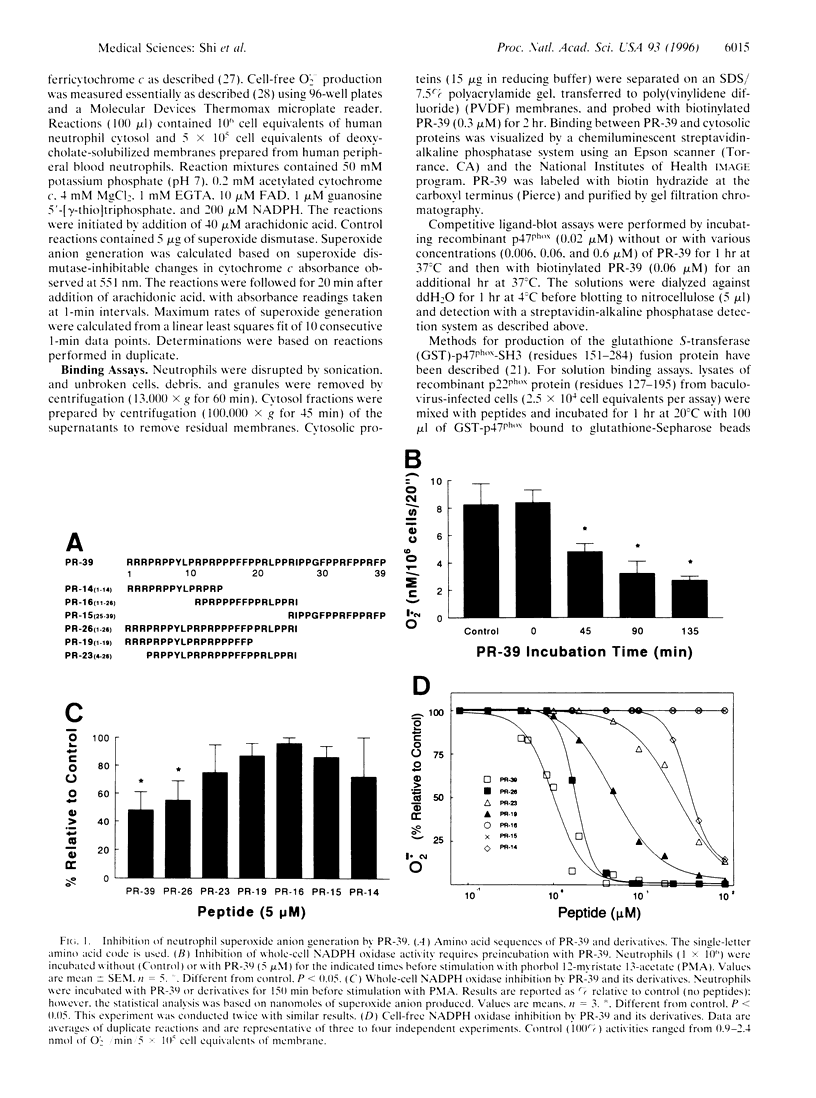
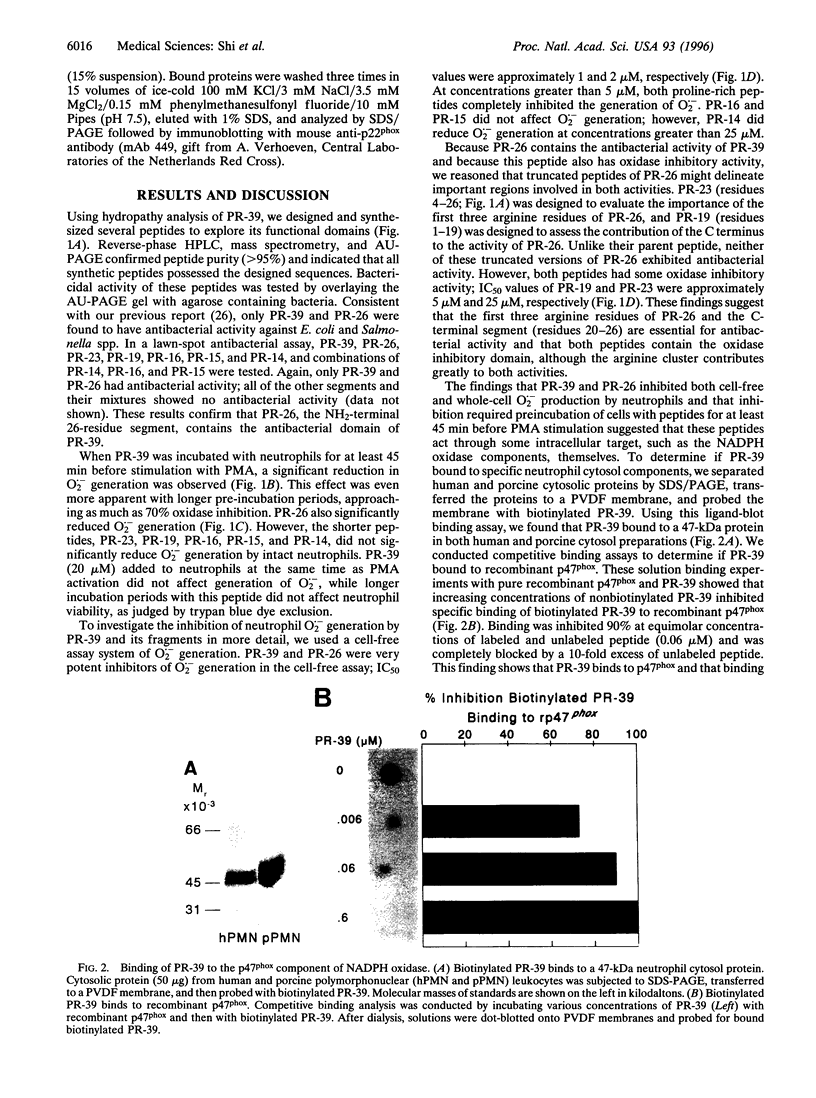
Abstract
Reactive oxygen intermediates generated by the phagocyte NADPH oxidase are critically important components of host defense. However, these highly toxic oxidants can cause significant tissue injury during inflammation; thus, it is essential that their generation and inactivation are tightly regulated. We show here that an endogenous proline-arginine (PR)-rich antibacterial peptide, PR-39, inhibits NADPH oxidase activity by blocking assembly of this enzyme through interactions with Src homology 3 domains of a cytosolic component. This neutrophil-derived peptide inhibited oxygen-dependent microbicidal activity of neutrophils in whole cells and in a cell-free assay of NADPH oxidase. Both oxidase inhibitory and direct antimicrobial activities were defined within the amino-terminal 26 residues of PR-39. Oxidase inhibition was attributed to binding of PR-39 to the p47phox cytosolic oxidase component. Its effects involve both a polybasic amino-terminal segment and a proline-rich core region of PR-39 that binds to the p47phox Src homology 3 domains and, thereby, inhibits interaction with the small subunit of cytochrome b558, p22phox. These findings suggest that PR-39, which has been shown to be involved in tissue repair processes, is a multifunctional peptide that can regulate NADPH oxidase production of superoxide anion O2-. thus limiting excessive tissue damage during inflammation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abo A., Boyhan A., West I., Thrasher A. J., Segal A. W. Reconstitution of neutrophil NADPH oxidase activity in the cell-free system by four components: p67-phox, p47-phox, p21rac1, and cytochrome b-245. J Biol Chem. 1992 Aug 25;267(24):16767–16770. [PubMed] [Google Scholar]
- Agerberth B., Lee J. Y., Bergman T., Carlquist M., Boman H. G., Mutt V., Jörnvall H. Amino acid sequence of PR-39. Isolation from pig intestine of a new member of the family of proline-arginine-rich antibacterial peptides. Eur J Biochem. 1991 Dec 18;202(3):849–854. doi: 10.1111/j.1432-1033.1991.tb16442.x. [DOI] [PubMed] [Google Scholar]
- Boman H. G., Agerberth B., Boman A. Mechanisms of action on Escherichia coli of cecropin P1 and PR-39, two antibacterial peptides from pig intestine. Infect Immun. 1993 Jul;61(7):2978–2984. doi: 10.1128/iai.61.7.2978-2984.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boman H. G. Peptide antibiotics and their role in innate immunity. Annu Rev Immunol. 1995;13:61–92. doi: 10.1146/annurev.iy.13.040195.000425. [DOI] [PubMed] [Google Scholar]
