Abstract
This study attempted to use collagen–Matrigel as extracellular matrix (ECM) to supply cells with three-dimensional (3D) culture condition and employ alginate-poly-l-lysine-alginate (APA) microcapsules to control the formation of alveolus-like structure in vitro. We tested mice foetal pulmonary cells (FPCs) by immunohistochemistry after 2D culture. The alveolus-like structure was reconstructed by seeding FPCs in collagen–Matrigel mixed with APA microcapsules 1.5 ml. A self-made mould was used to keep the structure from contraction. Meanwhile, it provided static stretch to the structure. After 7, 14 and 21 days of culture, the alveolus-like structure was analysed histologically and immunohistochemically, or by scanning transmission electron microscopy (TEM). We also observed these structures under inverted phase contrast microscope. The expression of pro-surfactant protein C (SpC) was detected by reverse transcription-polymerase chain reaction (RT-PCR). We obtained fibroblasts, epithelial cells and alveolar type II (AE2) cells in FPCs. In the reconstructed structure, seeding cells surrounding the APA microcapsules constructed alveolus-like structures, the size of them ranges from 200 to 300 μm. In each reconstructed lung tissue sheet, microcapsules had integrity. Pan-cytokeratin, vimentin and SpC positive cells were observed in 7- and 14-day cultured structures. TEM showed lamellar bodies of AE2 cells in the reconstructed tissues whereas RT-PCR expressed SpC gene. Primary mice FPCs could form alveolus-like structures in collagen–Matrigel/APA microcapsules engineered scaffolds, which could maintain a differentiated state of AE2 cells.
Keywords: tissue engineering, alveolus-like structure, microcapsule
Introduction
Lung disease, e.g. chronic obstructive pulmonary disorder (COPD) ranks No. 4 leading cause of death in the world [1]. For patients 65 years or below, lung transplantation may be considered appropriate. Yet before the scarce donated lung was acquired, they have to suffer from the pain for a long period [2]. The regeneration of distal lung tissue via tissue engineering thus raises the possibility to treat distal lung diseases as an alternative for organ shortage.
Gas exchange between air and blood mostly takes place in pulmonary alveoli (an important part of lung). Alveoli epithelial cells consist of type I (AE1) (>95%) and type II (AE2) (<5%) cells. AE2 cells synthesize and store surfactant protein in lamellar bodies and secrete it into the alveolar space. Such surfactant protein is responsible for stabilizing alveoli and decreasing surface tension, thus facilitating gas exchange [3, 4]. SpC is a hydrophobic protein. Its main role is to promote the absorption and distribution of phospholipids to the alveolar air/liquid interface, aid the formation of phospholipid monolayer and decrease the surface tension to a minimum.
Tissue engineering, a rising interdiscipline developed in late 1980s, has made breakthroughs in various tissues, e.g. bone, cartilage, kidney and cardiac muscle, etc. [5–12]. Scaffold is an important aspect of tissue engineering. Of critical importance in scaffold selection to develop of lung tissue are the elasticity and absorption kinetics of the material used. Porosity of the scaffold is also taken into consideration. In prior studies, many materials were used as scaffolds in lung tissue engineering, e.g. collagen [13–19], Matrigel [13, 20, 21], PLLA and PLGA [20]. Mondrinos et al. [20] acquired alveolar forming units (AFU) and SpC gene product by using Matrigel. PLLA and PLGA [20] were also employed as scaffolds to reconstruct 3D lung tissue in vitro, but these matrices failed to sustain the survival of distal lung epithelial cells. Gelfoam [22] was also used in rats to form porous structures similar to alveolar units. Degradable synthetic matrices used to engineer lung tissues include polyglycolic acid (PGA) [23] in the form of a felt sheet and PGA combined with pluronic F-127 (PF-127) [24]. A novel inverted colloidal crystal (ICC) [25] geometry, a recently identified scaffold with the potential to meet the requirement, is a biodegradable and highly elastic material with shape and pore size similar to that found in the alveolar structure itself.
