Abstract
In depression, the ability to experience daily life positive affect predicts recovery and reduces relapse rates. Interventions based on the experience sampling method (ESM-I) are ideally suited to provide insight in personal, contextualized patterns of positive affect. The aim of this study was to examine whether add-on ESM-derived feedback on personalized patterns of positive affect is feasible and useful to patients, and results in a reduction of depressive symptomatology. Depressed outpatients (n=102) receiving pharmacological treatment participated in a randomized controlled trial with three arms: an experimental group receiving add-on ESM-derived feedback, a pseudo-experimental group participating in ESM but receiving no feedback, and a control group. The experimental group participated in an ESM procedure (three days per week over a 6-week period) using a palmtop. This group received weekly standardized feedback on personalized patterns of positive affect. Hamilton Depression Rating Scale – 17 (HDRS) and Inventory of Depressive Symptoms (IDS) scores were obtained before and after the intervention. During a 6-month follow-up period, five HDRS and IDS assessments were completed. Add-on ESM-derived feedback resulted in a significant and clinically relevant stronger decrease in HDRS score relative to the control group (p<0.01; −5.5 point reduction in HDRS at 6 months). Compared to the pseudo-experimental group, a clinically relevant decrease in HDRS score was apparent at 6 months (B=−3.6, p=0.053). Self-reported depressive complaints (IDS) yielded the same pattern over time. The use of ESM-I was deemed acceptable and the provided feedback easy to understand. Patients attempted to apply suggestions from ESM-derived feedback to daily life. These data suggest that the efficacy of traditional passive pharmacological approach to treatment of major depression can be enhanced by using person-tailored daily life information regarding positive affect.
Keywords: Ecological momentary assessment, experience sampling method, intervention study, psychological feedback, depressive disorder, positive affect
According to the World Health Organization, depression is among the leading causes of disability 1. Improving the efficacy of pharmacotherapy and psychotherapy is considered a priority. Enlarging the window of observation of depressive symptomatology to out-of-the-office situations could result in a more detailed and personalized assessment of contextual influences on symptomatology, and hence may add to the effect of existing treatments.
Self-monitoring, comprising once-a-day retrospective paper-pencil assessments of mood, has been shown to reduce depressive symptomatology 2–4. However, because retrospectively obtained self-assessments are biased by mood-congruent emotional and cognitive biases 5, the use of prospective in-the-moment daily life assessments may be used to improve reliability, providing a much more fine-grained film of the dynamics of depressive symptomatology, which may aid in optimizing treatment decisions. Furthermore, digital instead of paper-and-pencil assessments have the advantage that data are immediately available.
Digitalized prospective, in-the-moment monitoring is commonly used in medical disciplines. Continuous monitoring, for example, is used in the treatment of hypertension and diabetes (i.e., 24h blood pressure or plasma glucose monitoring). In the field of mental health, however, this area remains to be explored. For mental health outcomes, the equivalent of mobile ambulatory assessment of medical outcomes has recently become available in the form of electronic momentary assessment techniques. These techniques represent the combination of experience sampling methodology (ESM) with new electronic tools, such as the PsyMate 6, allowing for direct electronic recording of the data. ESM consists of repeated assessments of affective experience and context in the flow of daily life 7–9.
Until recently, ESM has been used only in the context of research to identify moment-to-moment patterns and mechanisms of psychopathology 10–14. With the advent of personal digital assistants (PDAs) and web-based applications, however, real-life data are immediately available to patients and professional caregivers. This creates the possibility for ESM interventions (ESM-I) that can transform implicit real-life dynamic patterns to explicit, visualized and quantifiable configurations, through which dysfunctional patterns become modifiable. ESM-I has the additional benefit that it can be easily implemented in standard mental health care and does not require much additional investment of clinicians 6,11. Therefore, ESM-I constitutes a new viable approach to improve personalized mental health care and stands to become a widely used mobile-health tool in clinical practice 10–13,15.
A new and exciting development is the use of real-life self-monitoring with ESM-I in depressed patients to gain insight in personalized patterns of positive affect and the context in which they are experienced. Numerous recent studies 16–19 have shown the importance of the reward system and positive affect experience in resilience against depression. It was demonstrated that especially positive affect – more than its counterpart, negative affect – is crucial and necessary in predicting recovery from depression 20–23. Furthermore, a recent randomized controlled trial showed that allocation to an intervention that increased real-life positive affect experience was associated with a significant decline in depressive symptoms 22. Therefore, the next step in the treatment of depression is to examine whether self-monitoring can be used as an intervention to increase insights in personalized patterns of positive affect. Personalized feedback focused on positive affect and its context may help both the patient and the professional carer in their search for custom opportunities to increase the experience of that affect, thus enabling recovery from depression.
Although the above arguments suggest that ESM-I represents a novel approach with a potential to improve treatment in mental health care, feasibility and patient preference need to be considered as well. There is a need to know how patients experience this procedure and whether they are able and willing to participate.
Therefore, the aims of the current study were to examine whether: a) ESM-derived personalized feedback can be used, in combination with standard antidepressant medication, as an effective add-on treatment for depressive symptoms designed to increase patients' resources with regard to positive affective experience; b) ESM-I is considered feasible and useful by patients.
To our knowledge, this is the first randomized controlled trial using ESM as a novel therapeutic intervention in depressed patients, with a view to improve personalized treatment.
METHODS
Participants and design
Consecutively presenting depressed outpatients attending mental health care facilities serving the catchment areas of the Dutch cities of Eindhoven and Maastricht were approached by their health care professionals and recruited into the study. In addition, recruitment in the same catchment areas was also carried out independent of contact with mental health care services by distributing posters and flyers in health care facilities and local media. Recruitment occurred between January 2010 and February 2012.
