Abstract
Animals thrive in environments where food resources are abundant. While this correlation between population growth and food abundance is well established, much less is known about the influence of diet quality on physiological and developmental programs that support animal reproduction. Here we discuss dietary impact on fertility, and highlight a recent report on the activity of a nuclear receptor that protects against dietary metabolites to maintain germline stem cell integrity and reproduction.
Keywords: germline stem cells, sterility, diet, nuclear receptors, nhr-114, HNF4, C. elegans, gut microbiota
Introduction
Environmental variations affect the nutritional composition of food resources. A common example is animals encountering different quality of food depending on a dry or rainy season. This phenomenon is less evident in human agricultural populations, in which efforts are devoted to maintain consistent food resources, both in their physical and nutritional aspects. Concomitantly, while humans in the industrialized world compensate for environment-induced variations in their own diet by using nutritional supplements, natural animal populations likely rely on genetic programs, such as their physiology and behavior, to compensate for such changes.
The negative impact of natural dietary ingredients on health and fertility has traditionally been the domain of toxicologists or ecologists (for an in depth review on reproductive toxicological effects see ref. 1). For example, studies on the chemical defense of plants or animal preys have given deep insights into how organisms cope with diverse diets that potentially include harmful foods.2 However, only in recent years, developmental and cell biologists started to explore the impact of environmental compounds on the reproductive capacity of animals.3,4 Although it has been noted that the environment and diet can have a negative impact on the plasticity of developmental programs, the impact on germ cell developmental programs has remained relatively unclear.
Studies that investigate dietary impact on reproductive cells are greatly aided by the proven advantages offered by genetically tractable model organisms. In particular, the transparent roundworm Caenorhabditis elegans emerged as a powerful system to tackle such questions because: 1) food parameters are easily controlled in dietary experiments; 2) its gonads and germ cells are readily accessible; and 3) self-fertile hermaphrodites produce large progeny sizes to satisfy statistical needs.
A Novel Diet-Dependent Type of Sterility
Using C. elegans, we recently reported a genetic program that compensates for dietary variations and preserves the animal’s reproductive capacity.5 Our genetic work unveiled a novel diet-dependent sterility phenotype that affects only the reproductive cells and manifests in germline stem cell deterioration and in differentiation defects of female germ cells. Due to an accumulation of strong genomic and cellular defects, this diet-induced sterility is irreversible and, intriguingly, only apparent upon concomitant loss of the genetic activity that encodes the nuclear hormone receptor protein NHR-114.5 This presumed transcription factor is present in other nematodes, and is highly related to the mammalian Hepatocyte Nuclear Factor 4 (HNF4), which is an evolutionary conserved nuclear receptor involved in animal metabolism and gastrointestinal homeostasis.6,7
Nutrition and metabolism are long-standing intertwined science disciplines that have been largely ignored by developmental biologists. However, genetic evidence of dietary influence on animal ontogeny is accumulating and challenges the classical separatist view on physiology and developmental programs. Importantly, this dietary influence does not refer to caloric or nutrient related aspects, but rather refers to variable metabolites primarily produced in the gut. In addition to the intestinal cells, microbes of the intestinal tract are recognized as contributors to dietary metabolism, likely imposing changes in the physiology of a host organism.8 Moreover, intestinal microbes may produce diverse metabolites that possess the potential to positively or negatively influence their host. Hence, the emerging theme is that diet, in combination with gut microbes, influences a vast number of metazoan processes, suggesting that animals must possess mechanisms that integrate dietary and microbial “information” into their physiology and developmental pathways.8 For example, Toll-like receptor signaling in the mammalian intestinal epithelium suggests that genetic pathways detect and respond to intestinal microbial components to promote intestinal function and integrity.9 However, it is still unknown to what extent and how dietary variations and intestinal microbes influence animal reproduction. Moreover, given the important roles of intestinal microbes and their metabolites for human health,8 novel developmental diet-germ cell axis of communication may exist in animals to cope with dietary variation.
Virtually all laboratories across the world use as the standard diet of Caenorhabditis elegans a specific E. coli bacterial strain that was introduced to the “worm community” by Sydney Brenner.10 Surprisingly, in the absence of nhr-114 gene activity, we found that this E. coli B strain induces sterility; nhr-114-deficient animals are fertile on other diets.5 Remarkably, the sterility-inducing bacterial diet is converted into harmless food when grown on media supplemented with exogenous tryptophan, underscoring that the metabolic status of the bacteria drastically changes the reproductive capacity of an animal.5 Moreover, intestinal but not germ cell expression of NHR-114 protein prevents diet-dependent sterility. Therefore, our work suggests that somatic nuclear receptor nhr-114 activity has evolved to protect the germ cells from the negative effects of dietary variations and inconsistencies.
