Abstract
The apical surface of secretory tubular epithelia is a dynamic cellular domain where massive membrane turnover takes place during exocytosis and its subsequent compensatory endocytosis. This extensive membrane flow poses a difficulty in targeting secretory vesicles efficiently to a narrow apical domain. We have studied how actin filaments mediate the secretory process in the murine exocrine pancreas, which produces and secretes digestive enzymes that are deposited into the intestine. We show that cargo-filled secretory vesicles move over bundles of linear actin cables from their storage areas to the apical membrane of pancreatic acinar cells. mDia1, a linear actin nucleator of the Formin family, was identified as the generator of these structures. The active form of mDia1 localizes to the apical surface, and the microfilament bundles it forms emanate from the apical surface and extend into the cytoplasm, generating polarized secretion tracks. These bundles ensure orderly progression of exocytosis, since the apical targeting of pancreatic vesicles is compromised in their absence, and vesicles fuse with each other to generate compound, membrane-associated secretory structures.
Keywords: Formins, mDia, exocrine secretion, pancreatic acini, Lifeact
The exocrine pancreas is a tubular epithelium that is highly polarized, and serves as a classic model to study the cell-biological basis of secretion. Pancreatic acinar cells are clustered together to form lobes sharing a joint lumen. Acinar cells synthesize and store digestive enzymes that are secreted from their apical surface into the lumen.1,2 These enzymes are packed into large vesicles (~1 μm in diameter), which are stored in the vicinity of the luminal area. Each pancreatic acinar cell contains hundreds of secretory vesicles.3 Following a secretory stimulus, up to 30% of the cellular vesicle content is secreted over extended time periods of up to an hour.2,3 In spite of the massive addition of membrane surface at the apical domain, the overall size of this domain is maintained constant by a dynamic process of compensatory membrane endocytosis.4 Taken together, these observations underscore the challenge of directing secretion to a narrow and extremely dynamic apical domain over extended time periods. Studies from our lab have focused on the role of actin filaments as mediators of this apical targeting.5
In view of the multiple roles and forms of filamentous actin, it is difficult to dissect the distinct roles of actin solely on the basis of F-actin localization in fixed samples, or by use of general inhibitors of actin polymerization. By utilizing Lifeact-GFP for live imaging of F-actin,6 we obtained a sensitive imaging capacity that allowed us to examine the dynamics of actin-based structures during the secretory process. This approach enabled us to follow three distinct types of F-actin in the acinar cells:
• The “terminal web” is a thin microfilament mesh that lines the apical surface, and is thought to play an inhibitory role, which serves to attenuate sporadic, non-regulated secretion.7
• Just prior to fusion with the apical membrane, secretory vesicles are coated with actin filaments. This actin coat may mediate the contraction of the vesicle upon membrane fusion, to facilitate rapid release of the internal material to the lumen.8 We observed that the nucleation-promoting factor N-WASp, as well as Arp3, a subunit of the Arp2/3 nucleation complex, are both specifically localized to the circumference of the secretory vesicles at the time when the actin coat appears, suggesting that they represent the relevant nucleation machinery.
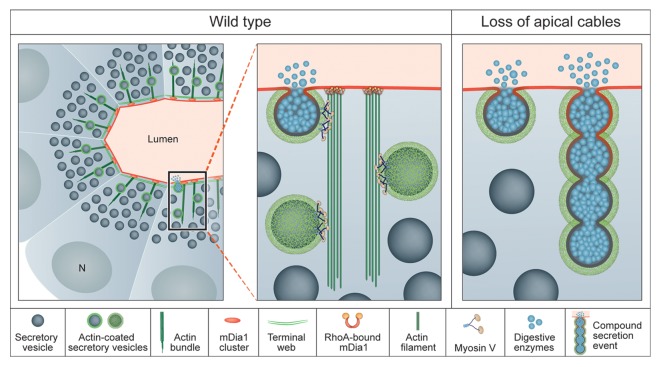
• Significantly, and apart from these well-established microfilament concentrations in acinar cells, the Lifeact-GFP tool enabled us to identify apical bundles of F-actin with an average length of 3–4 μm, which constitute a novel F-actin structure.9 These bundles of actin cables emanate from the apical membrane every 2.5 μm on average, are oriented perpendicular to the surface, and exhibit a high turnover rate. The function of these actin bundles is intimately linked to targeting of secretory vesicles, as these vesicles move along them on their way to the apical surface.
Our data suggests that the apical actin cables are generated by the formin mDia1, since the active form of mDia1 is localized to the apical surface, and bundle density correlates with the activity of mDia1. Disruption of bundle formation, either through treatment with Latrunculin A (LatA) or following expression of a dominant-negative form of mDia1, led to compromised targeting of the secretory process. Under these circumstances, secretory vesicles, which normally fuse individually with the apical cell surface, are much more likely to fuse with each other and generate compound, membrane-associated secretory structures9 (Fig. 1). These results indicate that, although the final route of secretory vesicles to the apical membrane is a short one, trafficking along actin bundles regulates the orderly targeting of vesicles, maintains steady speed of movement, and prevents collision of vesicles with one another.