Inclusion criteria were: age 18-65 years; a DSM-IV-TR diagnosis of depressive episode with a current total score on the Hamilton Depression Rating Scale - 17 (HDRS) 24 of at least 8 (i.e., above remission cut-off and including residual depressive states); receiving pharmacological treatment with antidepressants or mood stabilizers; adequate vision; sufficient Dutch language skills; no current or lifetime diagnosis of non-affective psychotic disorder, and no (hypo)manic or mixed episode within the past month.
The study protocol was approved by the Medical Ethics Committee of Maastricht University Medical Centre. Informed consent was obtained from all participants. The trial was registered at Netherlands Trial Register (Identifier: NTR1974).
A randomized controlled trial was conducted with three treatment arms. After baseline, patients were randomly allocated to the experimental, pseudo-experimental or control group. In addition to treatment as usual (TAU), the experimental group participated in an ESM procedure (three days per week over a 6-week period) using a palmtop. This group received weekly standardized feedback on personalized patterns of positive affect. Feedback was given to both the patient and the mental health professional. The pseudo-experimental group also participated in the ESM procedure (three days per week over a 6-week period) in addition to TAU, but without feedback. The control group received no additional intervention during TAU.
Randomization (allocation ratio 1:1:1) was stratified according to duration of antidepressant pharmacotherapy (new/switch vs. maintenance, i.e. receiving antidepressant or mood stabilizing medication for less vs. longer than 8 weeks prior to study entry), and current psychotherapy (yes or no). The randomization sequence in blocks of six (using the sequence generator on the Internet site http://random.org) was generated by the first author of this paper. An independent research assistant wrote the randomization code into sealed numbered envelopes. After completion of all baseline assessments, the interviewer allocated participants to their treatment condition based on the randomization code in the sealed envelope (opened in order of sequence). Interviewers were not blind to the patients' treatment allocation.
Procedure
Figure 1 shows participant flow and procedure throughout the trial. The study protocol consisted of a telephone interview, a screening, a baseline assessment (week 0), a six-week intervention period (weeks 1 to 6), a post assessment (week 7), and five follow-up assessments (at weeks 8, 12, 16, 20, 32). From baseline (week 0) onward, the overall study duration was 32 weeks.
Figure 1.
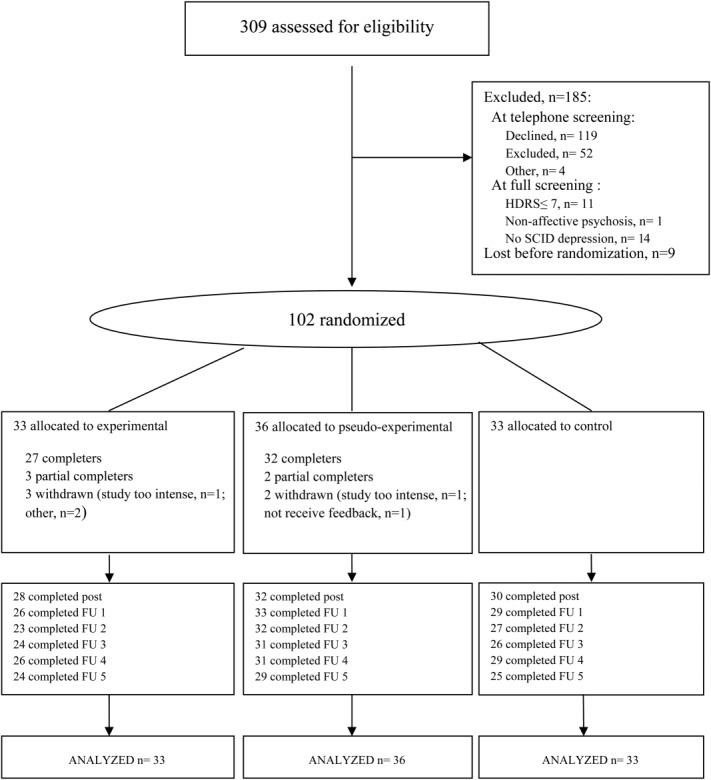
Flowchart of the study. HDRS - Hamilton Depression Rating Scale - 17, SCID - Structured Clinical Interview for DSM-IV Axis I Disorders, post - immediate post assessment, FU - follow-up assessment
The recruitment process started with a short telephone interview conducted by a psychologist or psychiatrist to establish whether inclusion criteria were likely met. During the screening, the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I) 25, the HDRS, and the 30-item Inventory of Depressive Symptoms (IDS-SR) 26 were administered. The HDRS semi-structured interview and the IDS self-report questionnaire, both assessing the severity of depressive symptoms, were completed at baseline, at post assessment and at follow-up. The IDS was used as a measure that is independent from the interpretation of the interviewer. ESM assessments took place as part of the baseline assessment (week 0), during the 6-week intervention period (weeks 1 to 6), and at the post assessment (week 7). The feasibility of the ESM measurement procedure and the desirability of the ESM-derived feedback on positive affect were evaluated through specific questions, with items rated on 7-point Likert scales (1=“not at all” to 7=“very”).
ESM was carried out according to previous studies 7,27–29. The recently developed PsyMate, a palmtop, was used to digitally collect daily life momentary assessments of positive affect in relation to momentary context and activity. The PsyMate was programmed to emit a beep 10 times per day at random intervals in each of ten 90-min time blocks between 7.30 and 22.30. At each beep, participants used the PsyMate to digitally complete a brief beep-questionnaire including current affect (four positive affect and six negative affect items) as well as current context and activities (“daily life activities”, “persons present”, “physical activity”, and “events”). PsyMate positive affect indicators included the adjectives “cheerful”, “satisfied”, “enthusiastic” and “relaxed” 22. Negative affect was indexed by the adjectives “down”, “suspicious”, “guilty”, “irritated”, “lonely” and “anxious”. The self-assessments were rated on 7-point Likert scales (ranging from 1=“not at all” to 7=“very”). Participants were instructed to complete the beep-questionnaire as quickly as possible after the beep.