- Cabiaux V., Agerberth B., Johansson J., Homblé F., Goormaghtigh E., Ruysschaert J. M. Secondary structure and membrane interaction of PR-39, a Pro+Arg-rich antibacterial peptide. Eur J Biochem. 1994 Sep 15;224(3):1019–1027. doi: 10.1111/j.1432-1033.1994.01019.x. [DOI] [PubMed] [Google Scholar]
- DeLeo F. R., Nauseef W. M., Jesaitis A. J., Burritt J. B., Clark R. A., Quinn M. T. A domain of p47phox that interacts with human neutrophil flavocytochrome b558. J Biol Chem. 1995 Nov 3;270(44):26246–26251. doi: 10.1074/jbc.270.44.26246. [DOI] [PubMed] [Google Scholar]
- Demling R. H. Current concepts on the adult respiratory distress syndrome. Circ Shock. 1990 Apr;30(4):297–309. [PubMed] [Google Scholar]
- Derossi D., Joliot A. H., Chassaing G., Prochiantz A. The third helix of the Antennapedia homeodomain translocates through biological membranes. J Biol Chem. 1994 Apr 8;269(14):10444–10450. [PubMed] [Google Scholar]
- Fawell S., Seery J., Daikh Y., Moore C., Chen L. L., Pepinsky B., Barsoum J. Tat-mediated delivery of heterologous proteins into cells. Proc Natl Acad Sci U S A. 1994 Jan 18;91(2):664–668. doi: 10.1073/pnas.91.2.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng S., Chen J. K., Yu H., Simon J. A., Schreiber S. L. Two binding orientations for peptides to the Src SH3 domain: development of a general model for SH3-ligand interactions. Science. 1994 Nov 18;266(5188):1241–1247. doi: 10.1126/science.7526465. [DOI] [PubMed] [Google Scholar]
- Finan P., Shimizu Y., Gout I., Hsuan J., Truong O., Butcher C., Bennett P., Waterfield M. D., Kellie S. An SH3 domain and proline-rich sequence mediate an interaction between two components of the phagocyte NADPH oxidase complex. J Biol Chem. 1994 May 13;269(19):13752–13755. [PubMed] [Google Scholar]
- Gabay J. E. Ubiquitous natural antibiotics. Science. 1994 Apr 15;264(5157):373–374. doi: 10.1126/science.8153623. [DOI] [PubMed] [Google Scholar]
- Gallo R. L., Ono M., Povsic T., Page C., Eriksson E., Klagsbrun M., Bernfield M. Syndecans, cell surface heparan sulfate proteoglycans, are induced by a proline-rich antimicrobial peptide from wounds. Proc Natl Acad Sci U S A. 1994 Nov 8;91(23):11035–11039. doi: 10.1073/pnas.91.23.11035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger D. N., Korthuis R. J. Physiologic mechanisms of postischemic tissue injury. Annu Rev Physiol. 1995;57:311–332. doi: 10.1146/annurev.ph.57.030195.001523. [DOI] [PubMed] [Google Scholar]
- Joseph G., Gorzalczany Y., Koshkin V., Pick E. Inhibition of NADPH oxidase activation by synthetic peptides mapping within the carboxyl-terminal domain of small GTP-binding proteins. Lack of amino acid sequence specificity and importance of polybasic motif. J Biol Chem. 1994 Nov 18;269(46):29024–29031. [PubMed] [Google Scholar]