Figure 1. Actin-coated vesicles are directed to the apical surface by actin-cable bundles generated by mDia1 (left, center). Following the loss of the actin bundles, either by treatment with LatA or by expression of a dominant-negative form of mDia1, the orderly apical targeting of vesicles is compromised and they fuse into one another (right).
The close association of secretory vesicles with the actin bundles suggests that this form of locomotion may be powered by myosin V motors. The predominant myosin V isoform in the exocrine pancreas, as in other epithelial tissues, is MyoVc.10,11 This is a “low duty ratio” and non-processive motor, i.e., one that spends most of the time during its catalytic cycle in weak association with actin, and cannot take multiple successive steps over the filament.12 Vesicle transport can thus be achieved by cooperative activity of multiple myosin V molecules, which progress along different cables within the same actin bundle.12 Trafficking along an actin bundle, rather than a single filament, increases the probability for re-joining of the motor with the filament. Notably, the velocity of exocrine vesicles moving over actin bundles that we report (35 nm/s) is similar to the measured velocity of MyoVc-dependent transport (24 nm/s).12
In conclusion, in secretory organs of mice and flies, apically generated actin cables provide essential tracks for the final stages of vesicle secretion. The apical localization of Dia-family actin nucleators is critical for actin bundle polarity.13 Trafficking of vesicles along these cables organizes the secretion process at highly restricted domains of the apical membrane of the cells.
Acknowledgments
This work was supported by a grant from the US-Israel BSF to BZS and ES. BZS is an incumbent of the Hilda and Cecil Lewis chair in Molecular Genetics.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/cib/article/25660
References
- 1.Motta PM, Macchiarelli G, Nottola SA, Correr S. Histology of the exocrine pancreas. Microsc Res Tech. 1997;37:384–98. doi: 10.1002/(SICI)1097-0029(19970601)37:5/6<384::AID-JEMT3>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 2.Williams JA. Regulation of pancreatic acinar cell function. Curr Opin Gastroenterol. 2006;22:498–504. doi: 10.1097/01.mog.0000239863.96833.c0. [DOI] [PubMed] [Google Scholar]
- 3.Valentijn KM, Gumkowski FD, Jamieson JD. The subapical actin cytoskeleton regulates secretion and membrane retrieval in pancreatic acinar cells. J Cell Sci. 1999;112:81–96. doi: 10.1242/jcs.112.1.81. [DOI] [PubMed] [Google Scholar]
- 4.Burgoyne RD, Morgan A. Secretory granule exocytosis. Physiol Rev. 2003;83:581–632. doi: 10.1152/physrev.00031.2002. [DOI] [PubMed] [Google Scholar]
- 5.Massarwa R, Schejter ED, Shilo BZ. Apical secretion in epithelial tubes of the Drosophila embryo is directed by the Formin-family protein Diaphanous. Dev Cell. 2009;16:877–88. doi: 10.1016/j.devcel.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 6.Riedl J, Crevenna AH, Kessenbrock K, Yu JH, Neukirchen D, Bista M, et al. Lifeact: a versatile marker to visualize F-actin. Nat Methods. 2008;5:605–7. doi: 10.1038/nmeth.1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Palade G. Intracellular aspects of the process of protein synthesis. Science. 1975;189:347–58. doi: 10.1126/science.1096303. [DOI] [PubMed] [Google Scholar]
- 8.Nightingale TD, Cutler DF, Cramer LP. Actin coats and rings promote regulated exocytosis. Trends Cell Biol. 2012;22:329–37. doi: 10.1016/j.tcb.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 9.Geron E, Schejter ED, Shilo BZ. Directing exocrine secretory vesicles to the apical membrane by actin cables generated by the formin mDia1. Proc Natl Acad Sci USA. 2013;110:10652–7. doi: 10.1073/pnas.1303796110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen X, Walker AK, Strahler JR, Simon ES, Tomanicek-Volk SL, Nelson BB, et al. Organellar proteomics: analysis of pancreatic zymogen granule membranes. Mol Cell Proteomics. 2006;5:306–12. doi: 10.1074/mcp.M500172-MCP200. [DOI] [PubMed] [Google Scholar]
- 11.Rodriguez OC, Cheney RE. Human myosin-Vc is a novel class V myosin expressed in epithelial cells. J Cell Sci. 2002;115:991–1004. doi: 10.1242/jcs.115.5.991. [DOI] [PubMed] [Google Scholar]
- 12.Takagi Y, Yang Y, Fujiwara I, Jacobs D, Cheney RE, Sellers JR, et al. Human myosin Vc is a low duty ratio, nonprocessive molecular motor. J Biol Chem. 2008;283:8527–37. doi: 10.1074/jbc.M709150200. [DOI] [PubMed] [Google Scholar]
- 13.Rousso T, Mostov KE, Schejter ED, Shilo BZ. Apical targeting of the formin Diaphanous in Drosophila tubular epithelia. eLife. 2013;2:e00666. doi: 10.7554/eLife.00666. [DOI] [PMC free article] [PubMed] [Google Scholar]