During both the 5-day ESM baseline assessment and the 5-day ESM post assessment, 10 beep-questionnaires were generated per day. The total number of beep-questionnaires therefore was 50 for both the ESM baseline and ESM post assessments. During the 6-week intervention period, participants completed 10 beep-questionnaires per day for three consecutive days (10 × 3 × 6=180 beep-questionnaires).
The ESM procedure was explained in an initial briefing session, and a practice run was performed to ensure that the participants understood the questions and the device. The debriefing to assess aspects of feasibility of the ESM procedure with PsyMate was scheduled immediately after the ESM baseline assessment.
Intervention
The experimental group received standardized ESM-derived feedback. Feedback sessions immediately followed the weekly ESM procedure. In these face-to-face sessions, feedback was provided by the researcher (a psychologist or psychiatrist). The feedback on participants' momentary affective state in specific daily life contexts and the association with depressive symptoms was given verbally, in writing and graphically (Figure 2). Feedback showed actual levels of positive affect (the mean of the items “cheerful”, “satisfied”, “enthusiastic” and “relaxed”) in the context of daily life activities (Figure 2a and 2b), events and social situations. The second part of the feedback showed changes in positive affect level (Figure 2c) and the number of depressive complaints over the course of the ESM intervention.
Figure 2.
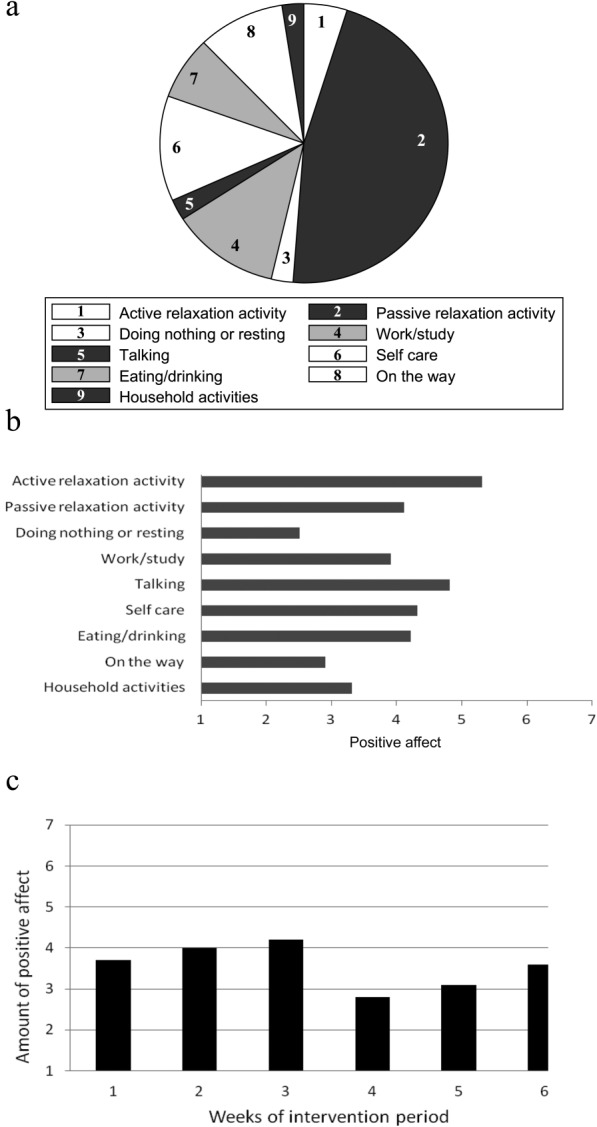
Examples of feedback graphs. (a) Amount of time spent doing different types of activities. (b) Amount of positive affect experienced per type of activity. (c) Mean level of positive affect over the 6-weeks intervention period
The ESM-derived feedback was divided in three modules. In each module, a novel element of feedback was added cumulatively. The first feedback sessions (1 and 2) were focused on positive affect experienced during activities. Feedback sessions 3 and 4 additionally focused on positive affect experienced after daily events, differentiating between affect experienced during events appraised with an internal vs. external locus of control. Finally, feedback sessions 5 and 6 additionally focused on positive affect experienced during social interactions in daily life.
Participants' opinion about the feedback procedures was evaluated at the post assessment.
The pseudo-experimental group was similar in procedure to the experimental group except that no feedback was given. To prevent any effects of different duration of the sessions, this group's sessions were filled with an alternative activity (an additional HDRS interview).
Statistical analysis
Statistical analyses were conducted using STATA 12.1 30. The data had a hierarchical structure, because multiple assessments of HDRS and IDS depressive symptoms were clustered within patients.
First, to examine the impact of treatment allocation on course of depressive symptoms, mean HDRS total scores were plotted over time (in weeks from baseline to last follow-up) for each of the three groups. The best fit was provided by a linear model (time) for the experimental and control group and a polynomial model (time and time2) for the pseudo-experimental group.
Next, the XTMIXED command was used to perform a multilevel regression analysis with the two-way interaction between time (in weeks) and treatment allocation as fixed effects, patients as random intercept and a random slope for time. The covariance was set to unstructured. The LINCOM command was used to calculate estimated between-group effects. A difference of three or more points on the HDRS was a priori considered as clinically relevant 31,32.
Power calculations using the STATA 30 SAMPSI command were based on previous work 33, and led to an initial sample size of 120 with a power of 84% to detect a 3-point difference in HDRS score 31,32. However, because many participants were excluded, inclusion rate was lower than expected. The eventual number of patients who participated in the trial was 102.
