Abstract
Root growth is critical for the effective exploitation of the rhizosphere and productive plant growth. Our recent work1 showed that root architecture was dependent upon the degree of symplastic connectivity between neighboring cells during the specification of lateral root primordia and was affected by genes regulating callose deposition at plasmodesmata (PD). Here we provide additional evidence that both symplastic connectivity and callose are also important during the later phase of lateral root development: emergence. Callose immunolocalization assays indicated that transient symplastic isolation of the primordium occur immediately prior to emergence through the overlaying tissues to produce the mature lateral root.1 Here we could corroborate these results by analyzing the mobility of a symplastic tracer and the expression of PD genes in lateral roots and in response to auxins. Moreover, we show that altering callose deposition affects the number of emerged lateral roots suggesting that PD regulation is important for emergence.
Keywords: Arabidopsis, intercellular transport, lateral root emergence, lateral root primordium, plasmodesmata, symplastic connectivity
The formation of lateral root primordia along the main root axis is controlled by interconnected signaling pathways involving multiple hormones.2-5 Lateral root (LR) emergence leads to root branching so increasing the functional capacity of roots to underpin increased plant growth. Our recent publication reported that these changes are coordinated in part by regulating symplastic connectivity between neighboring cells through the deposition of callose at plasmodesmata (PD).1 Hence, callose deposition at PD coincides with a reduction in molecular trafficking in and out of LR stage III and older primordia,1 which suggests a potential role for symplastic transport in the late stages of LR development.
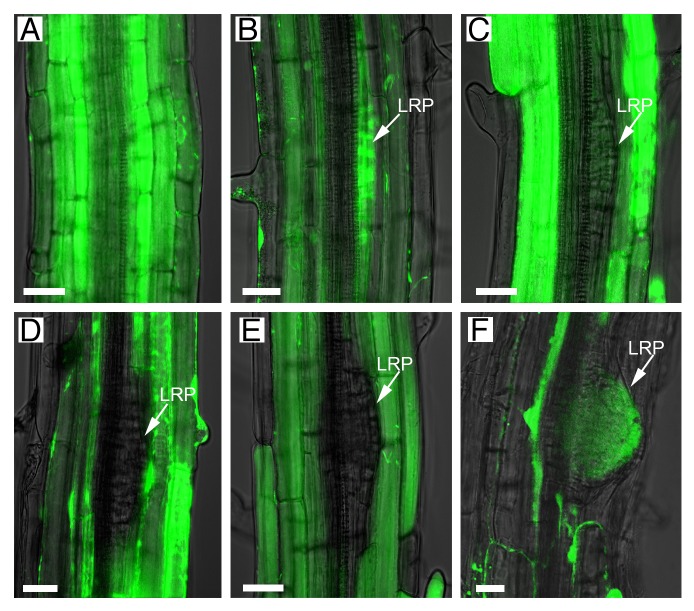
As an alternative to GFP1 in the examination of symplastic boundaries between the primordia overlaying tissues (epidermal, cortical, and endodermal) and the primordia, 10-d-old Arabidopsis roots were exposed to a chemical marker (carboxyfluorescein diacetate, CFDA). CFDA (which is non-fluorescent) produces fluorescein diacetate (FDA) inside the cells, which fluoresces green after excitation with the 488 nm laser.6 Apoplastic transport between the endodermis and the lateral root is blocked by the presence of the Casparian strip.7 FDA diffuses intracellularly via PD from the epidermis to the stele in the root meristem, elongation, and early differentiation zones (basal meristem) (Fig. 1A). Fluorescence was visible throughout the tissues and in early stage primordia, but it was absent inside primordia at stage III to V, suggesting the formation of a new symplastic boundary between the developing LR and the neighboring outer tissues (Fig. 1B-E). This symplasmic domain was temporary and was lost just before emergence when FDA diffused throughout the new LR (Fig. 1F).