Defects remain in precisely controlling alveolus-like constructs and maintaining AE2 differentiation despite recent developments. For this reason, selecting a new scaffold material is necessary. Microencapsulation was proposed as a means to protect the enclosed cells from the host immune system [26–28]. Smaller molecules, e.g. oxygen, nutrition and small proteins, can still rapidly equilibrate across microcapsules. Considering the cystoid construct of lung alveoli as well as its fine biocompatibility and controllability in physics parameter of microcapsules, we chose microcapsules to carry out space occupancy from the point of bionics. We could prepare microcapsules by changing the preparing process of the physical parameters to control the size and shape of the alveolar to construct tissue-engineered structures well. So we could effect on the character of alveolus-like structure by changing the physical microstructure of microcapsules. Among microcapsules, APA is most commonly used as microcapsule materials nowadays.
At present, collagen and Matrigel have been successfully used jointly as extracellular matrix (ECM) in the regeneration of cardiac muscle [29, 30] and uterine [31, 32]. ECM is important in 3D environment because it regulates cell behaviour by influencing cell proliferation, survival, shape and differentiation.
In our study, we have investigated the regeneration of lung tissues in vitro by using mice FPCs as seeding cells. Meanwhile, collagen–Matrigel is used as ECM to supply 3D culture condition for cells. APA microcapsules are employed to control the formation of alveolus-like structure.
Materials and methods
Mice
Timed-pregnant Balb/c mice were provided by the Experimental Animals Center of the Beijing Institute of Basic Medical Sciences and kept in a controlled environment with free access to food and water. All experiments were approved by the Animal Experimental Committee of the Beijing Institute of Basic Medical Sciences.
Isolation of FPCs
Unless mentioned otherwise, all cell culture materials were purchased from Fisher Scientific (Rome, Georgia). Foetal lungs were harvested from pups at gestational day 18 as previously described [15, 20]. Briefly, isolated lungs were rinsed in 1 × phosphate-buffered saline (PBS), minced, and digested with pre-warmed 0.25% trypsin in 1 × PBS for 25 min. at 37°C. Following the trypsin digestion, the enzymatic activity was quenched by addition of two volume equivalents of high glucose DMEM (Gibco, Invitrogen, Carlsbad, CA, USA) containing 10% foetal bovine serum (FBS; Hyclone, Logan, UT, USA), followed by extensive trituration performed with a Pasteur pipette. The resultant homogenates were filtered through a nylon mesh (150 μm; BD Falcon, San Jose, CA, USA) and centrifuged at 800 rpm for 5 min. The cell pellet was resuspended in 1.8 ml of distilled water for 30 sec. to lyse red blood cells, followed by addition of 0.2 ml 10 × PBS. The cells were then pelleted again, resuspended in a defined volume of DMEM containing 10% FBS, and counted in a haemocytometer.
Preparation of microcapsules
We used the three-step encapsulation method to prepare empty microcapsules as described previously [33]. We chose as follow parameters: electrostatic field was 6 kV/1.5 cm, the speed of the pump was 15 ml/hr, needle inner diameter was 0.09 mm. For APA microcapsules, 2 ml 2% sodium alginate was extruded through ID 0.5 nozzle of Encapsular (NISCO, Sweden) into the crosslinking agent (CaCl2). The microcapsules were transferred to a 50 ml sterile conical tube and washed successively with 0.55% CaCl2, 0.28% CaCl2, 0.85% saline, 0.1% CHES (2[N-cyclohexylamino]ethan-sulfonic acid) and 1.1% CaCl2. Next, the droplets were further crosslinked with 0.05% poly-L-lysine (PLL, MW 15000, Fluka, USA) in D-Hank’s for 6 min., washed with 0.1% CHES, 1.1% CaCl2, 0.85% saline and coated with 0.05% alginate for 4 min. to form another layer. Later, the microcapsules were washed in 0.85% saline, and the unpolymerized polysaccharide in the core of the microcapsule was dissolved in 0.55 mM sodium citrate for 6 min. Finally, after two rinses in 0.85% saline, the microcapsules were transferred to a 10 ml sterile conical tube and kept under normal culture conditions or 4°C.
Through changing physical parameters, such as voltage, flow rate, concentration of alginate, we could control the size of APA microcapsules when preparing them.
Preparation of the casting moulds
For the preparation of the moulds, the 2% sterile molten agar was poured into the wells of 12-well plates (1 ml per well). After solidification, four sterile glass capillary tubes (2 ± 0.15 mm in diameter) were plugged in the agar in the four corners at equal interval to form a square. The distance between the two neighboured tubes is the half of the diameter of the well.
