Abstract
Thymic stromal lymphopoietin (TSLP) endows human blood-derived CD11c+ dendritic cells (DCs) and Langerhans cells (LCs) obtained from human epidermis with the capacity to induce pro-allergic T cells. In this study, we investigated the effect of TSLP on umbilical cord blood CD34+-derived LC-like cells. These cells are often used as model cells for LCs obtained from epidermis. Under the influence of TSLP, both cell types differed in several ways. As defined by CD83, CD80 and CD86, TSLP did not increase maturation of LC-like cells when compared with freshly isolated LCs and epidermal émigrés. Differences were also found in the production of chemokine (C-C motif) ligand (CCL)17. LCs made this chemokine only when primed by TSLP and further stimulated by CD40 ligation. In contrast, LC-like cells released CCL17 in response to CD40 ligation, irrespective of a prior treatment with TSLP. Moreover, the CCL17 levels secreted by LC-like cells were at least five times higher than those from migratory LCs. After maturation with a cytokine cocktail consisting of tumour necrosis factor-α, interleukin (IL)-1β, IL-6 and prostaglandin (PG)E2 LC-like cells released IL-12p70 in response to CD40 ligation. Most importantly and in contrast to LC, TSLP-treated LC-like cells did not induce a pro-allergic cytokine pattern in helper T cells. Due to their different cytokine secretion and the different cytokine production they induce in naïve T cells, we conclude that one has to be cautious to take LC-like cells as a paradigm for ‘real’ LCs from the epidermis.
Keywords: LC-like cells, TSLP, cytokine, naïve T cells, Langerhans cells, human
Introduction
Dendritic cells (DCs) are critical for the onset and control of T-cell immune responses [1–4]. Langerhans cells (LCs) are DCs residing primarily within the epithelia of many organs, foremost in the epidermis of the skin [5]. The T-cell stimulatory function of LCs is well established in vitro [6]. There is also evidence that LCs function as antigen-presenting cells (APCs) in vivo, although this is currently under intense debate [5, 7].
The heterogeneity of DCs is reflected in the various ways of their generation from defined haematopoietic precursors. In human beings, there are several DC types: CD14+ blood monocyte-derived DCs, CD34+ hematopoietic progenitor cell-derived dermal/interstitial DCs, CD34+ haematopoietic progenitor cell-derived LCs, DC directly derived from peripheral blood and plasmacytoid DCs [8–14]. In vivo, the differentiation of LCs requires epithelial TGF-β1[15, 16]. In response to TGF-β1, LCs develop from early monocytic cells identified as lysozyme+, CD14+/–, CD11b– in serum-free cultures of CD34+ cells [11, 17–19]. In contrast to other myeloid and plasmacytoid DCs, LCs are unique in the expression of langerin (CD207), a type II lectin which is the major molecule of Birbeck granules [20, 21]. However, langerin seems not to be an exclusive LC marker, as in mice a dermal langerin+ DC subset was found [22–24] but a description of a human counterpart is still missing.
Thymic stromal lymphopoietin (TSLP) is an interleukin (IL)-7-like cytokine, which appears to be critically involved in allergic diseases [25]. It is highly expressed by keratinocytes in atopic dermatitis lesions, but not in other types of skin inflammation [26]. Human LCs treated with TSLP undergo phenotypic and functional maturation. Notably, CD4+ helper T cells [27] as well as CD8+ T cells [28], stimulated by TSLP-treated LCs and DCs secrete a pro-allergic pattern of cytokines with increased amounts of IL-4, IL-5, IL-13 and tumour necrosis factor (TNF)-α, but reduced levels of interferon (IFN)-γ and IL-10. Moreover, TSLP-treated LCs produce thymus and activation regulated chemokine (TARC)/CCL17, a Th2-T-cell-attracting chemokine [27]. These observations in human DCs are in accordance with mouse studies, which also revealed a pivotal role for TSLP and LCs in the pathogenesis of atopic diseases [29, 30].
So far, only relatively few papers studied the function of human LCs. This is most likely due to the difficulties in the isolation of purified LCs from the skin and due to the low yield of such highly enriched cells. Instead of LCs from human skin, many functional studies have been using CD34+ haematopoietic progenitor cell-derived LCs. Even though epidermal and CD34+ haematopoietic progenitor cell-derived LCs were shown to share phenotypical similarities, studies systematically comparing their cytokine secretion, chemokine responsiveness and induction of cytokine production in naïve T cells are still lacking.
In this study, we investigated the effect of TSLP on human CD34+ haematopoietic progenitor cell-derived LCs and compared it with that of skin emigrants. To this end, we generated LC-like cells under the aegis of granulocyte macrophage-colony stimulating factor (GM-CSF), TNF-α, stem cell factor (SCF) and flt-3. In order to obtain a synchronized maturation like in skin emigrants [27, 31] we exposed them additionally to a cytokine-cocktail consisting of TNF-α, IL-1β, IL-6 and PGE2[32].
Materials and methods
Media and reagents
LC-like cells were cultured in serum-free medium X-VIVO 15 (Lonza, Verviers, Belgium), containing 2 mM L-glutamine (Sigma Chemicals, St. Louis, MO, USA) and 50 μg/ml gentamycin (PAA, Linz, Austria). Recombinant (rh) TSLP (15 ng/ml final working concentration in all experiments) and anti-TSLP-antibody were purchased from R&D Systems (Minneapolis, MN, USA). For phenotypic analyses using flow cytometry, the following commercially available fluorescein isothiocyanate (FITC)-, phycoerythrin (PE)- and allophycocyanin (APC)-conjugated mouse anti-human mAbs were used: anti-CD1a, anti-CD1c, anti-CD40, anti-CD80, anti-CD86, anti-human leukocyte antigen (HLA)-DR (BD-Biosciences, San Diego, CA, USA), anti-CD83, anti-CD207/langerin (Beckman-Coulter, Fullerton, CA, USA), anti-CD34L (Ancell, Bayport, MN, USA) and anti-TSLP-R (Biolegend, San Diego, CA, USA). Isotype controls included the corresponding fluorochrome-conjugated mouse IgG1 (BD-Biosciences, Ancell), mouse IgG2a (BD-Bioscience) and mouse IgG2b (DAKO, Glostrup, Denmark) reagents.