In natural environments, feeding and reproduction of C. elegans is associated with colonization of rotten food, which inherently contains a diverse and variable microbial composition.11-13 Hence, our findings imply that loss of nhr-114 gene activity may result in restricting natural populations of C. elegans in its dietary options and may confine the reproduction of these populations to specific dietary environments. Therefore, in a natural environment the absence of nhr-114/HNF4 activity would be disastrous for the continuity of natural populations of C. elegans.
Germline Development is Sensitive to the Nutritional Status
Germ cells are fundamental to animal reproduction. Only germ cells specialize in passing on genetic information to the next generation; all other cells in multicellular organisms are somatic and support animal reproduction as a whole. Germ cells of most animals, including invertebrates and humans, develop initially during embryonic and juvenile stages, forming a germline tissue that reaches homeostasis in the adult. Germ cell developmental programs are in synchrony with gonadal development (a somatic tissue) and share following unique features: 1) an expansion of germ cell precursors into a proliferative pool of germline stem cells; 2) a cell cycle transition from mitosis to meiosis in the maturing germ cells; and 3) sex-specific differentiation into haploid gametes, i.e., sperm or oocytes.14 In the adult reproductive organs, the somatic support cells maintain the germline tissue and influence germ cell development through 2 types of systemic responses: fixed and transient. Such an example of a fixed response is the neuroendocrine control of oocyte maturation in mammals, which occurs under defined developmental circumstances and in a periodic fashion in the adult.15 In contrast, transient responses are highly variable because they rapidly integrate changing environmental cues from external sources and the organism’s physiology. Such an example of a transient response is the proliferative control of germline stem cells.16,17
Reports have shown that the feeding status of an animal affects germline tissue homeostasis and is a major trigger of transient responses in the reproductive organ.18-21 Work spearheaded in genetic model organisms revealed a molecular basis of dietary influences on germline stem cells.18-20 In response to food abundance, studies on invertebrate model systems identified genetic pathways that involve insulin signaling, tumor growth factor-β (TGF-β) signaling, and nuclear hormone receptors to control germ cell proliferation dynamics.17-20 For example, in Drosophila melanogaster germline stem cell proliferation activity is low in fasting conditions, but higher in feasting conditions.17,22 Similarly, in Caenorhabditis elegans, stem cell proliferation activity is controlled by nutrition as a consequence of neuronal insulin and germ cell intrinsic TGF-β activity.17,18 These observations in flies and nematodes resonate well with longitudinal studies on adult humans, which have suggested that nutrient-rich and well-balanced diets can help to boost female fertility.23
Intriguingly, germ cells fulfill their function relatively late in an organism’s life. This implies that by the time germ cells are used in reproduction, the entire body has already been exposed to various environmental and dietary influences. While the accumulation of somatic defects may decrease an individual’s health, germ cell defects have the potential to completely hamper an individual’s reproductive fitness. This is particularly problematic when the undifferentiated germ cell precursors—the germline stem cells—are affected. Therefore, it is reasonable to assume that genetic mechanisms may have evolved to shield germ cells from potentially irreversible damage caused by dietary influence.
Nuclear Receptors and Dietary/Microbial Interactions
Intestinal NHRs are ideal molecular candidates for protecting the organism against dietary insults. Nuclear receptors are key mediators of physiological responses against external and variable compounds.23 NHRs combine a DNA-binding domain (DBD) with a ligand-binding domain (LBD) that is capable of binding small lipophilic signaling molecules.23 Typically, upon ligand binding, a conformational change that renders the receptor transcriptionally active is induced that is instrumental for mounting specific transcriptional responses.23 Indeed, some intestinal nuclear receptors and HNF4 orthologs coordinate dietary homeostasis by coordinating nutrient uptake, eliminating toxic components and protecting against bacterial invasion,7 or modulating dietary responses.24
How precisely the activity of nhr-114/HNF4 protects the C. elegans germ cells is unknown. However, we found that the somatic activity of NHR-114 in the intestine is primarily responsible for protecting the integrity of the germline stem cells in the gonad.5 Moreover, global gene expression analysis established a correlation between nhr-114 absence, and a genetic downregulation of a detoxification response, suggesting that NHR-114/HNF4 may govern a transcriptional compensatory program that protects immature germ cells from dietary toxins or toxic metabolites. Since mammalian HNF4-alfa mediates similar detoxification responses in the gastrointestinal system,25,26 this activity may represent an evolutionary conserved intestinal function that also protects the physiology of specific tissues, such as the reproductive system (for a graphical summary see Fig. 1).
