Abstract
Ras GTPases and phosphatidylinositol 3-kinases mediate intracellular signaling in directed cell migration. During chemotaxis, cells spatially control the activation of Ras/PI (3,4,5)-trisphosphate (PIP3) signaling and translate extracellular chemical gradients into intracellular signal cascades. This process is called directional sensing, and enables persistent cell migration with extraordinary sensitivity in shallow, unstable gradients of chemoattractants. In our recent study, we identified a Rho GTPase and its guanine nucleotide exchange factor (GEF) as molecular modulators that transmit signals from G protein-coupled receptors to Ras/PIP3 signaling pathways. The proteins spatially stabilize Ras activation and PIP3 production toward higher concentrations of chemoattractants. Unlike known roles of Rho GTPases and GEFs, the function of these proteins in directional sensing is independent of the actin cytoskeleton and cell morphology. Our findings provide novel mechanistic insight into the precision of directional cell migration.
Keywords: Chemotaxis, PIP3, Rho GTPase, guanine nucleotide exchange factor, Dictyosteliums
A fundamental question in biology is how cells recognize the extracellular environment, translate information into intracellular signals, and produce appropriate physiological responses with extremely high accuracy. Chemotaxis provides an excellent experimental system to address this question.1-3 During chemotaxis, cells sense extracellular chemical gradients, generate intracellular signaling cascades, and reorganize and orient the actin cytoskeleton toward higher concentrations of chemoattractants.
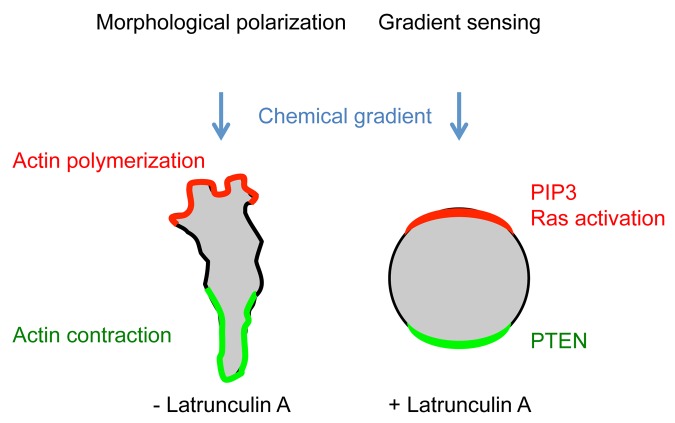
Chemical gradients in the extracellular space are dynamic and very unstable due to frequent perturbation. However, cells can accurately recognize the environment and produce persistent changes in polarity and direction of movement toward higher concentrations of attractants. It has been shown that cells move toward a change in concentration as small as 2% from the front to back of a cell.1,4 Two mechanisms ensure the precision of chemotaxis: morphological polarization and directional sensing.1,4-10 For morphological polarization, migrating cells establish a leading edge, which contains dynamic actin filaments formed by polymerization, and generate lamellipodia and filopodia to pull the cell forward. On the other hand, the trailing edge contains less dynamic actin filaments and myosin II for contraction to push the cytoplasm forward. The leading edge is enriched for signaling components, leading to increased sensitivity to chemoattractants, compared with the trailing edge. Morphological polarization can be created and maintained in the absence of a chemical gradient and is therefore sufficient to initiate cell migration. In contrast, directional sensing is independent of the actin cytoskeleton and enables cells to sense the direction of a chemical gradient. This sensing mechanism is crucial for directed cell migration in the physiological environment, where chemical gradients are unstable, and ensures the high accuracy of migration by consistently sensing the direction of gradients. In other words, directional sensing functions as a compass for directed cell migration.
To experimentally separate morphological polarization and directional sensing, latrunculin A, which inhibits actin polymerization, has been used. In the presence of latrunculin A, cells lose their elongated shape and become round. However, cells can still sense chemical gradients and direct amplified intracellular signals toward higher concentrations of attractants. These signaling events include the activation of Ras GTPases and the production of PIP3, which can be visualized using specific biosensors, such as a green fluorescent protein (GFP)-ras binding domain (RBD) fusion protein that specifically associates with GTP-bound forms of Ras and a GFP-PH domain from CRAC (PHcrac) fusion protein that specifically binds to PIP3. These probes are recruited to the leading edge of migrating cells, whereas they form a crescent-like structure at the plasma membrane on the side of cells facing the source of attractants in the presence of latrunculin A. Directional sensing acts downstream of G-protein coupled receptors and is evolutionarily conserved in humans and Dictyostelium However, molecular components that link the receptors and Ras/PIP3 signaling in directional sensing remain unknown.