Figure 1. Symplastic connectivity between the outer tissues and the primordia is regulated during lateral root development. A symplastic fluorescent dye (green; FDA), generated from CFDA applied exogenously, moved freely through cell layers in non-lateral root regions and into stage I–II primordia (A-B). Movement was blocked after transition from stage III to IV primordia (C-E) and restored just before emergence (F). Images in the brightfield channel (superimposed) were used to distinguish the lateral root primordia (LRP, arrowed in B-F). Scale bars: 20 µm.
Increase levels of the hormone auxin are associated with both the initiation and the emergence phase of LR development.4 Screening microarray data8 for proteins involved in callose deposition and induced after treatment with auxins, identified Plasmodesmata Callose-Binding protein 1 (PDCB1). PDCB1 functions in callose stabilization at PD,9 and its expression appears to be greatly upregulated 6h after treatment with the synthetic auxin 1-naphthaleneacetic acid (NAA). This is a relatively late transcriptional response since LR initiation genes (such as cell-cycle related genes) are induced as early as 2h post-treatment with NAA.8 However, PDCB1 response to auxins is blocked in plants carrying a gain-of-function mutation in SOLITARY-ROOT/IAA14 (slr1) that supresses lateral root initiation,8 which supports a role for PDCB1 in LR development.
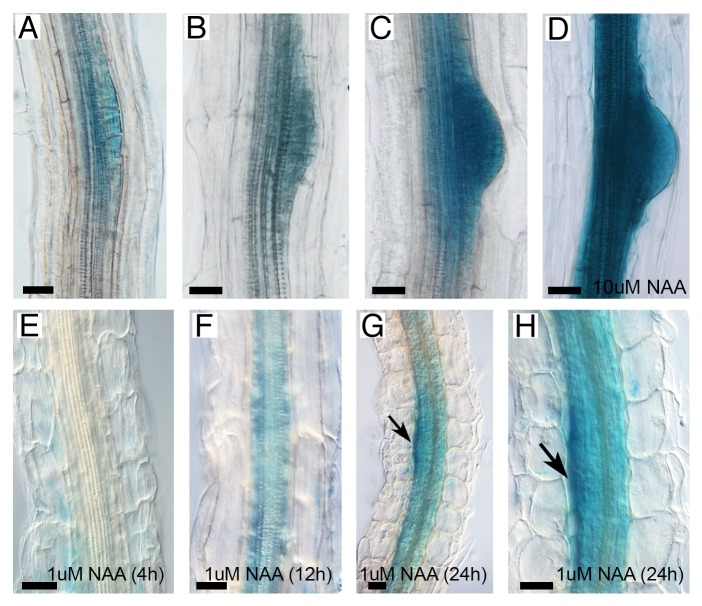
To confirm the microarray results, we determined the expression of PDCB1 in LR and studied its response to auxin treatment using transcriptional reporter lines (Fig. 2). Using promoter::GUS reporter lines we found PDCB1 expression in stage III–VI LR primordia, which was strongly upregulated when we transferred these seedlings to medium containing 10 µM NAA for 24h (Fig. 2A-D). To study PDCB1 regulation by auxins in more detail, we germinated transgenic seeds expressing the PDCB1 transcriptional reporter in 10 µM 1-N- naphthylphthalamic acid (NPA) for 4 d before transfer to 1 µM NAA. In wild type seedlings, NPA treatment inhibits basipetal auxin transport, which blocks LR initiation.3 After transfer to NAA, LR are induced synchronously from xylem-pole pericycle cells. Interestingly, short periods of NAA treatment did not visibly change PDCB1 expression (4h shown in Fig. 2E). After 8h, GUS activity started to appear in dividing pericycle cells and staining was clearly seen 12h after treatment (Fig. 2F). Gene expression was stronger 24h after transfer to NAA, reaching a maximum in the upper boundary of the primordia (arrowed, Fig. 2G-H). These patterns of expression in response to auxins are consistent with PDCB1 playing a role in late rather than early initiation stages of LR development where it might function in the deposition of callose around the primordium before emergence.