Isolation of umbilical cord blood CD34+ cells
Umbilical cord blood samples were collected from uncomplicated full-term deliveries following informed consent from the mother. CD34+ cells were isolated by a magnetic beads separation method according to the manufacturer’s instructions (Miltenyi Biotec, Bergisch-Gladbach, Germany). The purity of isolated CD34+ cells was generally >90% as verified by flow cytometry.
In vitro culture of LC–like cells derived from CD34+ cells
DCs were generated from CD34+ progenitor cells enriched from mononuclear fractions of umbilical cord blood samples. A total of 1–2 × 104 cells were seeded into 24-well plates and maintained in X-VIVO 15 (Lonza) supplemented with 2 mM L-glutamine (Sigma), 100 U/ml penicillin, 100 μg/ml streptomycin (Irvine Scientific, Santa Ana, CA, USA), rh TGF-β1 (0.5 ng/ml, specific activity 3.5 × 104 U/mg, R&D Systems), rh GM-CSF (100 ng/ml, specific activity 5.6 × 106 IU/mg, Leukine™, Berlex, Wayne, NJ, USA), rh TNF-α (50 U/ml, specific activity 1 × 108 U/mg, kindly provided by Dr. G. R. Adolf, Bender, Vienna, Austria, rh SCF (20 ng/ml, specific activity 5 × 105 U/mg, PeproTech, London, UK) and rh flt-3 (100 ng/ml, PeproTech) as described previously [17], with slight modifications. After 8 days of culture, cells were harvested, and CD1a+ cells were enriched using standard immunomagnetic techniques (Miltenyi Biotec). The purity of isolated CD1a+ cells was >95%. A total of 2 × 104 CD1a+ cells were plated into 24-well plates and were cultured in X-VIVO 15 with the additives noted above. At day 12, cells were harvested, counted and analysed for the expression of CD83. Subsequently, cells were incubated in parallel for additional 8 hrs either with medium alone, medium containing TSLP to induce maturation (‘mature LC-like cells’) or a maturation-inducing cytokine-cocktail consisting of the inflammatory cytokines TNF-α, IL-1β, IL-6 and PGE2[32, 33]. Such treated cells were then used for T-cell co-cultures.
Purification of naïve T cells and co-cultures with LC-like cells
Naïve CD4+ T cells were generated from anonymous blood bank donors. Peripheral blood mononuclear cells were incubated with a mixture of mAbs, including HLA-DR, CD8, CD14, CD16, CD19, CD56, CD123, CD235a/glycophorin A (BD-Biosciences) and CD45 RO (DAKO). Petri-dishes were coated for 1 hr with AffiniPure goat antimouse IgG (10 μg/ml, Jackson ImmunoResearch Laboratories, Avondale, PA, USA). Naïve CD4+ CD45RA+ T cells were isolated using a panning technique as described previously [34]. The panning step was repeated twice to obtain >95% pure CD4+ T cells. LC-like cells cultured under different conditions (medium alone, with TSLP or neutralizing anti-TSLP antibody) were washed twice and co-incubated with naïve CD4+CD45RA+ T cells in 48-well plates at a 6:1 ratio (6 × 105 T cells; 1 × 105 LCs) for 6 days.
Analysis of T-cell cytokine production
For ELISA assays, 6-day co-cultured cells were transferred to fresh wells and re-stimulated with plate-bound anti-CD3 (10 μg/ml, BD-Biosciences) and anti-CD28 mAbs (2 μg/ml, BD-Biosciences) for 30 hrs. Culture supernatants were frozen at −80°C until levels of IL-4, IL-5, IL-10, IL-13, TNF-α and IFN-γ were measured with ELISA kits (IL-4, IL-5, IL-10, IL-13 and TNF-α from Bender MedSystems, Vienna, Austria; IFN-γ from BD-Biosciences). To determine the intracellular cytokine production, the primed CD4+ T cells were re-stimulated at day 6 with 50 ng/ml phorbol myristate acetate (PMA) and 2 μg/ml ionomycin for 6 hrs. GolgiStop (1 μg/ml, BD-Biosciences) was also added during this 6 hr incubation. Cells were then washed and stained with a combination of PE-labelled anti-IL-4, anti-IL-13 mAbs, FITC-labelled anti-IFN-γ mAbs and APC-labelled anti-TNF-α, and anti-IL-10 mAbs (all from BD-Biosciences) by using a cell permeabilization kit (Fix&Perm™, An der Grub, Kaumberg, Austria).
Mixed leucocyte reaction
Graded doses of gamma irradiated (30 Gy) LC-like cells were added to 1.5 × 105 allogeneic CD4+CD45RA+ T cells, and cells were cultured in 96-well flat-bottom culture plates for 6 days. Proliferation was quantified by addition of 1 μCi [3H]thymidine (specific activity 247.9 GBq/mmol = 6.7 Ci/mmol, New England Nuclear, Boston, MA, USA) during the last 16 hrs of the culture period, and finally incorporated radioactivity was measured with a scintillation counter (Perkin Elmer Life Sciences, Boston, MA, USA). Results were shown as means of triplicate wells.