Construction of alveolus-like structure
The isolated mouse FPCs were resuspended by concentrated culture medium (2 × H-DMEM, 200 U/ml penicillin and 200 mg/ml streptomycin). Then prepared 0.5 ml microcapsules were added in cell suspension. The 0.4 ml liquid collagen type I (1.5 mg/ml) prepared from rat tails were mixed with above-mentioned mixture. The pH of the mixture was neutralized immediately by titration with 0.1M NaOH to 7.2. A basement membrane protein mixture, Matrigel (Sigma-Aldrich, St. Louis, MO, USA), was supplemented at a final concentration of 10%. The final concentration of FPCs was 5×106 cells/ml. The mixture was then transferred into 12-well plate casting moulds. It incubated for 30 min. at 37°C to allow gelation and to form tissue sheet. Thereafter, 2 ml culture medium (H-DMEM, 20% FBS, 100 U/ml penicillin, and 100 mg/ml streptomycin) was added to each well. Culture medium was replaced by fresh medium after 24 hrs. After that, the medium was changed every other day.
For control group, the cells and scaffolds complex had no APA microcapsules.
Histological and immunohistochemistry
At the culture days of 7, 14 and 21 days, the sheets were frozen in OCT (Sakura Finetek) at −70°C. Frozen sheets were cut into 4 μm thick for haematoxylin and eosin staining, which was performed according to regular procedures.
For immunohistochemical staining, the following primary antibodies were used: pan-cytokeratin (CK) (1:800; Santa Cruz, Santa Cruz, CA, USA) as epithelial cell marker, vimentin (1:1000; Sigma-Aldrich) as fibroblast marker and pro-surfactant protein C (SpC) (1:1200; Chemicon, Temecula, CA, USA) as AE2 cells marker. After incubated with the primary antibodies overnight at 4°C, the sections were incubated with biotin-labelled secondary antibody and streptavidin-horseradish peroxidase (S-A/HRP) (Zymed, San Francisco, CA, USA). Then the sections detected with Diaminbenzidine (DAB) (Sigma-Aldrich). Negative controls were performed by omitting primary antibodies.
Transmission electron microscopy (TEM)
For TEM, the lung tissue sheets were fixed in 2.5% glutaraldehyde, post-fixed with 1% osmium tetroxide and embedding in Epon 812. Ultrathin sections were cut with a diamond knife and picked up on copper grids, counterstained with uranyl acetate and lead citrate, and examined with a Philips Technai 10 transmission electron microscope (Philip, Holland, Amsterdam, the Netherlands).
Reverse transcriptase-polymerase chain reaction
Reverse transcriptase polymerase chain reaction (RT-PCR) was utilized to detect steady-state mRNA expression of surfactant protein C (SpC) for AE2 cells. RT-PCR was carried out as previously described [20] with some modifications. Briefly, total RNA was isolated from sheets and subsequent purification of the RNA-containing aqueous extraction phase on an RNAprep pure Cell Kit (Tiangen, Beijing, China), according to the manufacturer’s protocols. The quality of isolated RNA was assessed by measuring the ratio of OD260/OD280 and by electrophoresis in 1% agarose gels with ethidium bromide containing loading buffer. The isolated RNA was reverse transcribed performed with a commercial Quantscropt RT kit (Tiangen), according to the manufacturer’s instructions; the resultant complimentary DNA was used for PCR amplification. Briefly, 1 μl cDNA was added to a reaction mixture containing 12.5 μl 2 × GC-rich PCR Master Mix (Tiangen), 9.5 μl ddH2O and 2 μl (10 μM) forward and reverse primers optimized for each gene of interest in preliminary experiments. For all genes, a 35 cycle two-step PCR routine with a 45 sec. denaturation step at 94°C and an 80 sec. combined annealing and extension step at 68°C was used (conditions specified by Clontech, Mountain View, CA, for their Atlas gene arrays). Negative controls run for all PCR reactions included no reverse transcription samples to check for genomic DNA, as well as reactions without the addition of the cDNA templates. The primer sequences used in characterization of FPC populations are shown in Table 1 (Clontech Atlas, Mouse 1.2 Array II, Cat. #7857–1; BD Biosciences ClonTech, Palo Alto, CA, USA).