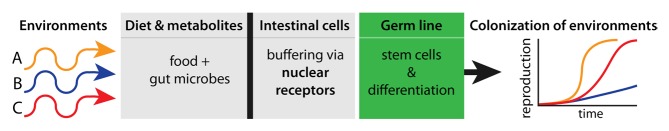
Figure 1. Different environmental conditions (arrows) affect dietary composition, which is further metabolized by gut microbes. The activity of gastrointestinal nuclear receptors may detect toxins or toxic metabolites and elicit a genetic program that specifically shields germ cells from negative impact. This protection results in the capacity to efficiently reproduce and increase population size in diverse environments and may, therefore, underlie the colonization of new territories.
Concluding Remarks
Organisms live and evolve in variable environments. Therefore, the capacity of a natural population to feed on diverse diets may provide an ecological advantage over those populations that feed on more restrictive diets. For example, genetic programs that support the metabolism of diverse foods encountered in new environments may facilitate colonization of new territories. A diversified diet and proper germ cell function are 2 main factors that contribute to the reproductive success of an animal and both ensure the survival of the next generation. Since fertility and reproduction rates are tightly linked to the availability of food resources, genetically programmed systemic responses must have evolved to coordinate germ cell homeostasis with food abundance. However, a better molecular understanding of how diet is linked to reproductive stem cells will still be required to understand the underlying physiological soma-to-germ cell interactions that support fertility. This topic is of increasing importance given that human dietary habits are rapidly changing on a global scale with a continuous population growth.27
Overall, our findings provide a paradigm for understanding dietary impact on germ cell development.5 Firstly, our work shows that germline stem cells are exceptionally vulnerable to dietary metabolites. Secondly, that the dietary impact is at the level of cellular and genomic integrity and the process of cell division, which contrasts to the previously reported nutritional effect on dynamics of germ cell proliferation. Thirdly, our finding that the metabolic status of the dietary bacteria specifically impacts the animal reproductive tissue adds a new angle to microbe-host interactions. This raises the question of whether the human gut microbiota may also impact fertility and similar genetic programs are in place to protect mammalian germline stem cells.
Acknowledgments
We thank Mark Leaver and the 2 anonymous reviewers for their helpful comments. Work in the Eckmann lab is supported by the Deutsche Forschungsgemeinschaft (EC369/2–1 and FOR855) and the Max Planck Society.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/cib/article/26004
References
- 1.Mortimer D, Barratt CL, Björndahl L, de Jager C, Jequier AM, Muller CH. What should it take to describe a substance or product as ‘sperm-safe’. Hum Reprod Update. 2013;19(Suppl 1):i1–45. doi: 10.1093/humupd/dmt008. [DOI] [PubMed] [Google Scholar]
- 2.Glendinning JI. How do predators cope with chemically defended foods? Biol Bull. 2007;213:252–66. doi: 10.2307/25066643. [DOI] [PubMed] [Google Scholar]
- 3.Allard P, Colaiácovo MP. Bisphenol A impairs the double-strand break repair machinery in the germline and causes chromosome abnormalities. Proc Natl Acad Sci USA. 2010;107:20405–10. doi: 10.1073/pnas.1010386107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Susiarjo M, Sasson I, Mesaros C, Bartolomei MS. Bisphenol a exposure disrupts genomic imprinting in the mouse. PLoS Genet. 2013;9:e1003401. doi: 10.1371/journal.pgen.1003401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gracida X, Eckmann CR. Fertility and germline stem cell maintenance under different diets requires nhr-114/HNF4 in C. elegans. Curr Biol. 2013;23:607–13. doi: 10.1016/j.cub.2013.02.034. [DOI] [PubMed] [Google Scholar]
- 6.Watt AJ, Garrison WD, Duncan SA. HNF4: a central regulator of hepatocyte differentiation and function. Hepatology. 2003;37:1249–53. doi: 10.1053/jhep.2003.50273. [DOI] [PubMed] [Google Scholar]