In our recent study, we discovered that a Rho GTPase and Rho GEF pair mediates directional sensing in Dictyostelium cells.11 We systematically deleted 13 of 19 Rho GTPases and 20 of 43 Dbl domain-containing Rho GEFs in the Dictyostelium genome and found that only one knockout strain for each group, i.e., those lacking RacE (Rho) or GxcT (Rho GEF), was defective in chemotaxis. RacE-null cells and GxcT-null cells failed to spatially stabilize Ras activation and PIP3 production in a chemoattractant gradient, leading to lateral pseudopod extension and impaired chemotaxis. These defects were specific to spatial orientation of Ras and PIP3 signaling, but not to the level of activation. Overexpression of wild-type and a constitutively active form of RacE, but not a dominant negative form, increased the spatial stability of Ras activation and PIP3 production. Furthermore, GxcT interacts with RacE with a strong preference for a dominant negative mutant, RacE(T25N), and only weakly binds to a constitutively active form, RacE(G20V), suggesting that GxcT functions as a GEF for RacE.
Suggesting that an active form of RacE restricts Ras/PIP3 signaling, GFP-RacE(G20V) was located on the side of the cell facing away from the source of the gradient in the presence of latrunculin A.11 This localization depended on the effector loop, which is associated with downstream effectors of Rho GTPases. The amino acid sequence of this effector loop is highly specific to each type of Rho GTPase, and is identical for Dictyostelium RacE and human RhoA. Phylogenetic analyses also showed that RacE is most closely related to human RhoA. Indeed, a constitutively active form, RhoA(Q63L), was located on the side of the cell facing away from the source of the gradient in the presence of latrunculin A in Dictyostelium cells, similar to RacE(G20V). Therefore, our data suggest a conserved mechanism by which an active form of RacE negatively controls the distribution of Ras activation and PIP3 production from the back of cells.
It has been shown that Rho family GTPases, including Rho, Rac, and Cdc42 in mammals, act as downstream effectors of Ras GTPases and PIP3 to control distinct types of actin cytoskeleton remodeling in cell migration.12,13 In our study, we revealed an actin-independent role for Rho GTPases in conveying spatial information upstream of Ras and PIP3 signaling.11 It will be of great interest to further dissect molecular mechanisms by which Rho GTPases regulate Ras and PI3 kinases in directional sensing during chemotaxis.
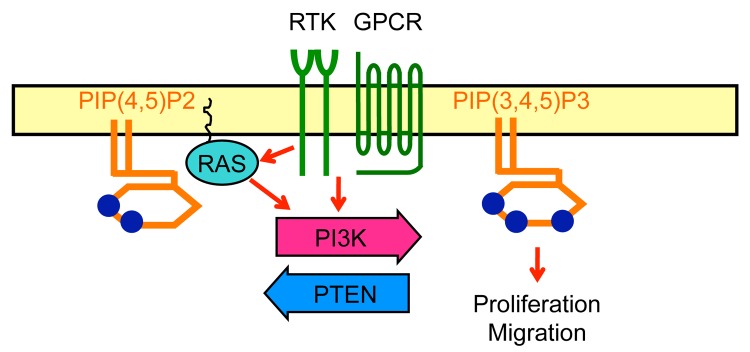
Figure 1. Ras and PIP3 signaling. PIP3, a plasma membrane phospholipid which is generated by PI3Ks and degraded by PTEN, stimulate cell proliferation and migration. RTK, receptor tyrosine kinase; GPCR, G-protein coupled receptor.
Figure 2. Morphological polarization and directional sensing. Migrating cells establish elongated cell morphology and a polarized pattern of the actin cytoskeleton. The cell shape increases the ability of cells to recognize chemoattractants at the leading edge. In the absence of the actin cytoskeleton, cells can still sense a chemical gradient and produce amplified signal cacades toward higher concentrations of chemoattractants.