RESULTS
The characteristics of the included subject sample at screening are shown in Table1. There were no large or significant differences in socio-demographic characteristics between the groups, but at screening there were some differences in clinical features. Compared to the pseudo-experimental and control group, patients in the experimental group used lithium more often and displayed lower HDRS and IDS total scores (Table1). Group differences in HDRS and IDS or lithium use were non-significant at baseline (two weeks later, i.e., just before start of the intervention) (F(2;98)=1.00, p=0.37; F(2;98)=1.52, p=0.22, and χ2(2)=4.65, p=0.10, respectively).
Table 1.
Demographic and clinical characteristics of the study sample at screening
| Total (n=102) | Experimental (n=33) | Pseudo-experimental (n=36) | Controls (n=33) | Test parameter | df | p | |
|---|---|---|---|---|---|---|---|
| Age (mean±SD) | 48.0±10.2 | 48.7±10.2 | 46.7±9.6 | 48.9±10.9 | χ2=2.06 | 2 | 0.36 |
| Sex (M/F) | 46/56 | 17/16 | 14/22 | 15/18 | χ2=1.11 | 2 | 0.57 |
| Educational level | |||||||
| Low | 25 | 6 | 9 | 10 | |||
| Middle | 38 | 12 | 14 | 12 | χ2=1.73 | 4 | 0.79 |
| High | 39 | 15 | 13 | 11 | |||
| Full or part-time work | 35 | 13 | 10 | 12 | χ2=1.12 | 2 | 0.57 |
| Living with partner/own family | 53 | 18 | 17 | 18 | χ2=0.50 | 2 | 0.78 |
| Bipolar disorder | 9 | 5 | 2 | 2 | χ2=2.43 | 2 | 0.39 |
| DSM-IV Axis I comorbidity | 40 | 12 | 16 | 12 | χ2=0.64 | 2 | 0.73 |
| HDRS total score (mean±SD) | 15.8±4.6 | 14.1±4.5 | 16.2±4.8 | 17.0±4.3 | F=3.64 | 2;99 | 0.03 |
| IDS total score (mean±SD) | 36.2±10.4 | 32.9±10.2 | 36.4±10.0 | 39.2±10.5 | F=3.19 | 2;99 | 0.045 |
| GAF symptoms (mean±SD) | 56.3±7.7 | 58.0±7.5 | 55.9±7.6 | 55.0±7.8 | F=1.35 | 2;95 | 0.26 |
| GAF disability (mean±SD) | 54.6±10.9 | 54.0±10.5 | 55.9±11.5 | 53.9±10.9 | F=0.34 | 2;95 | 0.71 |
| Antidepressant | |||||||
| Start/switch | 19 | 5 | 6 | 8 | χ2=1.04 | 2 | 0.66 |
| Maintenance | 83 | 28 | 30 | 25 | |||
| Current use of benzodiazepines | 30 | 7 | 10 | 13 | χ2=2.70 | 2 | 0.27 |
| Current use of antipsychotics | 26 | 6 | 8 | 12 | χ2=3.18 | 2 | 0.24 |
| Current use of hypnotics | 22 | 5 | 9 | 8 | χ2=1.19 | 2 | 0.55 |
| Current use of lithium | 11 | 7 | 1 | 3 | χ2=6.23 | 2 | 0.049 |
| Current psychotherapy | 10 | 4 | 4 | 2 | χ2=0.77 | 2 | 0.77 |
HDRS – Hamilton Depression Rating Scale, IDS – Inventory of Depressive Symptoms, GAF – Global Assessment of Functioning
Educational level – low: no/primary/low secondary, middle: high school/low vocational, high: higher vocational/university
Of the 102 randomized patients, 93 completed at least one HDRS assessment during the post-intervention assessment period of approximately 6 months. There were no large or significant between-group differences in completion of at least one HDRS assessment during this period (χ2(2)= 0.93, p=0.62). Similar findings were obtained for the IDS (χ2(2)= 0.93, p=0.62).
Of the 69 patients allocated to the experimental or pseudo-experimental group, 59 (85.5%) completed the 6-week intervention period, comprising 6 × 3 ESM assessment days and six corresponding intervention sessions. There was no large or significant difference in baseline depressive symptoms between patients who fully completed the intervention period and those who did not (HDRS: B=0.76, p=0.72; IDS: B=1.03, p=0.80). The average number of completed beep-questionnaires in these 59 patients was 135.5±16.5 out of 180, indicating a completion rate of 75.3%. There were no significant differences between the experimental vs. the pseudo-experimental group in either the mean number of completed beep-questionnaires over the entire intervention period (t=0.91, df=57, p=0.18), or the number of patients who completed all six intervention sessions (χ2(1)=0.69, p=0.50). Feedback sessions lasted significantly longer (mean: 48.9±11.2 min, range 27-105 min) compared to the pseudo-experimental interview sessions (mean: 39.5±12.9 min, range 15–90 min) (B=9.57, p<0.001).
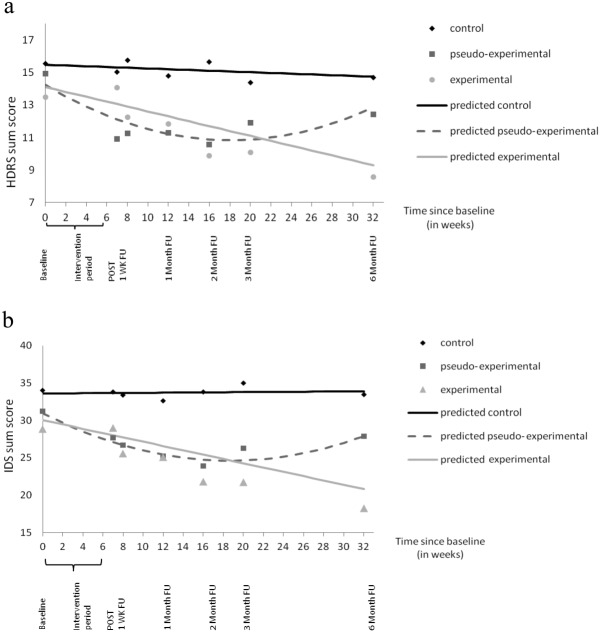
Figure 3 displays the results of the multilevel regression analysis of the interaction between treatment allocation and time on HDRS and IDS scores. The experimental group demonstrated a significantly greater weekly decline in depressive symptoms over the complete study period compared to the control group (HDRS: B=−0.15, p<0.001; IDS: B=−0.29, p=0.002). Between-group comparisons demonstrated that the decline in depressive symptoms in the experimental group compared to the control group became significant at week 8 (IDS) and 11 (HDRS) and lasted until the end of the study (week 32). Over time, differences between the experimental and control group became stronger, reaching a −5.5 HDRS point difference and a −13.1 IDS point difference at week 32.