Determination of LC-derived IL-12 and CCL17
LC-like cells were cultured with or without addition of TSLP and of cytokine-maturation cocktail for 2 days, i.e. from day 12 to day 14 of culture. The resulting LC-like cells were plated at 1 × 106/ml in the presence or absence of CD40 ligand-expressing cells for additional 48 hrs from day 14 to day 16. Murine myeloma cells transfected with the human CD154/CD40 ligand molecule (P3xTBA7 cells) were used to ligate the CD40 molecule on the surface of LC-like cells [35]. Wild-type cells served as a negative control (P3x63Ag8.653-WT). Both cells were kind gifts from Dr. R. A. Kroczek (Berlin, Germany). Supernatants were collected after 48 hrs and stored at −80°C until assayed for IL-12p70 and TARC by ELISA (BD-Biosciences for IL-12p70, R&D Systems for TARC).
Results
Characterization of CD34+ derived LC-like cells
The initial inocula of 1–2 × 106 CD34+ cells multiplied 48.2-fold ± 23.6 S.D. (n = 10) within 12 days of culture. Cells grew in large clusters, as described previously [17]. On day 12, the percentage of CD1a+ cells ranged between 51–67% of all viable cells. In eight individual experiments with cord blood from different donors the percentage of langerin+ cells within the CD1a+ population was detected by flow cytometry and showed a range of 51–88.4% (mean 71.6%) (Fig. 1A).
Fig 1.

Phenotype of CD34+-derived LC-like cells activated with TSLP. LC-like cells were identified by their CD1a expression. (A) Expression of CD1a and CD207 on day 12 of culture. Fluorescence of CD1a-gated cells is depicted in the histograms. Filled histograms represent staining of experimental antibodies, open histograms the corresponding isotype controls. (B) CD34+-derived LC-like cells lacked CD83 expression on day 12 (upper row). After disruption of cell clusters during enrichment for CD1a, all LC-like cells matured by day 14 irrespective of the activation stimulus (lower row). (C) When maturation stimuli were added without mechanical disturbance of cells, only the cytokine cocktail consisting of TNF-α, IL-1β, IL-6 and PGE2- induced maturation, whereas TSLP did not. One representative experiment of three is shown in (A), (B) and (C).
TSLP does not promote maturation of CD34+-derived LC-like cells – in contrast to epidermis-derived LC
On day 12 of culture, CD34+-derived LC-like cells strongly resembled immature epidermal LCs in that they lacked the expression of CD83, but highly expressed CD1a and CD1c. However, when day 12 LC-like cells were enriched for CD1a by magnetic activated cell separation (MACS) separation and further cultured for 48 hrs with medium alone or medium containing either TSLP or maturation-inducing cytokine cocktail (TNF-α, IL-1β, IL-6, PGE2), all three populations acquired CD83 expression (Fig. 1B). Apparently, the maturation stimulus given by the mechanical dissociation of cell clusters and the breaking of e-cadherin bonds between cells [36, 37] was too strong in order to allow any judgement of an additive TSLP effect. In contrast, when maturation stimuli were simply added to the cultures on day 12 without any further manipulation, the cytokine cocktail induced distinct maturation of DCs as compared to controls in medium alone. In this setting it became evident that TSLP had no maturation effect on these cells as judged from the unchanged expression levels of CD83, −86, −80, −40, −207 (langerin) and −208 [dendritic cell lysosome-associated membrane glycoprotein (DC-LAMP)] (Fig. 1C).
TSLP induces expression of TSLP-R, but not of OX40-L
Although OX40-L, a known trigger for Th2 cell polarization in blood-derived DCs [38], was induced after stimulation with cytokine cocktail, TSLP-stimulated LC-like cells as well as the controls did not display OX40-L expression (Fig. 2A). Contrarily, LC-like cells were capable to up-regulate the expression of TSLP-R after TSLP stimulation (Fig. 2B). Up-regulation of TSLP-R was also seen in cocktail-matured LC-like cells (Fig. 2B) and in mature epidermal LCs (Fig. 3). Interestingly, the latter expressed TSLP-R regardless of the presence or absence of TSLP.
Fig 2.

OX40-L and TSLP-R expression of CD34+-derived LC-like cells. LC-like cells were identified by their CD1a expression. Fluorescence of CD1a-gated cells is depicted in the histograms. Filled histograms represent staining of experimental antibodies, open histograms the corresponding isotype controls. (A) OX40L and CD83 expression on day 12 and 14 was up-regulated only by cytokine cocktail, but not by TSLP, whereas (B) TSLP-R (surface staining) was induced by both maturation stimuli. One representative experiment of three is shown.
Fig 3.
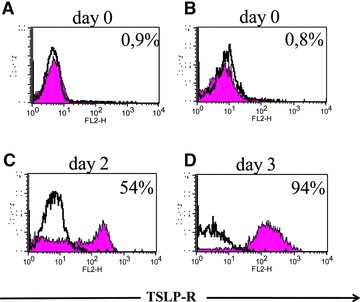
TSLP-R expression in mature LCs from human skin. Fluorescence of CD1a-gated cells is depicted in the histograms. Filled histograms represent staining of experimental antibodies, open histograms the corresponding isotype controls. (A) Intracellular TSLP-R staining of LCs freshly isolated from human epidermis. (B) Surface expression of TSLP-R on freshly isolated LCs on day 0 and (C) after two days of culture. (D) Surface expression of TSLP-R on epidermal emigrants after 3 days of culture. All LCs were gated on CD1a. One representative experiment of four is shown.
TSLP enhances survival of epidermal LCs, but not of CD34+-derived LC-like cells
Previously, Soumelis et al. [26] revealed a survival effect of TSLP on blood CD11c+ DCs in 24 hr cultures. Likewise, our group demonstrated that TSLP could maintain the survival of LCs freshly isolated from human epidermis in 48 hr cultures [27] and Figure 4B. However, in contrast to epidermal LCs, TSLP did not enhance the survival of CD34+-derived LC-like cells. As shown in Figure 4A, the number of remaining, viable LC-like cells was not higher when CD1a-enriched cells were stimulated on day 12 with TSLP for 24 hrs (data not shown) or 48 hrs. In the cocktail-matured group, the cell viability even decreased after 48 hrs stimulation (Fig. 4A). Overall, the number of viable LC-like cells was similar within the control, TSLP- and cocktail-stimulated groups (data not shown).