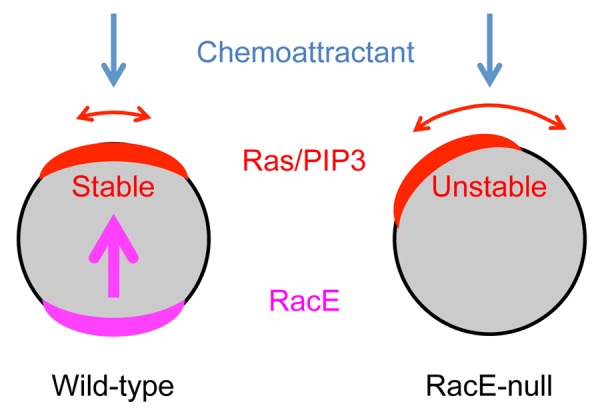
Figure 3. RacE stabilizes directional sensing. Wild-type cells efficiently direct Ras/PIP3 signaling toward the source of chemoattractants. In contrast, the accuracy of directional sensing decreases in RacE-null cells. RacE spatially restricts the position of Ras activation and PIP3 production from the back of cells.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We are grateful to H Sesaki for critically reading the manuscript. This work was supported by a NIH grant (GM084015).
Footnotes
Previously published online: www.landesbioscience.com/journals/cib/article/27681
References
- 1.Swaney KF, Huang CH, Devreotes PN. Eukaryotic chemotaxis: a network of signaling pathways controls motility, directional sensing, and polarity. Annu Rev Biophys. 2010;39:265–89. doi: 10.1146/annurev.biophys.093008.131228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Petrie RJ, Doyle AD, Yamada KM. Random versus directionally persistent cell migration. Nat Rev Mol Cell Biol. 2009;10:538–49. doi: 10.1038/nrm2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Franz CM, Jones GE, Ridley AJ. Cell migration in development and disease. Dev Cell. 2002;2:153–8. doi: 10.1016/S1534-5807(02)00120-X. [DOI] [PubMed] [Google Scholar]
- 4.Wang Y, Chen CL, Iijima M. Signaling mechanisms for chemotaxis. Dev Growth Differ. 2011;53:495–502. doi: 10.1111/j.1440-169X.2011.01265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang F. The signaling mechanisms underlying cell polarity and chemotaxis. Cold Spring Harb Perspect Biol. 2009;1:a002980. doi: 10.1101/cshperspect.a002980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Janetopoulos C, Firtel RA. Directional sensing during chemotaxis. FEBS Lett. 2008;582:2075–85. doi: 10.1016/j.febslet.2008.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Devreotes P, Janetopoulos C. Eukaryotic chemotaxis: distinctions between directional sensing and polarization. J Biol Chem. 2003;278:20445–8. doi: 10.1074/jbc.R300010200. [DOI] [PubMed] [Google Scholar]
- 8.Insall RH, Machesky LM. Actin dynamics at the leading edge: from simple machinery to complex networks. Dev Cell. 2009;17:310–22. doi: 10.1016/j.devcel.2009.08.012. [DOI] [PubMed] [Google Scholar]
- 9.Vicente-Manzanares M, Horwitz AR. Cell migration: an overview. Methods Mol Biol. 2011;769:1–24. doi: 10.1007/978-1-61779-207-6_1. [DOI] [PubMed] [Google Scholar]
- 10.Bourne HR, Weiner O. A chemical compass. Nature. 2002;419:21. doi: 10.1038/419021a. [DOI] [PubMed] [Google Scholar]
- 11.Wang Y, Senoo H, Sesaki H, Iijima M. Rho GTPases orient directional sensing in chemotaxis. Proc Natl Acad Sci U S A. 2013;110:E4723–32. doi: 10.1073/pnas.1312540110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sit ST, Manser E. Rho GTPases and their role in organizing the actin cytoskeleton. J Cell Sci. 2011;124:679–83. doi: 10.1242/jcs.064964. [DOI] [PubMed] [Google Scholar]
- 13.Jaffe AB, Hall A. Rho GTPases: biochemistry and biology. Annu Rev Cell Dev Biol. 2005;21:247–69. doi: 10.1146/annurev.cellbio.21.020604.150721. [DOI] [PubMed] [Google Scholar]