Figure 3.
Mean depression scores and predicted lines plotted over time. (a) Hamilton Depression Rating Scale (HDRS). (b) Inventory of Depressive Symptoms (IDS). POST – immediate post assessment, FU – follow-up assessment
The pseudo-experimental group followed a different pattern: it displayed significantly lower HDRS and IDS scores compared to the control group, starting directly after the intervention period (week 7). However, the initial decrease in depressive symptoms did not persist until the last assessment: after week 26 (HDRS) and week 28 (IDS), the difference in depressive symptoms between the pseudo-experimental group and the control group was no longer significant (Figure 3). Compared to the pseudo-experimental group, patients in the experimental group demonstrated clinically relevant lower HDRS scores, a priori defined as a decrease of 3 or more points 31,32, at the end of the study (weeks 31 and 32) (B=−3.1, p=0.08 and B=−3.6, p=0.053, respectively).
Patients in the control group did not demonstrate a change in HDRS and IDS scores over the course of the study (B=–0.02, p=0.56, and B=0.01, p=0.92, respectively).
Table2 displays the results of the patient estimated feasibility of ESM-I. Results indicated that the procedure was not very stressful with respect to number of beeps per day, time to fill out a beep-questionnaire, or sound of the beep. Instructions on how to use the PsyMate were rated as very clear. Table2 also displays the results regarding participants' opinions on ESM-derived feedback, indicating that feedback on positive affect was relatively easy to understand. Also, participants appreciated getting ESM-derived feedback and tried to apply the suggestions from the feedback to their daily lives.
Table 2.
Patient estimated feasibility of the procedure and opinions on the feedback
| Mean (min-max) (scale 1-7) | SD | N (subjects) | ||
|---|---|---|---|---|
| Feasibility of the procedure | ||||
| Was the text on screen readable? | 5.8 (2-7) | 1.4 | 102 | |
| Was it difficult to switch PsyMate on? | 1.6 (1-6) | 1.2 | 102 | |
| Was the PsyMate difficult to control? | 1.4 (1-5) | 0.8 | 102 | |
| Were the verbal instructions you received about using the PsyMate clear? | 6.6 (3-7) | 0.7 | 102 | |
| Were the written instructions you received with the PsyMate clear? | 6.5 (1-7) | 1.0 | 96 | |
| Were the questions you answered on the PsyMate difficult or unclear? | 2.6 (1-6) | 1.5 | 102 | |
| Did you find it annoying or stressful to use the PsyMate? | ||||
| With respect to the number of beeps per day? | 3.1 (1-7) | 1.6 | 102 | |
| With respect to the time it took to answer the questions for a single beep? | 2.5 (1-7) | 1.5 | 102 | |
| With respect to the noises/sound volume? | 2.0 (1-7) | 1.5 | 102 | |
| ESM-derived feedback | ||||
| I found it easy to understand the explanation given with the feedback | 6.1 (4-7) | 0.6 | 25 | |
| The researcher was able to answer my questions well when there was something I didn't understand | 6.4 (6-7) | 0.5 | 24 | |
| I was annoyed that I wasn't allowed to get answers about (help) questions that were about my specific problems | 2.3 (1-6) | 1.6 | 22 | |
| Would you like to have received more specific advice following the feedback you were given? | 3.2 (1-6) | 2.0 | 25 | |
| I appreciated getting a summary of the feedback | 6.2 (5-7) | 0.7 | 25 | |
| I found it easy to understand the feedback summary | 6.2 (3-7) | 0.9 | 25 | |
| I was happy to get feedback in the form of graphs | 6.5 (4-7) | 0.7 | 24 | |
| I found it easy to understand the information in the graphs | 6.3 (3-7) | 0.9 | 25 | |
| I have tried to apply the suggestions from the feedback in my daily life | 5.4 (3-7) | 1.1 | 25 | |
| The amount of information in the feedback was exactly right | 5.4 (4-7) | 0.9 | 25 | |
| The duration of the contact reserved for feedback was exactly right | 6.2 (4-7) | 0.4 | 25 | |
DISCUSSION
This study shows that the use of add-on momentary assessment technology may be effective as a therapeutic tool to complement standard antidepressant treatment. Allocation to the intervention group with ESM-derived feedback on positive affect was associated with a linear decrease in HDRS depressive symptoms over time that persisted until the last follow-up six months later. The difference with the pseudo-experimental group was clinically relevant and borderline significant.
Although the use of ESM-derived feedback in the treatment of depression has been suggested before 8,10,11,34,35, the present endeavor is, to our knowledge, the first randomized controlled trial that systematically examined ESM-I as a therapeutic tool to provide depressed patients with insight into personalized patterns of positive affect. Relative to receiving passive pharmacological treatment only, depressed patients who received additional feedback on personalized opportunities for positive affect demonstrated a clinically relevant and persistent decrease in depressive symptomatology. This could reflect increased insight and accompanying change towards behavioral patterns increasing positive affect. In contrast, the effects in the pseudo-experimental group (self-monitoring without feedback) did not appear to persist. Because these patients may have thought that they were receiving the experimental intervention, this could reflect a placebo response. Another speculation is that these findings result from a short-lived behavioral activation effect attributable to the weekly in-the-office appointments in the intervention period. Moreover, these appointments, in which patients had the opportunity to share their depressive feelings, may have been experienced as more supportive than the experimental feedback appointments, which would explain the stronger immediate reduction in symptoms in this group. Finally, although speculative, the effect of continuous self-monitoring (i.e. without ESM-derived feedback) on depressive symptoms may also be explained by an increased momentary emotional awareness 36. This may make ESM-I an interesting tool to use in mindfulness based cognitive therapy, as suggested by Telford et al 36.
