendonk E, Plasterk RHA. The complete family of genes encoding G proteins of Caenorhabditis elegans. Nat Genet. 1999;21:414–9. doi: 10.1038/7753. [DOI] [PubMed] [Google Scholar]
- 19.Zwaal RR, Mendel JE, Sternberg PW, Plasterk RHA. Two neuronal G proteins are involved in chemosensation of the Caenorhabditis elegans Dauer-inducing pheromone. Genetics. 1997;145:715–27. doi: 10.1093/genetics/145.3.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Esposito G, Amoroso MR, Bergamasco C, Di Schiavi E, Bazzicalupo P. The G protein regulators EGL-10 and EAT-16, the Giα GOA-1 and the G(q)α EGL-30 modulate the response of the C. elegans ASH polymodal nociceptive sensory neurons to repellents. BMC Biol. 2010;8:138–50. doi: 10.1186/1741-7007-8-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bargmann CI. Chemosensation in C. elegans. WormBook. 2006:1–29. doi: 10.1895/wormbook.1.123.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dhaka A, Uzzell V, Dubin AE, Mathur J, Petrus M, Bandell M, Patapoutian A. TRPV1 is activated by both acidic and basic pH. J Neurosci. 2009;29:153–8. doi: 10.1523/JNEUROSCI.4901-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]