Figure 2.PDCB1 is expressed in lateral roots and upregulated after exposure to auxins. Expression was reported by GUS activity using the reporter lines pPDCB1::GUS (A-H). Staining of 10-d-old seedling revealed gene expression in stages II–III (A), IV–V (B), and VI (C) lateral root primordia. 24h treatment with 10 µM NAA greatly upregulates PDCB1 expression in emerging lateral roots and dividing pericycle cells (D). Staining of seedlings grown in 10 µM NPA and transferred to 1 µM NAA for 4h (E), 12h (F), and 24h (G-H) showed time-dependent PDCB1 response to exogenous auxins. Arrow in (G-H) indicates maxima of gene expression in the upper boundary of the primordia. Scale bars: 20 µm.
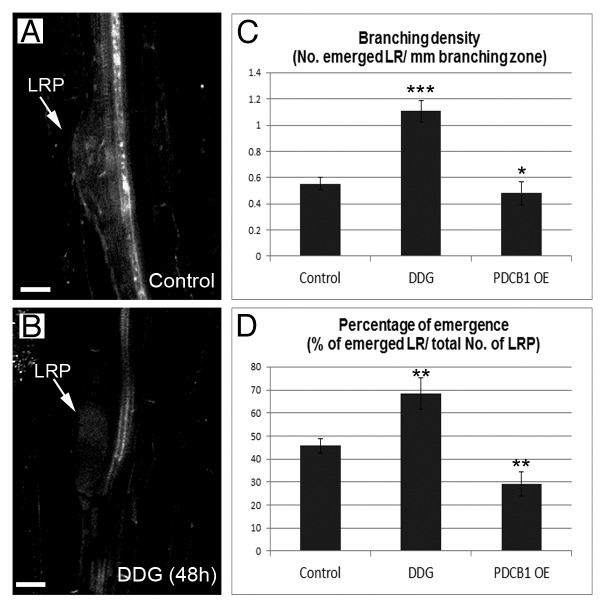
To study further the importance of regulating callose during emergence, we investigated the phenotype of plants overexpressing PDCB1 (PDCB1 OE) and of plants exposed to the chemical 2-deoxy-d-glucose (DDG, a glucose analog that inhibits callose synthesis).10,11 PDCB1 OE plants accumulate excessive callose, which influences LR patterning.1,9 The number of emerged LR divided by the length of the branching zone (branching density) and the ratio between emerged and non-emerged LR (percentage of emergence) were used as parameters to evaluate phenotypic differences in emergence. Six-day-old seedlings transferred to medium containing DDG displayed a reduction in callose deposited around stage IV primordia after 48h (Fig. 3A-B). These seedlings displayed an improvement in branching density and percentage of emerged LR (Fig. 3C-D). Conversely, these parameters were significantly reduced in PDCB1 OE plants (Fig. 3C-D). These results highlight the importance of regulating callose deposition around the primordia and in the neighboring tissue during LR emergence.
Figure 3. Altering callose deposition in developing lateral roots regulates branching density and percentage of emergence. Callose (white, stained with aniline blue) failed to deposit around stage IV–V primordia (LRP) treated for 48h with 50uM DDG in comparison to control (untreated) roots (A-B). Scale bars: 20 µm. Branching density (C) and percentage of emergence (D) were calculated in 9-d-old wild-type roots, untreated (Control) and treated with 50uM DDG for 72h, and in PDCB1 OE plants. Error bars represent SD. Statistical significance are represented as *, P < 0.1; **, P < 0.01; ***, P < 0.001.
Lateral root emergence and extension delivers the full root potential for plant performance. It has been shown that the movement of signals and the mechanical properties of the cell-wall determine LR outgrowth.5,12,13 However, to date there has been no direct experimental evidence to demonstrate the importance of symplastic connectivity in the emergence process.