Although interventions based on momentary assessment technology have been developed for several mental disorders and health promoting behaviors 15,37–43, actual implementation in mental health care is still limited 12. Examples are interventions directed to practice anxiety reducing techniques 39, remind patients to use previously learned skills 43, or remind patients about medication adherence 41. Interventions that provided insights derived from momentary assessments were developed for attention deficit-hyperactivity disorder 38 and migraine attacks 44.
Mild to severely depressed patients 45,46 were able and motivated to complete ESM measurements for a longer period of time (18 days), and became actively involved in their recovery process by trying to implement suggestions derived from ESM-feedback into their personal daily life. So, the current results suggest that ESM interventions as an add-on treatment may be both feasible and effective for patients suffering from mild to severe depressive disorder, including residual depressive states that are associated with substantial morbidity 47–52.
A first limitation of this study is that neither patients nor researchers were blind with regard to treatment allocation. If knowledge of allocation resulted in biased depression ratings by the patient one would, in contrast to the current results, expect that the experimental group (relative to the control group) demonstrated the largest decrease in HDRS depression at the post-intervention assessment. Knowledge of allocation by researchers did not result in biased HDRS depression ratings, because analyses using the IDS self-rating depression scale yielded similar results. Second, although we showed clinically significant effect size differences between the experimental and the pseudo-experimental group, these differences were not conclusive by conventional alpha. This may relate to the fact that the sample was somewhat smaller (n=102) compared to the sample size (n=120) required to obtain power of >0.80. Finally, given that more face-to-face time may reduce depressive complaints, the longer duration (approximately 10 min) of the feedback sessions may have had an influence on the results. However, given the resemblance between the two groups with respect to weekly ESM assessments and subsequent weekly face-to-face contact with the researcher, it is unlikely that this had a large impact on the results.
Using ESM-I in mental health care has the potential to bridge the gap between the therapist office and patients' daily life, by bringing the patients' daily life into the therapist office, and creating the opportunity to extend the therapeutic setting to patients' daily life. The latter may be achieved by a web-based interactive ESM-I application that provides in-the-moment feedback based on previously assessed individual patterns of affect and behavior. This may result in helpful person-tailored insights that not only foster individualized therapy but also the diagnostic process, monitoring of early change in response to medication alterations, or identifying individual affective patterns indicating recovery or relapse 53. This approach could be integrated with cognitive behavior therapy 54 and may create a 24/7 access to and provision of care. Currently, web-based interactive ESM-I applications are in development and studies are required to examine treatment efficacy as well as cost-efficiency.
Although the present findings suggest that providing ESM-derived feedback to depressed patients is feasible and leads to a lasting decrease in depressive symptomatology, these results need to be replicated.
Acknowledgments
M. Wichers was supported by the Netherlands Organization for Scientific Research (VENI grant no. 916.76.147). The study was funded by the Dutch Health Research Council (ZON-MW grants nos. 171001002 and 91501003). The tool with which momentary assessments were performed (the PsyMate) is developed under the auspices of the Maastricht University technology transfer office, partially supported by unrestricted grants from Servier and Janssen-Cilag. The research leading to these results has received funding from the European Community's Seventh Framework Program under grant agreement no. HEALTH-F2–2009-241909 (Project EU-GEI). The authors thank all patients for participating and all collaborating mental health centers for their support in recruiting patients. They also thank W. Beuken and T. Driessen for administrative support, and P. Höhn, K. Akkermans, R. Diederen, K. Gielen, M. Hendriks and E. Pols for support in data acquisition. The first two authors contributed equally to this work.