Table 1.
cDNA primer sequences used for reverse transcriptase-polymerase chain reaction to test the expression of SpC gene in alveolus-like structures
| Gene | Forward primer | Reverse primer | Product length |
|---|---|---|---|
| SpC | AGCGAGCAGACACCATCGCTACC | CTCGGAACCAGTATCATGCCCTTC | 242 bp |
Results
Two-dimensional culture and assessment
Upon primary isolation, we cultured FPCs in 2D condition. Under inverted phase contrast microscope we observed that cells survived well, showing fusiform and slabstone mixing growth (Fig. 1A). The immunohistochemical analysis of primary foetal pulmonary cells demonstrated the presence of mixed cultures comprising fibroblast, epithelial cells and AE2 cells, etc. Epithelial cells and AE2 cells expressed pan-CK and SpC, respectively (Fig. 1 B and D). Cells were vimentin positive, suggesting the presence of fibroblasts (Fig. 1C).
Fig 1.
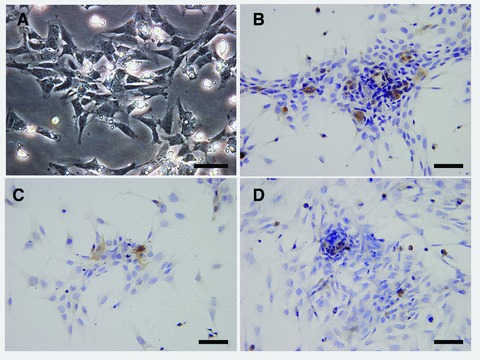
The identification of the mice foetal pulmonary cells (FPCs). (A) Primary FPCs, cultured in 2D in vitro, were observed under the inverted phase contrast microscope. The immunohistochemical analysis of isolation of primary FPCs following 2 days cultured in 2D in vitro. (B) Pan-cytokeratin (CK)-positive epithelia cells were observed, haematoxylin nuclear counterstaining. (C) Vimentin-positive fibrolasts were showed, haematoxylin nuclear counterstaining. (D) Pro-surfactant protein C (SpC) staining of alveolar type II (AE2) cells, haematoxylin nuclear counterstaining. Bars = 50 μm.
Gross observation
In the static stretch casting mould, lung tissue sheets condensed after 2 days of culture and contracted gradually into a tetragonal shape (Fig. 2A). After cultured 14 days in vitro, the sheets contracted in a butterfly-like shape upon stretch (Fig. 2B). In 21 days of culture, they maintained their butterfly-like shape (Fig. 2C). The four glass columns standing in the moulds resisted to contract and prevented the constructs from thickening, providing a static stretch at the same time.
Fig 2.

Gross observation of reconstructed 3D lung tissue sheets in vitro. (A) The formation of tissue sheet was observed after reconstructing for 7 days. (B) The sheets contracted and became butterfly-like shape gradually after 14 days of culture. (C) After cultured 21 days, tissue sheets maintained their butterfly-like shape.
Inverted phase contrast microscope observation
The diameter of prepared APA microcapsules using aforesaid physical parameters was 200–300 μm. We observed the lung tissue sheets under phase contrast microscope (Fig. 3A–C). The formation of cystic form structure was observed inside a 3D scaffold, especially in the centre of the tissue sheets. APA microcapsules had better integrity in 3D-culture system of the static stretch casting mould. Meanwhile, seeding cells grew well around microcapsules in sheets after cultured 7, 14 and 21 days (Fig. 3A–C). However, the amount of cells in 21-day-cultured sheet was less than that in 7 and 14 days. We noticed that seeding cells in part of sheets accumulated for growth (Fig. 3A and B, white arrow).
Fig 3.
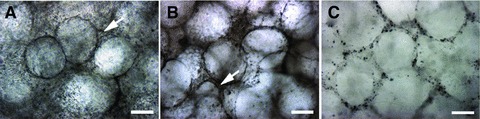
The reconstructed alveolus-like structures in vitro were observed under phase contrast microscope. The structures were observed after 7 days (A) and 14 days (B). Alginate-poly-l-lysine-alginate (APA) microcapsules had better integrity in static stretch casting mould 3D culture system; meanwhile, seeding cells grew well around microcapsules in sheets. Cells gathered to grow (white arrow) could be observed in some parts of the alveolus-like structures. (C) After cultured for 21 days, APA microcapsules were intact, but the amount of cells in sheet became small. Bars = 200 μm.