Fig 4.
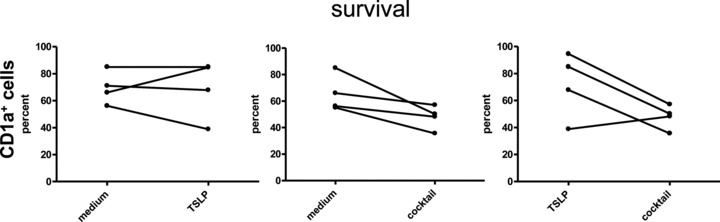
Viability and survival of TSLP primed CD34+-derived LC-like cells. CD1a-enriched LC-like cells derived from CD34+ cord blood progenitors were cultured with or without addition of TSLP and of cytokine-maturation cocktail for 2 days. (A) Cell yields as expressed by the percent of surviving LC-like cells are indicated. TSLP had no significant influence on the number or viability of LC-like cells. (B) A comparison of cell yields of CD1a-enriched LC-like cells and of epidermal LCs is shown. Both cell populations were cultured with and without addition of TSLP. Mean of four experiments for LC-like cells and of five experiments for epidermal LCs is depicted.
TSLP does not increase the immunostimulatory capacity of LC-like cells – in contrast to epidermis-derived LC
The immunostimulatory capacity of LC-like cells was tested by allogeneic mixed leucocyte reaction. LC-like cells under all different culture conditions efficiently stimulated naïve CD4+ CD45RA+ T cells. However, this immunostimulatory capacity was significantly stronger in cocktail-stimulated LC-like cells than in the unstimulated or TSLP-stimulated ones (Fig. 5).
Fig 5.
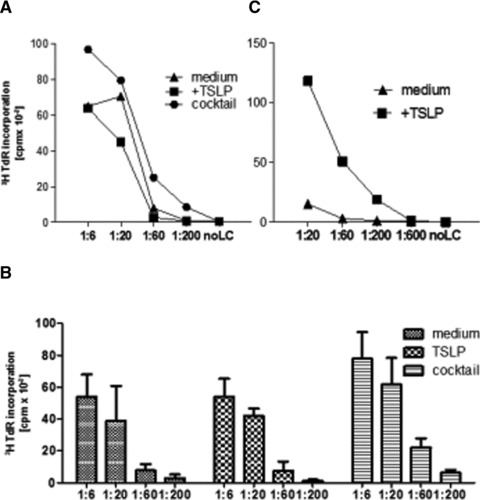
Naïve CD4+ cell proliferation induced by TSLP-treated LC-like cells. Various numbers of LC-like cells were co-cultured with 2 × 105 naïve allogeneic T cells for 6 days. (A) Proliferation was quantified by [3H]thymidine incorporation (cpm). Data represent one of four independent experiments. (B) Mean and standard deviation of four experiments is shown. DC: T-cell ratio is depicted in decreasing ratios. (C) Various numbers of epidermal LCs were co-cultured with 2 × 105 naïve allogeneic T cells for 6 days. Proliferation was quantified by [3H]thymidine incorporation (cpm). Data represent one of three independent experiments.
TSLP does not prime CD34+-derived LC-like cells to produce TARC/CCL17 and IL-12 in response to CD40 ligation – in contrast to epidermis-derived LC
To investigate the capacity of CD34+-derived LC-like cells to produce TARC, a typical Th2 attracting chemokine, and IL-12p70, a typical Th1 cytokine, cells were co-cultured with CD40L-transfected cells, and released cytokines were measured by ELISA. In response to CD40 ligation, LC-like cells secreted high levels of CCL17/TARC (more than 50 ng/ml and 106 cells), and unlike LC, they did this irrespective of the presence or absence of TSLP and cytokine cocktail (Fig. 6A). Furthermore, immature CD34+-LC-like cells released moderate levels of IL-12p70 in response to CD40 ligation. Lower amounts of IL-12p70 were detected in LC-like cells after maturation with cytokine cocktail. CD34+-derived LC-like cells cultured in the presence of TSLP, in turn, took an intermediate position as far as their IL-12p70 release was lower than that of immature cells, but higher than that of cytokine-matured cells (Fig. 6B). However, these differences did not reach statistical significance. Without CD40 ligation little CCL17/TARC and no IL-12p70 were secreted by LC-like cells.
Fig 6.

CCL17 and IL-12 p70 production by TSLP-treated LC-like cells in response to CD40 ligation. LC-like cells were cultured with or without addition of TSLP and in the presence of maturation-inducing cytokine cocktail for 2 days (from day 12 to day 14). The resulting LC-like cells were plated at 1 × 106/ml with or without CD40 ligand-expressing cells for additional 48 hrs. Supernatants were measured for CCL17 and IL-12p70. (A) CD40 ligation led to a pronounced CCL17 secretion regardless of pre-treatment with TSLP or cytokine cocktail. Six experiments are shown. (B) In contrast to LCs from human skin, immature LC-like cells released IL-12p70 in response to CD40 ligation. TSLP-treated LC-like cells secreted lower amount of IL12p70 but the lowest amount was found in the cytokine cocktail-treated population. Eight experiments are summarized in the graphs. Error bars indicate S.D.s.