References
- 1.World Health Organization. Depression. A global public health concern. Geneva: World Health Organization; 2012. [Google Scholar]
- 2.Dimidjian S, Hollon SD, Dobson KS, et al. Randomized trial of behavioral activation, cognitive therapy, and antidepressant medication in the acute treatment of adults with major depression. J Consult Clin Psychol. 2006;74:658–70. doi: 10.1037/0022-006X.74.4.658. [DOI] [PubMed] [Google Scholar]
- 3.Fuchs CZ, Rehm LP. A self-control behavior therapy program for depression. J Consult Clin Psychol. 1977;45:206–15. doi: 10.1037//0022-006x.45.2.206. [DOI] [PubMed] [Google Scholar]
- 4.Lewinsohn PM. A behavioral approach to depression. In: Friedman RJ, Katz M, editors. The psychology of depression: contemporary theory and research. Vol. 1974. Oxford: Wiley; pp. 157–78. [Google Scholar]
- 5.Koster EHW, Raedt De R, Leyman L, et al. Mood-congruent attention and memory bias in dysphoria: exploring the coherence among information-processing biases. Behav Res Ther. 2010;48:219–25. doi: 10.1016/j.brat.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 6.Myin-Germeys I, Birchwood M, Kwapil T. From environment to therapy in psychosis: a real-world momentary assessment approach. Schizophr Bull. 2011;37:244–7. doi: 10.1093/schbul/sbq164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Csikszentmihalyi M, Larson R. Validity and reliability of the experience-sampling method. J Nerv Ment Dis. 1987;175:526–36. doi: 10.1097/00005053-198709000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Rot aan het M, Hogenelst K, Schoevers RA. Mood disorders in everyday life: a systematic review of experience sampling and ecological momentary assessment studies. Clin Psychol Rev. 2012;32:510–23. doi: 10.1016/j.cpr.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 9.Myin-Germeys I, Oorschot M, Collip D, et al. Experience sampling research in psychopathology: opening the black box of daily life. Psychol Med. 2009;39:1533–47. doi: 10.1017/S0033291708004947. [DOI] [PubMed] [Google Scholar]
- 10.Wichers M, Hartmann JA, Kramer IMA, et al. Translating assessments of the film of daily life into person-tailored feedback interventions in depression. Acta Psychiatr Scand. 2011;123:402–3. doi: 10.1111/j.1600-0447.2011.01684.x. [DOI] [PubMed] [Google Scholar]
- 11.Wichers M, Simons CJP, Kramer IMA, et al. Momentary assessment technology as a tool to help patients with depression help themselves. Acta Psychiatr Scand. 2011;124:262–72. doi: 10.1111/j.1600-0447.2011.01749.x. [DOI] [PubMed] [Google Scholar]
- 12.Trull TJ, Ebner-Priemer U. Ambulatory assessment. Annu Rev Clin Psychol. 2013;9:151–76. doi: 10.1146/annurev-clinpsy-050212-185510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trull TJ, Ebner-Priemer UW. Using experience sampling methods/ecological momentary assessment (ESM/EMA) in clinical assessment and clinical research: introduction to the special section. Psychol Assess. 2009;21:457–62. doi: 10.1037/a0017653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thewissen V, Bentall RP, Oorschot M, et al. Emotions, self-esteem, and paranoid episodes: an experience sampling study. Br J Clin Psychol. 2011;50:178–95. doi: 10.1348/014466510X508677. [DOI] [PubMed] [Google Scholar]
- 15.Heron KE, Smyth JM. Ecological momentary interventions: incorporating mobile technology into psychosocial and health behaviour treatments. Br J Health Psychol. 2010;15:1–39. doi: 10.1348/135910709X466063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wichers MC, Barge-Schaapveld DQCM, Nicolson NA, et al. Reduced stress-sensitivity or increased reward experience: the psychological mechanism of response to antidepressant medication. Neuropsychopharmacology. 2009;34:923–31. doi: 10.1038/npp.2008.66. [DOI] [PubMed] [Google Scholar]
- 17.Wichers M, Peeters F, Geschwind N, et al. Unveiling patterns of affective responses in daily life may improve outcome prediction in depression: a momentary assessment study. J Affect Disord. 2010;124:191–5. doi: 10.1016/j.jad.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 18.Dunn BD. Helping depressed clients reconnect to positive emotion experience: current insights and future directions. Clin Psychol Psychother. 2012;19:326–40. doi: 10.1002/cpp.1799. [DOI] [PubMed] [Google Scholar]
- 19.Garland EL, Fredrickson B, Kring AM, et al. Upward spirals of positive emotions counter downward spirals of negativity: insights from the broaden-and-build theory and affective neuroscience on the treatment of emotion dysfunctions and deficits in psychopathology. Clin Psychol Rev. 2010;30:849–64. doi: 10.1016/j.cpr.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Geschwind N, Nicolson NA, Peeters F, et al. Early improvement in positive rather than negative emotion predicts remission from depression after pharmacotherapy. Eur Neuropsychopharmacol. 2011;21:241–7. doi: 10.1016/j.euroneuro.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 21.Dichter GS, Felder JN, Petty C, et al. The effects of psychotherapy on neural responses to rewards in major depression. Biol Psychiatry. 2009;66:886–97. doi: 10.1016/j.biopsych.2009.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Geschwind N, Peeters F, Drukker M, et al. Mindfulness training increases momentary positive emotions and reward experience in adults vulnerable to depression: a randomized controlled trial. J Consult Clin Psychol. 2011;79:618–28. doi: 10.1037/a0024595. [DOI] [PubMed] [Google Scholar]
- 23.Fredrickson BL, Cohn MA, Coffey KA, et al. Open hearts build lives: positive emotions, induced through loving-kindness meditation, build consequential personal resources. J Pers Soc Psychol. 2008;95:1045. doi: 10.1037/a0013262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.First M, Spitzer R, Gibbon M, et al. SCID-I. Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I) Washington: American Psychiatric Press; 1996. [Google Scholar]
- 26.Rush AJ, Gullion CM, Basco MR, et al. The Inventory of Depressive Symptomatology (IDS): psychometric properties. Psychol Med. 1996;26:477–86. doi: 10.1017/s0033291700035558. [DOI] [PubMed] [Google Scholar]
- 27.Delespaul P. Assessing schizophrenia in daily life: the experience sampling method. Maastricht: University of Limburg; 1995. [Google Scholar]