- Lehrer R. I., Lichtenstein A. K., Ganz T. Defensins: antimicrobial and cytotoxic peptides of mammalian cells. Annu Rev Immunol. 1993;11:105–128. doi: 10.1146/annurev.iy.11.040193.000541. [DOI] [PubMed] [Google Scholar]
- Leto T. L., Adams A. G., de Mendez I. Assembly of the phagocyte NADPH oxidase: binding of Src homology 3 domains to proline-rich targets. Proc Natl Acad Sci U S A. 1994 Oct 25;91(22):10650–10654. doi: 10.1073/pnas.91.22.10650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leto T. L., Garrett M. C., Fujii H., Nunoi H. Characterization of neutrophil NADPH oxidase factors p47-phox and p67-phox from recombinant baculoviruses. J Biol Chem. 1991 Oct 15;266(29):19812–19818. [PubMed] [Google Scholar]
- Lim W. A., Richards F. M., Fox R. O. Structural determinants of peptide-binding orientation and of sequence specificity in SH3 domains. Nature. 1994 Nov 24;372(6504):375–379. doi: 10.1038/372375a0. [DOI] [PubMed] [Google Scholar]
- Malech H. L., Gallin J. I. Current concepts: immunology. Neutrophils in human diseases. N Engl J Med. 1987 Sep 10;317(11):687–694. doi: 10.1056/NEJM198709103171107. [DOI] [PubMed] [Google Scholar]
- Mann D. A., Frankel A. D. Endocytosis and targeting of exogenous HIV-1 Tat protein. EMBO J. 1991 Jul;10(7):1733–1739. doi: 10.1002/j.1460-2075.1991.tb07697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin E., Ganz T., Lehrer R. I. Defensins and other endogenous peptide antibiotics of vertebrates. J Leukoc Biol. 1995 Aug;58(2):128–136. doi: 10.1002/jlb.58.2.128. [DOI] [PubMed] [Google Scholar]
- Martínez-Cayuela M. Oxygen free radicals and human disease. Biochimie. 1995;77(3):147–161. doi: 10.1016/0300-9084(96)88119-3. [DOI] [PubMed] [Google Scholar]
- McPhail L. C., Qualliotine-Mann D., Agwu D. E., McCall C. E. Phospholipases and activation of the NADPH oxidase. Eur J Haematol. 1993 Nov;51(5):294–300. doi: 10.1111/j.1600-0609.1993.tb01611.x. [DOI] [PubMed] [Google Scholar]
- McPhail L. C. SH3-dependent assembly of the phagocyte NADPH oxidase. J Exp Med. 1994 Dec 1;180(6):2011–2015. doi: 10.1084/jem.180.6.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotrosen D., Yeung C. L., Leto T. L., Malech H. L., Kwong C. H. Cytochrome b558: the flavin-binding component of the phagocyte NADPH oxidase. Science. 1992 Jun 5;256(5062):1459–1462. doi: 10.1126/science.1318579. [DOI] [PubMed] [Google Scholar]
- Schreck R., Rieber P., Baeuerle P. A. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-kappa B transcription factor and HIV-1. EMBO J. 1991 Aug;10(8):2247–2258. doi: 10.1002/j.1460-2075.1991.tb07761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selsted M. E., Ouellette A. J. Defensins in granules of phagocytic and non-phagocytic cells. Trends Cell Biol. 1995 Mar;5(3):114–119. doi: 10.1016/s0962-8924(00)88961-8. [DOI] [PubMed] [Google Scholar]