TSLP-treated CD34+-derived LC-like cells do not induce an inflammatory Th2 cytokine profile in naïve CD4+ helper T cells – in contrast to epidermis-derived LC
The capacity of TSLP-stimulated LC-like cells to polarize naïve CD4+ T cells was compared with that of LC-like cells, which were generated in the presence and in the absence of TSLP. The addition of a neutralizing anti-TSLP antibody to cells cultured in the absence of TSLP, did not change the cytokine expression of T cells (data not shown). Naïve CD4+/CD45RA+ T cells purified from human peripheral blood were cultured with LC-like cells at a 6:1 ratio for 6 days. Co-cultured cells were re-stimulated for 30 hrs with anti-CD3 and anti-CD28 and released cytokines were measured by ELISA. Compared to untreated LC-like cells (cultured in medium alone), TSLP-stimulated LC-like cells induced naïve CD4+ T cells to produce higher amounts of IFN-γ and of IL-13, albeit not statistically significant. Similarly, TSLP stimulation of LC-like cells failed to lead to a significant increase of IL-4, IL-5, TNF-α or a decrease of IL-10 release by the T cells (Fig. 7A). The exact same cytokine secretion pattern was revealed by flow cytometry analyses of intracellular cytokines (Fig. 7B).
Fig 7.
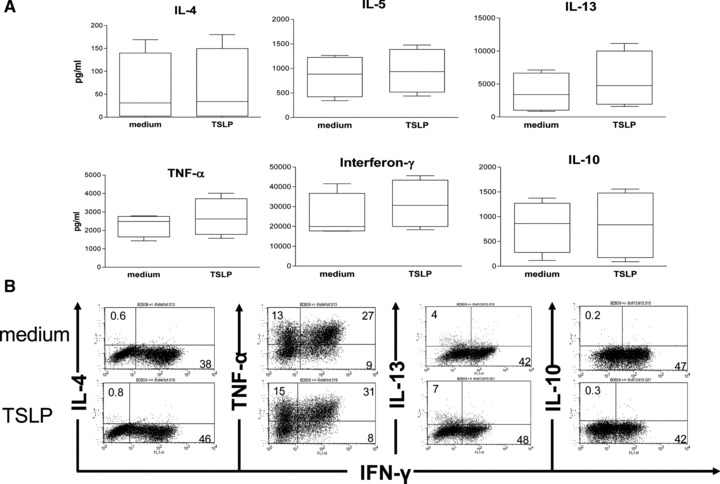
No effect on the Th1 versus Th2 balance by TSLP-treated CD34+-derived LC-like cells. (A) LC-like cells, which had been cultured in the presence or absence of TSLP for 2 days (from day 12 to day 14), were co-cultured with allogeneic CD4+ CD45RA+ naïve T cells. After 6 days of co-culture, cells were re-stimulated for additional 30 hrs with plate-bound anti-CD3 and anti-CD28 antibodies. No significant differences in the release of IL-4, IL-5, IL-13, TNF-α, IFN-γ and IL-10 by naïve CD4+ T cells were detected by ELISA. Error bars indicate S.D.s. Data represent four independent experiments. (B) Intracellular cytokine staining of naïve CD4+ T cells after 6 hrs re-stimulation with PMA and ionomycin confirmed the cytokine secretion profiles measured by ELISA. One representative experiment of three is shown. Note that experiments in (A) and in (B) are from different donors.
Discussion
In this study, we described and compared the distinct effects of TSLP on human CD34+-derived LC-like cells and human epidermal LCs. Contrary to what had been reported for LCs from human epidermis [27], TSLP stimulation failed to increase viability and to promote maturation of LC-like cells. Furthermore, TSLP did not increase the immunostimulatory capacity of these cells and did not prime them to produce TARC/CCL17, and most importantly, it did not induce an inflammatory Th2 cytokine profile in CD4+ helper T cells.
LC-like cells generated from human umbilical cord blood CD34+ progenitor cells are used frequently as a model for human LCs from human skin [17, 39, 40]. They express langerin and CD1a costimulatory molecules and display Birbeck granules, but lack markers associated with dermal DCs like CD11c, dendritic cell-specific intercellular adhesion molecule-3-grabbing non-integrin (DC-SIGN) and the mannose receptor. This study provides several opportunities to compare cord blood derived CD34+ LC-like cells with ‘true’ epidermal LCs. In contrast to freshly isolated LCs and to epidermal émigrés [27], LC-like cells did not respond to TSLP with an increased maturation as defined by the expression of maturation-associated molecules such as CD83, CD80, CD86 and CD208/DC-LAMP (Fig. 1C). Although migratory LCs matured uniformly, cord blood-derived LC-like cells of day 12 of culture did not. A homogenous maturation of LC-like cells occurred only in the presence of a strong maturation stimulus, which is similar to the generation of mature CD34+-derived DCs [33]. Thus, it seems mandatory to take into account and accurately define the state of maturation of LC-like cells whenever taking them as an example for epidermal LCs.
It is known that OX40 ligand on TSLP-treated blood DCs is critically involved in T-cell polarization [38] and that LCs may be different in this regard. We [27] demonstrated that only a varying portion of all migratory LCs express OX40 ligand, regardless of the presence of TSLP during migration. The multitude of other, largely undefined, cytokines in the explant cultures may neutralize this effect of TSLP on LCs. Interestingly, LC-like cells showed analogy with epidermal emigrants. They did not express this particular molecule, irrespective whether TSLP was present or not. Surprisingly, LC-like cells matured with cytokine-cocktail displayed OX40 ligand expression. Indeed, Krause et al. [41] reported that PGE2, an important compound of the cytokine cocktail, is responsible for the OX40 ligand expression in monocyte-derived and peripheral blood myeloid DCs. This might also be likely for LC-like cells.
To address the question if the different expression of TSLP-R in LC of human skin and LC-like cells could explain their varying response to TSLP, we performed surface and intracellular FACS staining of TSLP-R. We could not detect TSLP-R expression in freshly isolated LCs from human skin (Fig. 3) or in immature LC-like cells by flow cytometry analysis, though (Fig. 2B). Conversely, matured LCs from skin (Fig. 3) and matured LC-like cells displayed TSLP-R expression in a similar manner. Using flow cytometry Lu et al. [42] showed that blood derived DCs expressed TSLP-R in very low levels. Like blood-derived DCs, a higher expression of TSLP-R was observed only after activation of epidermal LCs and LC-like cells. Likewise, quantitative PCR revealed no rise in TSLP-R expression in activated CD34+-derived CD1a+ DCs [43]. Based on these findings, we conclude that TSLP-R expression in freshly isolated LCs from human skin and immature LC-like cells might be too weak to be detected by flow cytometry. The expression profiles of TSLP-R shown by quantitative PCR [43] together with the expression after activation of epidermal LCs and LC-like cells indicate an up-regulation rather than a neo-expression of TSLP-R.