Histology and immunohistochemistry
Haematoxylin and eosin staining of sheets revealed branching spherical hollow structures (Fig. 4B–D). These spherical hollow structures, reminiscent of sacculation, were close to the histology of native mouse lung (Fig. 4A) in appearance. After 7 and 14 days of culture, haematoxylin and eosin staining demonstrated that reconstructed tissue sheets had alveolus-like structures (Fig. 4B and C). The nuclei of cells were integral. Cytoplasm could be stained by eosin. The alveolus-like structure was maintained in a 21-day-cultured sheet, but necrosis coincided. The disaggregation of nuclei was observed (Fig. 4D).
Fig 4.
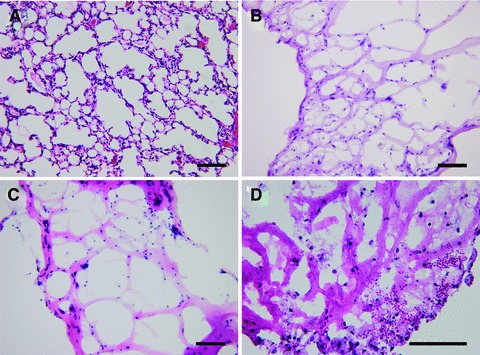
Haematoxylin and eosin staining of mouse normal lung tissue and reconstructed lung tissue sheets in vitro. (A) The picture of mouse normal lung tissue. (B) The tissue sheets had an alveolus-like structure after cultured for 7 days. (C) The alveolus-like structure was shown in 14 days cultured sheet. (D) The alveolus-like structure was maintained in 21 days cultured sheet, but necrosis was observed. Bars = 100 μm.
Pan-CK, vimentin and SpC positive cells were observed in tissue sheets cultured for 7 and 14 days (Fig. 5A–F). Immunohistochemically, pan-CK-positive cells were concentrated as monolayer (Fig. 5A and D) around the alveolus-like structure. Vimentin positive cells were evenly distributed in tissue sheets (Fig. 5B and E). In addition, AE2 cells were localized in collagen/Matrigel-APA constructs, as illustrated by pro-surfactant protein C immunostaining (Fig. 5C and F). We found pan-CK-positive cells and SpC-positive cells decreased in 21-day-cultured lung tissue sheets (Fig. 5G and I). No vimentin-positive results in 21-day-cultured lung tissue sheets (Fig. 5H).
Fig 5.
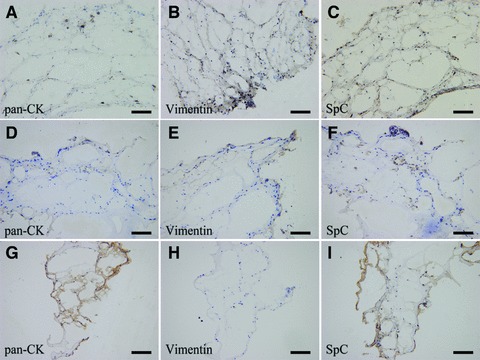
Immunohistochemical analysis of reconstructed lung tissue sheets in vitro. (A, D) Pan-CK-positive cells were concentrated as a monolayer around of the alveolus-like structures in sheets after 7 and 14 days of culture. (B, E) Vimentin positive cells were distributed in reconstructed tissue sheet after 7 and 14 days of culture. (C, F) SpC-positive cells were detected in sheets after 7 and 14 days of culture. (G, I) Pan-CK-positive cells and SpC-positive cells decreased in 21-day-cultured lung tissue sheets. (H) There were no vimentin-positive results in 21-day-cultured lung tissue sheets. Bars = 100 μm.
Reverse transcriptase-polymerase chain reaction
To demonstrate the importance of 3D culture for maintaining distal epithelial gene expression associated with the function of alveolus-like morphogenesis and sacculation (Fig. 4), we detected the expression of SpC gene in collagen–Matrigel cultures with or without APA microcapsules for 7, 14 and 21 days, respectively, in vitro.
The RT-PCR analysis of reconstructed lung tissue sheets with or without microcapsules after 7 and 14 days demonstrated the continuous expression of mRNA for the AE2-specific marker SpC (Fig. 6C). We concluded that the AE2 cells in tissue sheets with APA microcapsules maintained a differentiated state. Nevertheless, we failed to detect the expression of SpC gene in 21-day-cultured sheets with or without APA microcapsules.
Fig 6.