- Shasby D. M., Vanbenthuysen K. M., Tate R. M., Shasby S. S., McMurtry I., Repine J. E. Granulocytes mediate acute edematous lung injury in rabbits and in isolated rabbit lungs perfused with phorbol myristate acetate: role of oxygen radicals. Am Rev Respir Dis. 1982 Apr;125(4):443–447. doi: 10.1164/arrd.1982.125.4.443. [DOI] [PubMed] [Google Scholar]
- Shi J., Goodband R. D., Chengappa M. M., Nelssen J. L., Tokach M. D., McVey D. S., Blecha F. Influence of interleukin-1 on neutrophil function and resistance to Streptococcus suis in neonatal pigs. J Leukoc Biol. 1994 Jul;56(1):88–94. doi: 10.1002/jlb.56.1.88. [DOI] [PubMed] [Google Scholar]
- Shi J., Ross C. R., Chengappa M. M., Blecha F. Identification of a proline-arginine-rich antibacterial peptide from neutrophils that is analogous to PR-39, an antibacterial peptide from the small intestine. J Leukoc Biol. 1994 Dec;56(6):807–811. doi: 10.1002/jlb.56.6.807. [DOI] [PubMed] [Google Scholar]
- Shi J., Ross C. R., Chengappa M. M., Sylte M. J., McVey D. S., Blecha F. Antibacterial activity of a synthetic peptide (PR-26) derived from PR-39, a proline-arginine-rich neutrophil antimicrobial peptide. Antimicrob Agents Chemother. 1996 Jan;40(1):115–121. doi: 10.1128/aac.40.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumimoto H., Kage Y., Nunoi H., Sasaki H., Nose T., Fukumaki Y., Ohno M., Minakami S., Takeshige K. Role of Src homology 3 domains in assembly and activation of the phagocyte NADPH oxidase. Proc Natl Acad Sci U S A. 1994 Jun 7;91(12):5345–5349. doi: 10.1073/pnas.91.12.5345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taffs R. E., Sitkovsky M. V. Modulation of the effector functions of cytolytic T-lymphocytes with synthetic peptide inhibitors of protein kinases. J Pharm Sci. 1992 Jan;81(1):37–44. doi: 10.1002/jps.2600810108. [DOI] [PubMed] [Google Scholar]
- Vallette F. M., Juin P., Pelleschi M., Henry J. P. Basic peptides can be imported into yeast mitochondria by two distinct targeting pathways. Involvement of the peptide-sensitive channel of the outer membrane. J Biol Chem. 1994 May 6;269(18):13367–13374. [PubMed] [Google Scholar]
- Zasloff M. Antibiotic peptides as mediators of innate immunity. Curr Opin Immunol. 1992 Feb;4(1):3–7. doi: 10.1016/0952-7915(92)90115-u. [DOI] [PubMed] [Google Scholar]
- de Mendez I., Adams A. G., Sokolic R. A., Malech H. L., Leto T. L. Multiple SH3 domain interactions regulate NADPH oxidase assembly in whole cells. EMBO J. 1996 Mar 15;15(6):1211–1220. [PMC free article] [PubMed] [Google Scholar]