In addition, the different CCL17 and IL-12 production of migratory LCs and LC-like cells in response to TSLP treatment (Fig. 6) strongly suggests expression of functional TSLP-R (Figs 2B and 3). Specifically, differences were found in the production of CCL17. Epidermal LCs secreted this chemokine only when primed by TSLP and stimulated by CD40 ligation. LC-like cells, in contrast, released CCL17 in response to CD40 ligation, irrespective of a prior treatment with TSLP. Apart from this, the levels of CCL17 secreted by LC-like cells were at least five times higher than those from migratory LCs.
IL-12 is a crucial factor to direct Th1 polarization. Although much is known about the secretion of the bioactive IL-12p70 heterodimer by in vitro generated human DCs, there are only few reports dealing with the production of IL-12p70 in human LCs. Peiser et al. [44] illustrated that LCs from human skin secrete moderate amounts of IL12p70 after combined stimulation with CD40 ligand and ligands for TLR 2, 4 and 5. In our previous studies, we did not detect IL-12p70 after stimulation of LCs with CD40 ligation only [27, 45]. Similar results were obtained by other groups [31, 46, 47]. Taken all these results together, it appears that mature LCs produce little if any IL-12p70, even after CD40 ligation. The Th1-biasing function of LCs may be mediated by their ability to produce IL-23 [31] or by the secretion of minute, immeasurable amounts of IL-12 that may reach effective concentration in the small volume of the immunological synapse upon T-cell contact. Unlike mature migratory LCs, LC-like cells released IL-12p70 in response to CD40 ligation even when they were matured with the cytokine cocktail consisting of TNF-α, IL-1β, IL-6 and PGE2.
It is important to emphasize that TSLP-treated epidermal LCs and LC-like cells induced different cytokine patterns in helper T cells. Whereas TSLP-treated epidermal LCs induced a pro-allergic T-cell phenotype (high levels of IL-13, IL-4, IL-5, TNF-α and low levels of IFN-γ and IL-10), in naïve CD4+ T cells [27], TSLP-treated LC-like cells did not do so.
The finding that LC-like cells differed from human epidermal LCs in their cytokine secretion after TSLP stimulation is in agreement to the work of Peiser et al. [44] showing a different cytokine release in monocyte-derived and epidermal LCs.
Taking all this into account, we conclude that one has to be cautious to use LC-like cells as a paradigm for epidermal LCs, particularly regarding their different cytokine secretion and the different cytokine production they induce in naïve T cells. Therefore, it appears mandatory to validate all data obtained with CD34-derived LC-like cells with LC extracted from the epidermis at some point. This is important in the light of the current interest to harness the properties of LC for immunotherapy of allergic disorders [48] or cancer [49–51].
Acknowledgments
This work was supported by the COMET Centre ONCOTYROL (project #1.3, Cell Therapy Unit) and funded by the Federal Ministry for Transport Innovation and Technology (BMVIT) and the Federal Ministry of Economics and Labour/the Federal Ministry of Economy, Family and Youth (BMWA/BMWFJ), the Tiroler Zukunftsstiftung and the State of Styria represented by the Styrian Business Promotion Agency (and supported by the Innsbruck Medical University, TILAK – Tiroler Landeskrankenanstalten GmbH (TILAK-Hospital Holding Company), Miltenyi Biotec GmbH and CellGenix Technologie Transfer GmbH). We also thank all colleagues from the Department of Plastic and Reconstructive Surgery who contributed to the supply of skin for this study. S. Dubrac was supported by grants from the Austrian Science Fund (FWF P21449) and Innsbruck Medical University (MFI 4301).
Conflict of interest
The authors confirm that there are no conflicts of interest.
References
- 1.Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature. 2007;449:419–26. doi: 10.1038/nature06175. [DOI] [PubMed] [Google Scholar]
- 2.Steinman RM. Dendritic cells in vivo: a key target for a new vaccine science. Immunity. 2008;29:319–24. doi: 10.1016/j.immuni.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 3.Mellman I, Steinman RM. Dendritic cells: specialized and regulated antigen processing machines. Cell. 2001;106:255–8. doi: 10.1016/s0092-8674(01)00449-4. [DOI] [PubMed] [Google Scholar]
- 4.Lanzavecchia A, Sallusto F. Regulation of T cell immunity by dendritic cells. Cell. 2001;106:263–6. doi: 10.1016/s0092-8674(01)00455-x. [DOI] [PubMed] [Google Scholar]
- 5.Romani N, Clausen BE, Stoitzner P. Langerhans cells and more: langerin-expressing dendritic cell subsets in the skin. Immunol Rev. 2010;234:120–41. doi: 10.1111/j.0105-2896.2009.00886.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schuler G, Steinman RM. Murine epidermal Langerhans cells mature into potent immunostimulatory dendritic cells in vitro. J Exp Med. 1985;161:526–46. doi: 10.1084/jem.161.3.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kissenpfennig A, Malissen B. Langerhans cells – revisiting the paradigm using genetically engineered mice. Trends Immunol. 2006;27:132–9. doi: 10.1016/j.it.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 8.Steinman RM, Idoyaga J. Features of the dendritic cell lineage. Immunol Rev. 2010;234:5–17. doi: 10.1111/j.0105-2896.2009.00888.x. [DOI] [PubMed] [Google Scholar]