The initial “connected” phase between the lateral root and the overlaying tissue appears to be lost soon after primordia transition to stage III–IV since the movement of a symplasmic dye into the LR primordium is restricted (Fig. 1). We also found that PDCB1 expression is induced by auxins in late stages of primordium development (Fig. 2). Since PDCB1 acts as a callose binding protein, we propose a role for this gene in the formation of symplastic domains around stage III–V primordia. Future experiments using knockout mutants for PDCB1 (currently not available) will test the veracity of this statement. Perturbing callose deposition around pre-formed primordia (following DDG treatment) resulted in an increase in emerged LR (Fig. 3). Conversely, increasing callose ectopically (in the PDCB1 OE line) reduced branching density. This is likely a consequence of modifying callose in the tissues overlaying the primordia since our previous work demonstrated that mutations in PD callose-degrading enzymes expressed in the stele and within young LR primordia do not affect the emergence process.1 Together these results suggest that callose deposition in the overlaying tissue must be tightly regulated to determine the number of LR that will emerge. This could be relevant during the root response to changes in the below ground environment: the capacity to rapidly modify the number of emerged LR determines plant adaptation and survival in adverse soil conditions.2
Our molecular diffusion and cell biological data suggest that callose is deposited around developing primordia before emergence and this is associated with restricted symplastic connectivity. It has been suggested recently that water flow into the primordia (through aquaporin activity) is necessary for organ emergence.13 A reduction in symplastic connectivity, in the boundaries of emerging primordia, might be required to maintain the osmotic potential that facilitates organ emergence by blocking water release into the overlaying tissue. Whether there are additional effects, such as isolating the primordia from physico-chemical properties of the apoplast or from developmental processes triggered in neighboring mature tissues, is not yet known. Alternatively, altering callose deposition in the overlaying tissue might affect the movement of signals that trigger cell-wall modifications in the endodermal and cortical tissue to allow the emergence of new lateral roots. Since a wide range of environmental cues regulates callose deposition14-16 this mechanism might provide the plasticity to modify root branching pattern in changing soil conditions. Future work will determine which are the signals and proteins regulating this process in vivo.
Materials and Methods
Plant material, growth, and treatments
Unless specified, Arabidopsis thaliana Columbia (Col-0) plants were used. Construction of PDCB1 overexpression (p35S::mCherry-PDCB1) and transcriptional reporter (pPDCB1::GUS) lines was described before.1,9
Seeds were sterilized and germinated in long day conditions on plates containing MS medium. For NAA treatment, seedlings were germinated in MS supplemented with 10 µM NPA (Sigma) and transferred after 4 d to MS supplemented with 1 µM NAA (Sigma).
For DDG treatment, seedlings were germinated in MS plates and transferred after 6 d to MS plates supplemented with 50 µM 2-deoxy-d-glucose (Sigma).
GUS staining, dye loading, and aniline blue staining
Standard protocols were used for GUS assays.9 CFDA loading studies, to assess symplastic permeability, were performed as described before.1 Similarly, the protocol followed for aniline blue staining of callose has been published.1
Analysis of branching density and percentage of emergence
Cleared root preparations were characterized as described.1 We recorded the total number of primordia and emerged lateral roots (new meristems sticking out of the main root) as well as the length of the branching zone (the length of the root portion where LRs are emerged) in 9-d-old seedlings to calculated branching density (number of emerged lateral roots divided by the length of the branching zone) and percentage of emergence (percentage of emerged lateral roots from the total number of lateral root primordia initiated). Standard deviation (SD) and P-values (Student T-test) were calculated using Microsoft Excel.