- 7.Schmidt DR, Mangelsdorf DJ. Nuclear receptors of the enteric tract: guarding the frontier. Nutr Rev. 2008;66(Suppl 2):S88–97. doi: 10.1111/j.1753-4887.2008.00092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nicholson JK, Holmes E, Kinross J, Burcelin R, Gibson G, Jia W, Pettersson S. Host-gut microbiota metabolic interactions. Science. 2012;336:1262–7. doi: 10.1126/science.1223813. [DOI] [PubMed] [Google Scholar]
- 9.Abreu MT. Toll-like receptor signalling in the intestinal epithelium: how bacterial recognition shapes intestinal function. Nat Rev Immunol. 2010;10:131–44. doi: 10.1038/nri2707. [DOI] [PubMed] [Google Scholar]
- 10.Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kiontke K, Sudhaus W. Ecology of Caenorhabditis species. WormBook 2006; 1-14; [DOI] [PMC free article] [PubMed]
- 12.Félix MA, Braendle C. The natural history of Caenorhabditis elegans. Curr Biol. 2010;20:R965–9. doi: 10.1016/j.cub.2010.09.050. [DOI] [PubMed] [Google Scholar]
- 13.Braendle C, Milloz J, Félix MA. Mechanisms and evolution of environmental responses in Caenorhabditis elegans. Curr Top Dev Biol. 2008;80:171–207. doi: 10.1016/S0070-2153(07)80005-6. [DOI] [PubMed] [Google Scholar]
- 14.Wolpert L. Principles of development. Oxford; New York: Oxford University Press, 2011. [Google Scholar]
- 15.Jones RE, Lopez KH. Chapter Three - The Menstrual Cycle. Human Reproductive Biology (Third Edition). San Diego: Academic Press, 2006:73-95. [Google Scholar]
- 16.Drummond-Barbosa D, Spradling AC. Stem cells and their progeny respond to nutritional changes during Drosophila oogenesis. Dev Biol. 2001;231:265–78. doi: 10.1006/dbio.2000.0135. [DOI] [PubMed] [Google Scholar]
- 17.Michaelson D, Korta DZ, Capua Y, Hubbard EJ. Insulin signaling promotes germline proliferation in C. elegans. Development. 2010;137:671–80. doi: 10.1242/dev.042523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dalfó D, Michaelson D, Hubbard EJ. Sensory regulation of the C. elegans germline through TGF-β-dependent signaling in the niche. Curr Biol. 2012;22:712–9. doi: 10.1016/j.cub.2012.02.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.LaFever L, Drummond-Barbosa D. Direct control of germline stem cell division and cyst growth by neural insulin in Drosophila. Science. 2005;309:1071–3. doi: 10.1126/science.1111410. [DOI] [PubMed] [Google Scholar]
- 20.Angelo G, Van Gilst MR. Starvation protects germline stem cells and extends reproductive longevity in C. elegans. Science. 2009;326:954–8. doi: 10.1126/science.1178343. [DOI] [PubMed] [Google Scholar]
- 21.Seidel HS, Kimble J. The oogenic germline starvation response in C. elegans. PLoS ONE. 2011;6:e28074. doi: 10.1371/journal.pone.0028074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chavarro J, Willett W, Skerrett PJ. The fertility diet. New York: McGraw-Hill, 2008. [Google Scholar]
- 23.Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schütz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P, et al. The nuclear receptor superfamily: the second decade. Cell. 1995;83:835–9. doi: 10.1016/0092-8674(95)90199-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Watson E, MacNeil LT, Arda HE, Zhu LJ, Walhout AJ. Integration of metabolic and gene regulatory networks modulates the C. elegans dietary response. Cell. 2013;153:253–66. doi: 10.1016/j.cell.2013.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kamiyama Y, Matsubara T, Yoshinari K, Nagata K, Kamimura H, Yamazoe Y. Role of human hepatocyte nuclear factor 4alpha in the expression of drug-metabolizing enzymes and transporters in human hepatocytes assessed by use of small interfering RNA. Drug Metab Pharmacokinet. 2007;22:287–98. doi: 10.2133/dmpk.22.287. [DOI] [PubMed] [Google Scholar]
- 26.Nakata K, Tanaka Y, Nakano T, Adachi T, Tanaka H, Kaminuma T, Ishikawa T. Nuclear receptor-mediated transcriptional regulation in Phase I, II, and III xenobiotic metabolizing systems. Drug Metab Pharmacokinet. 2006;21:437–57. doi: 10.2133/dmpk.21.437. [DOI] [PubMed] [Google Scholar]
- 27.Godfray HC, Beddington JR, Crute IR, Haddad L, Lawrence D, Muir JF, Pretty J, Robinson S, Thomas SM, Toulmin C. Food security: the challenge of feeding 9 billion people. Science. 2010;327:812–8. doi: 10.1126/science.1185383. [DOI] [PubMed] [Google Scholar]