- 9.Caux C, Vanbervliet B, Massacrier C, et al. CD34+ hematopoietic progenitors from human cord blood differentiate along two independent dendritic cell pathways in response to GM-CSF+TNFa. J Exp Med. 1996;184:695–706. doi: 10.1084/jem.184.2.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shortman K, Liu YJ. Mouse and human dendritic cell subtypes. Nat Rev Immunol. 2002;2:151–61. doi: 10.1038/nri746. [DOI] [PubMed] [Google Scholar]
- 11.Strobl H, Riedl E, Scheinecker C, et al. TGF-b1 promotes in vitro development of dendritic cells from CD34+ hemopoietic progenitors. J Immunol. 1996;157:1499–507. [PubMed] [Google Scholar]
- 12.Gatti E, Velleca MA, Biedermann BC, et al. Large-scale culture and selective maturation of human Langerhans cells from granulocyte colony-stimulating factor-mobilized CD34+ progenitors. J Immunol. 2000;164:3600–7. doi: 10.4049/jimmunol.164.7.3600. [DOI] [PubMed] [Google Scholar]
- 13.Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor a. J Exp Med. 1994;179:1109–18. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Romani N, Gruner S, Brang D, et al. Proliferating dendritic cell progenitors in human blood. J Exp Med. 1994;180:83–93. doi: 10.1084/jem.180.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Borkowski TA, Letterio JJ, Farr AG, et al. A role for endogenous transforming growth factor b1 in Langerhans cell biology: the skin of transforming growth factor b1 null mice is devoid of epidermal Langerhans cells. J Exp Med. 1996;184:2417–22. doi: 10.1084/jem.184.6.2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Borkowski TA, Letterio JJ, Mackall CL, et al. A role for TGFb1 in Langerhans cell biology – further characterization of the epidermal Langerhans cell defect in TGFb1 null mice. J Clin Invest. 1997;100:575–81. doi: 10.1172/JCI119567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Strobl H, Bello-Fernandez C, Riedl E, et al. flt3 ligand in cooperation with transforming growth factor- b1 potentiates in vitro development of Langerhans-type dendritic cells and allows single-cell dendritic cell cluster formation under serum-free conditions. Blood. 1997;90:1425–34. [PubMed] [Google Scholar]
- 18.Caux C, Massacrier C, Dubois B, et al. Respective involvement of TGF-b and IL-4 in the development of Langerhans cells and non-Langerhans dendritic cells from CD34+ progenitors. J Leukoc Biol. 1999;66:781–91. doi: 10.1002/jlb.66.5.781. [DOI] [PubMed] [Google Scholar]
- 19.Jaksits S, Kriehuber E, Charbonnier AS, et al. CD34+ cell-derived CD14+ precursor cells develop into Langerhans cells in a TGF-b1-dependent manner. J Immunol. 1999;163:4869–77. [PubMed] [Google Scholar]
- 20.Valladeau J, Duvert-Frances V, Pin JJ, et al. The monoclonal antibody DCGM4 recognizes langerin, a protein specific of Langerhans cells, and is rapidly internalized from the cell surface. Eur J Immunol. 1999;29:2695–704. doi: 10.1002/(SICI)1521-4141(199909)29:09<2695::AID-IMMU2695>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 21.Valladeau J, Ravel O, Dezutter-Dambuyant C, et al. Langerin, a novel C-type lectin specific to Langerhans cells, is an endocytic receptor that induces the formation of Birbeck granules. Immunity. 2000;12:71–81. doi: 10.1016/s1074-7613(00)80160-0. [DOI] [PubMed] [Google Scholar]
- 22.Poulin LF, Henri S, de Bovis B, et al. The dermis contains langerin+ dendritic cells that develop and function independently of epidermal Langerhans cells. J Exp Med. 2007;204:3119–31. doi: 10.1084/jem.20071724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ginhoux F, Collin M, Bogunovic M, et al. Blood-derived dermal langerin+ dendritic cells survey the skin in the steady state. J Exp Med. 2007;204:3133–46. doi: 10.1084/jem.20071733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bursch LS, Wang L, Igyarto B, et al. Identification of a novel population of langerin+ dendritic cells. J Exp Med. 2007;204:3147–56. doi: 10.1084/jem.20071966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu YJ. Thymic stromal lymphopoietin: master switch for allergic inflammation. J Exp Med. 2006;203:269–73. doi: 10.1084/jem.20051745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Soumelis V, Reche PA, Kanzler H, et al. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat Immunol. 2002;3:673–80. doi: 10.1038/ni805. [DOI] [PubMed] [Google Scholar]
- 27.Ebner S, Nguyen VA, Forstner M, et al. Thymic stromal lymphopoietin converts human epidermal Langerhans cells into antigen presenting cells that induce pro-allergic T cells. J Allergy Clin Immunol. 2007;119:982–90. doi: 10.1016/j.jaci.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 28.Gilliet M, Soumelis V, Watanabe N, et al. Human dendritic cells activated by TSLP and CD40L induce proallergic cytotoxic T cells. J Exp Med. 2003;197:1059–63. doi: 10.1084/jem.20030240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yoo J, Omori M, Gyarmati D, et al. Spontaneous atopic dermatitis in mice expressing an inducible thymic stromal lymphopoietin transgene specifically in the skin. J Exp Med. 2005;202:541–9. doi: 10.1084/jem.20041503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Elentner A, Finke D, Schmuth M, et al. Langerhans cells are critical in the development of atopic dermatitis-like inflammation and symptoms in mice. J Cell Mol Med. 2009;13:2658–72. doi: 10.1111/j.1582-4934.2009.00797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morelli AE, Rubin JP, Erdos G, et al. CD4+ T cell responses elicited by different subsets of human skin migratory dendritic cells. J Immunol. 2005;175:7905–15. doi: 10.4049/jimmunol.175.12.7905. [DOI] [PubMed] [Google Scholar]