Acknowledgments
We thank Jim Barnes for reading and correction of the manuscript. The John Innes Centre is grant-aided by the Biotechnology and Biological Science Research.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/cib/article/26531
References
- 1.Benitez-Alfonso Y, Faulkner C, Pendle A, Miyashima S, Helariutta Y, Maule A. Symplastic intercellular connectivity regulates lateral root patterning. Dev Cell. 2013;26:136–47. doi: 10.1016/j.devcel.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 2.Benková E, Bielach A. Lateral root organogenesis - from cell to organ. Curr Opin Plant Biol. 2010;13:677–83. doi: 10.1016/j.pbi.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 3.Himanen K, Boucheron E, Vanneste S, de Almeida Engler J, Inzé D, Beeckman T. Auxin-mediated cell cycle activation during early lateral root initiation. Plant Cell. 2002;14:2339–51. doi: 10.1105/tpc.004960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lavenus J, Goh T, Roberts I, Guyomarc’h S, Lucas M, De Smet I, Fukaki H, Beeckman T, Bennett M, Laplaze L. Lateral root development in Arabidopsis: fifty shades of auxin. Trends Plant Sci. 2013;18:450–8. doi: 10.1016/j.tplants.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 5.Péret B, De Rybel B, Casimiro I, Benková E, Swarup R, Laplaze L, Beeckman T, Bennett MJ. Arabidopsis lateral root development: an emerging story. Trends Plant Sci. 2009;14:399–408. doi: 10.1016/j.tplants.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 6.Oparka KJ, Prior D, Wright KM. Symplastic communication between primary and developing lateral roots of Arabidopsis thaliana. J Exp Bot. 1995;46:187–97. doi: 10.1093/jxb/46.2.187. [DOI] [Google Scholar]
- 7.Geldner N. The endodermis. Annu Rev Plant Biol. 2013;64:531–58. doi: 10.1146/annurev-arplant-050312-120050. [DOI] [PubMed] [Google Scholar]
- 8.Parizot B, De Rybel B, Beeckman T. VisuaLRTC: a new view on lateral root initiation by combining specific transcriptome data sets. Plant Physiol. 2010;153:34–40. doi: 10.1104/pp.109.148676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simpson C, Thomas C, Findlay K, Bayer E, Maule AJ. An Arabidopsis GPI-anchor plasmodesmal neck protein with callose binding activity and potential to regulate cell-to-cell trafficking. Plant Cell. 2009;21:581–94. doi: 10.1105/tpc.108.060145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li W, Zhao Y, Liu C, Yao G, Wu S, Hou C, Zhang M, Wang D. Callose deposition at plasmodesmata is a critical factor in restricting the cell-to-cell movement of Soybean mosaic virus. Plant Cell Rep. 2012;31:905–16. doi: 10.1007/s00299-011-1211-y. [DOI] [PubMed] [Google Scholar]
- 11.Radford JE, White RG. Inhibitors of myosin, but not actin, alter transport through Tradescantia plasmodesmata. Protoplasma. 2011;248:205–16. doi: 10.1007/s00709-010-0244-3. [DOI] [PubMed] [Google Scholar]
- 12.Lucas M, Kenobi K, von Wangenheim D, Vobeta U, Swarup K, De Smet I, Van Demme D, Lawrence T, Péret B, Moscardi E, et al. Lateral root morphogenesis is dependent on the mechanical properties of the overlaying tissues. Proc Natl Acad Sci USA. 2013;110:5229–34. doi: 10.1073/pnas.1210807110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Péret B, Li G, Zhao J, Band LR, Voß U, Postaire O, Luu DT, Da Ines O, Casimiro I, Lucas M, et al. Auxin regulates aquaporin function to facilitate lateral root emergence. Nat Cell Biol. 2012;14:991–8. doi: 10.1038/ncb2573. [DOI] [PubMed] [Google Scholar]
- 14.Benitez-Alfonso Y, Jackson D, Maule A. Redox regulation of intercellular transport. Protoplasma. 2011;248:131–40. doi: 10.1007/s00709-010-0243-4. [DOI] [PubMed] [Google Scholar]
- 15.Rinne PL, Welling A, Vahala J, Ripel L, Ruonala R, Kangasjärvi J, van der Schoot C. Chilling of dormant buds hyperinduces FLOWERING LOCUS T and recruits GA-inducible 1,3-beta-glucanases to reopen signal conduits and release dormancy in Populus. Plant Cell. 2011;23:130–46. doi: 10.1105/tpc.110.081307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zavaliev R, Ueki S, Epel BL, Citovsky V. Biology of callose (β-1,3-glucan) turnover at plasmodesmata. Protoplasma. 2011;248:117–30. doi: 10.1007/s00709-010-0247-0. [DOI] [PMC free article] [PubMed] [Google Scholar]