- 28.Wichers M, Myin-Germeys I, Jacobs N, et al. Genetic risk of depression and stress-induced negative affect in daily life. Br J Psychiatry. 2007:218–23. doi: 10.1192/bjp.bp.106.032201. [DOI] [PubMed] [Google Scholar]
- 29.Myin-Germeys I, Os van J, Schwartz JE, et al. Emotional reactivity to daily life stress in psychosis. Arch Gen Psychiatry. 2001;58:1137–44. doi: 10.1001/archpsyc.58.12.1137. [DOI] [PubMed] [Google Scholar]
- 30.StataCorp. 2011. Stata Statistical Software: Release 12. College Station: Statacorp LP,
- 31.Hegerl U, Mergl R. The clinical significance of antidepressant treatment effects cannot be derived from placebo-verum response differences. J Psychopharmacol. 2010;24:445–8. doi: 10.1177/0269881109106930. [DOI] [PubMed] [Google Scholar]
- 32.NICE. Depression: management of depression in primary and secondary care. Clinical practice guideline no. 23. London: National Institute for Clinical Excellence; 2004. [Google Scholar]
- 33.Barge-Schaapveld DQ, Nicolson NA. Effects of antidepressant treatment on the quality of daily life: an experience sampling study. J Clin Psychiatry. 2002;63:477–85. doi: 10.4088/jcp.v63n0603. [DOI] [PubMed] [Google Scholar]
- 34.Palmier-Claus J. The clinical uses of momentary assessment. Acta Psychiatr Scand. 2011;124:241–2. doi: 10.1111/j.1600-0447.2011.01761.x. [DOI] [PubMed] [Google Scholar]
- 35.Ebner-Priemer UW, Trull TJ. Ecological momentary assessment of mood disorders and mood dysregulation. Psychol Assess. 2009;21:463–75. doi: 10.1037/a0017075. [DOI] [PubMed] [Google Scholar]
- 36.Telford C, McCarthy-Jones S, Corcoran R, et al. Experience sampling methodology studies of depression: the state of the art. Psychol Med. 2011;42:1119–29. doi: 10.1017/S0033291711002200. [DOI] [PubMed] [Google Scholar]
- 37.Hareva DH, Okada H, Kitawaki T, et al. Supportive intervention using a mobile phone in behavior modification. Acta Med Okayama. 2009;63:113–20. doi: 10.18926/AMO/31830. [DOI] [PubMed] [Google Scholar]
- 38.Tryon WW, Tryon GS, Kazlausky T, et al. Reducing hyperactivity with a feedback actigraph: initial findings. Clin Child Psychol Psychiatry. 2006;11:607–17. doi: 10.1177/1359104506067881. [DOI] [PubMed] [Google Scholar]
- 39.Newman MG, Kenardy J, Herman S, et al. Comparison of palmtop-computer-assisted brief cognitive-behavioral treatment to cognitive-behavioral treatment for panic disorder. J Consult Clin Psychol. 1997;65:178–83. doi: 10.1037//0022-006x.65.1.178. [DOI] [PubMed] [Google Scholar]
- 40.Litt MD, Kadden RM, Kabela-Cormier E. Individualized assessment and treatment program for alcohol dependence: results of an initial study to train coping skills. Addiction. 2009;104:1837–8. doi: 10.1111/j.1360-0443.2009.02693.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Granholm E, Ben-Zeev D, Link PC, et al. Mobile Assessment and Treatment for Schizophrenia (MATS): a pilot trial of an interactive text-messaging intervention for medication adherence, socialization, and auditory hallucinations. Schizophr Bull. 2012;38:414–25. doi: 10.1093/schbul/sbr155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miklowitz DJ, Price J, Holmes EA, et al. Facilitated integrated mood management for adults with bipolar disorder. Bipolar Disord. 2012;14:185–97. doi: 10.1111/j.1399-5618.2012.00998.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Depp CA, Mausbach B, Granholm E, et al. Mobile interventions for severe mental illness: design and preliminary data from three approaches. J Nerv Ment Dis. 2010;198:715–21. doi: 10.1097/NMD.0b013e3181f49ea3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sorbi MJ, Mak SB, Houtveen JH, et al. Mobile web-based monitoring and coaching: feasibility in chronic migraine. J Med Internet Res. 2007;9 doi: 10.2196/jmir.9.5.e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zimmerman M, Martinez JH, Friedman M, et al. How can we use depression severity to guide treatment selection when measures of depression categorize patients differently? J Clin Psychiatry. 2012;73:1287–91. doi: 10.4088/JCP.12m07775. [DOI] [PubMed] [Google Scholar]
- 46.American Psychiatric Association Task Force for the Handbook of Psychiatric Measures. Handbook of psychiatric measures. Washington: American Psychiatric Association; 2000. [Google Scholar]
- 47.Cuijpers P, Graaf de R, Dorsselaer van S. Minor depression: risk profiles, functional disability, health care use and risk of developing major depression. J Affect Disord. 2004;79:71–9. doi: 10.1016/S0165-0327(02)00348-8. [DOI] [PubMed] [Google Scholar]
- 48.Judd LL, Akiskal HS, Maser JD, et al. Major depressive disorder: a prospective study of residual subthreshold depressive symptoms as predictor of rapid relapse. J Affect Disord. 1998;50:97–108. doi: 10.1016/s0165-0327(98)00138-4. [DOI] [PubMed] [Google Scholar]
- 49.Rush A, Trivedi M, Wisniewski S, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR* D report. Am J Psychiatry. 2006;163:1905–17. doi: 10.1176/ajp.2006.163.11.1905. [DOI] [PubMed] [Google Scholar]
- 50.Cuijpers P, Vogelzangs N, Twisk J, et al. Differential mortality rates in major and subthreshold depression: meta-analysis of studies that measured both. Br J Psychiatry. 2013;202:22–7. doi: 10.1192/bjp.bp.112.112169. [DOI] [PubMed] [Google Scholar]
- 51.Cuijpers P, Smit F, Straten Van A. Psychological treatments of subthreshold depression: a meta-analytic review. Acta Psychiatr Scand. 2007;115:434–41. doi: 10.1111/j.1600-0447.2007.00998.x. [DOI] [PubMed] [Google Scholar]
- 52.Ormel J, Petukhova M, Chatterji S, et al. Disability and treatment of specific mental and physical disorders across the world. Br J Psychiatry. 2008;192:368–75. doi: 10.1192/bjp.bp.107.039107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Os van J, Delespaul P, Wigman J, et al. Beyond DSM and ICD: Introducing "precision diagnosis" for psychiatry using momentary assessment technology. World Psychiatry. 2013;12:113–7. doi: 10.1002/wps.20046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kelly J, Gooding P, Pratt D, et al. Intelligent real-time therapy: harnessing the power of machine learning to optimise the delivery of momentary cognitive-behavioural interventions. J Ment Health. 2012;21:404–14. doi: 10.3109/09638237.2011.638001. [DOI] [PubMed] [Google Scholar]