- 32.Jonuleit H, Kühn U, Müller G, et al. Pro-inflammatory cytokines and prostaglandins induce maturation of potent immunostimulatory dendritic cells under fetal calf serum-free conditions. Eur J Immunol. 1997;27:3135–42. doi: 10.1002/eji.1830271209. [DOI] [PubMed] [Google Scholar]
- 33.Nguyen VA, Ebner S, Fürhapter C, et al. Adhesion of dendritic cells derived from CD34+ progenitors to resting human dermal microvascular endothelial cells is down-regulated upon maturation and partially depends on CD11a-CD18, CD11b-CD18 and CD36. Eur J Immunol. 2002;32:3638–50. doi: 10.1002/1521-4141(200212)32:12<3638::AID-IMMU3638>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 34.Koch F, Kämpgen E, Schuler G, et al. Effective enrichment of murine epidermal Langerhans cells by a modified –“mismatched”– panning technique. J Invest Dermatol. 1992;99:803–7. doi: 10.1111/1523-1747.ep12614764. [DOI] [PubMed] [Google Scholar]
- 35.Graf D, Korthäuer U, Mages HW, et al. Cloning of TRAP, a ligand for CD40 on human T cells. Eur J Immunol. 1992;22:3191–4. doi: 10.1002/eji.1830221226. [DOI] [PubMed] [Google Scholar]
- 36.Riedl E, Stöckl J, Majdic O, et al. Ligation of E-cadherin on in vitro-generated immature Langerhans-type dendritic cells inhibits their maturation. Blood. 2000;96:4276–84. [PubMed] [Google Scholar]
- 37.Jiang A, Bloom O, Ono S, et al. Disruption of E-cadherin-mediated adhesion induces a functionally distinct pathway of dendritic cell maturation. Immunity. 2007;27:610–24. doi: 10.1016/j.immuni.2007.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ito T, Wang YH, Duramad O, et al. TSLP-activated dendritic cells induce an inflammatory T helper type 2 cell response through OX40 ligand. J Exp Med. 2005;202:1213–23. doi: 10.1084/jem.20051135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ratzinger G, Baggers J, de Cos MA, et al. Mature human Langerhans cells derived from CD34+ hemopoietic progenitors stimulate greater cytolytic T lymphocyte activity in the absence of bioactive IL-12p70, by either single peptide presentation or cross-priming, than do dermal-interstitial or monocyte-derived dendritic cells. J Immunol. 2004;173:2780–91. doi: 10.4049/jimmunol.173.4.2780. [DOI] [PubMed] [Google Scholar]
- 40.van der Aar AM, Sylva-Steenland RM, Bos JD, et al. Cutting edge: loss of TLR2, TLR4, and TLR5 on Langerhans cells Abolishes Bacterial Recognition. J Immunol. 2007;178:1986–90. doi: 10.4049/jimmunol.178.4.1986. [DOI] [PubMed] [Google Scholar]
- 41.Krause P, Bruckner M, Uermosi C, et al. Prostaglandin E(2) enhances T-cell proliferation by inducing the costimulatory molecules OX40L, CD70, and 4-1BBL on dendritic cells. Blood. 2009;113:2451–60. doi: 10.1182/blood-2008-05-157123. [DOI] [PubMed] [Google Scholar]
- 42.Lu N, Wang YH, Wang YH, et al. TSLP and IL-7 use two different mechanisms to regulate human CD4+ T cell homeostasis. J Exp Med. 2009;206:2111–9. doi: 10.1084/jem.20090153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reche PA, Soumelis V, Gorman DM, et al. Human thymic stromal lymphopoietin preferentially stimulates myeloid cells. J Immunol. 2001;167:336–43. doi: 10.4049/jimmunol.167.1.336. [DOI] [PubMed] [Google Scholar]
- 44.Peiser M, Wanner R, Kolde G. Human epidermal Langerhans cells differ from monocyte-derived Langerhans cells in CD80 expression and in secretion of IL-12 after CD40 cross-linking. J Leukoc Biol. 2004;76:616–22. doi: 10.1189/jlb.0703327. [DOI] [PubMed] [Google Scholar]
- 45.Ebner S, Ratzinger G, Krösbacher B, et al. Production of IL-12 by human monocyte-derived dendritic cells is optimal when the stimulus is given at the onset of maturation, and is further enhanced by IL-4. J Immunol. 2001;166:633–41. doi: 10.4049/jimmunol.166.1.633. [DOI] [PubMed] [Google Scholar]
- 46.Nakagawa S, Koomen CW, Bos JD, et al. Differential modulation of human epidermal Langerhans cell maturation by ultraviolet B radiation. J Immunol. 1999;163:5192–200. [PubMed] [Google Scholar]
- 47.Berthier-Vergnes O, Bermond F, Flacher V, et al. TNF-a enhances phenotypic and functional maturation of human epidermal Langerhans cells and induces IL-12 p40 and IP-10/CXCL-10 production. FEBS Lett. 2005;579:3660–8. doi: 10.1016/j.febslet.2005.04.087. [DOI] [PubMed] [Google Scholar]
- 48.Dubrac S, Schmuth M, Ebner S. Atopic dermatitis: the role of Langerhans cells in disease pathogenesis. Immunol Cell Biol. 2010;88:400–9. doi: 10.1038/icb.2010.33. [DOI] [PubMed] [Google Scholar]
- 49.Ueno H, Schmitt N, Klechevsky E, et al. Harnessing human dendritic cell subsets for medicine. Immunol Rev. 2010;234:199–212. doi: 10.1111/j.0105-2896.2009.00884.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stoitzner P, Sparber F, Tripp CH. Langerhans cells as targets for immunotherapy against skin cancer. Immunol Cell Biol. 2010;88:431–7. doi: 10.1038/icb.2010.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Romani N, Thurnher M, Idoyaga J, et al. Targeting of antigens to skin dendritic cells: possibilities to enhance vaccine efficacy. Immunol Cell Biol. 2010;88:424–30. doi: 10.1038/icb.2010.39. [DOI] [PMC free article] [PubMed] [Google Scholar]