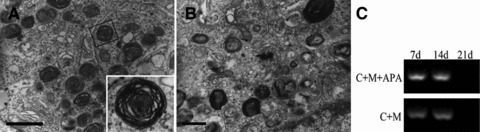
The analysis of alveolus-like structures used transmission electron micrograph and RT-PCR. The lamellar bodies of foetal pulmonary cells cultured in collagen–Matrigel/APA microcapsules scaffolds under static conditions for (A) 7 days, bar = 1 μm and (B) 14 days, bar = 500 nm. (C) The type II alveolar epithelial marker SpC gene expression was detected by RT-PCR in 3D scaffolds with or without APA microcapsules (C: collagen; M: Matrigel; APA: APA microcapsules).
Transmission electron microscopy
The ultrastructural analysis by TEM of reconstructed lung tissue sheets cultured 7 and 14 days in vitro showed the presence of sporadic lamellar bodies which stored various surfactants. In 7-day cultured tissue sheets, many lamellar bodies existed around the nucleus (Fig. 6 A). The number of lamellar bodies in 14-day cultured tissue sheets decreased (Fig. 6B). The result of lamellar bodies was also evident in previous reports [16]. In our study, the existence of lamellar bodies indicated AE2 cells were proved to maintain differentiated state in alveolus-like structures.
Discussion
In our study, we used type I collagen–Matrigel as scaffold materials and employed APA microcapsules to effect on space occupancy. Seeding cells mixed in above-mentioned materials to reconstruct lung tissue sheets in vitro. Meanwhile, we exerted static stretch in culture system. Histological examination, immunohistochemistry, RT-PCR and TEM tests demonstrated that collagen/Matrigel-APA microcapsules scaffolds system could supply FPCs with a well-grown condition and formation of alveolus-like structure and maintain an AE2 differentiated state. Our results may prove significant for engineering distal pulmonary tissues for the future. In addition, engineered lung tissue will play important roles in other fields in vitro, e.g. drug screening, the establishment of lung development model and the study of lung injury mechanism.
Based on prior studies [20], we used mice foetal pulmonary cells (FPCs) as seeding cells to reconstruct lung tissues in vitro. The dissociated FPCs used in this investigation represented a heterogeneous cell population that included epithelial cells, fibroblasts and smooth muscle cells, etc. The 2D condition provides the basis of 3D condition in the study of lung tissue engineering. We tested seeding cells cultured in 2D condition by immunohistochemistry (Fig. 1B–D). As shown in said pictures, primarily dissociated FPCs contained epithelial cells, fibroblasts and AE2 cells.
AE2 cells play important roles in the repairs and regeneration of lung tissue. Normal repairs of the epithelial layer occur through the proliferation of alveolar type II cells. Their subsequent differentiation to alveolar type I cells is necessary for the proper lung function. So AE2 cell was nicknamed the stem cell-like population of the lung [34–36]. The AE2 cell has parallel board of layered structures, known as the osmiophilic lamellar body (the main component of the small body of phospholipids). Cells released substances by way of granule exocytosis, spreading to form a layer on the alveolar surface film known as surface-active substance (surfactant). The decreased density of surfactants could reduce alveolar surface tension to prevent the excessive alveolar collapse during expiration. The phenomenon reversed during inspiration. Surfactants were constantly produced by AE2 cells, transported by pinocytotic of AE1 cells, and continuously updated. Based on its importance, it is necessary to investigate AE2 cells in reconstructed lung tissue in vitro. In our study, reconstructed lung tissue sheets were cultured for 7, 14 and 21 days in vitro. Immunohistochemical staining proved the expression of SpC gene product (Fig. 5C and F). In addition, SpC gene could be identified by RT-PCR (Fig. 6C). Lamellar bodies were observed by TEM (Fig. 6A and B). These results were homologous with the prior study by Mondrinos et al. [20] except the expression of SpC gene.
Collagen plays a dominant role in maintaining the biological and structural integrity of ECM. It is highly dynamic, undergoing constant remodelling for proper physiological functions [37]. Such a natural material is most commonly used as a bio-scaffold and merited for its biocompatible, nontoxic and degradable traits. Matrigel, a scaffold composed of basement membrane proteins, is commercially available used to culture a wide variety of cell types. Its essential components are laminin, type IV collagen, heparan sulphate, proteoglycan, entactin, TGFb1, IGF-1 and other ingredients. Matrigel could supply essential ECM components and various cytokine for seeding cells. Collagen combined with Matrigel as scaffold has been widely used in tissue engineering [6, 38–39]. Collagen and Matrigel used as FPCs scaffolds in lung tissue engineering, respectively, have been reported. So far no report is found on their combined use in lung tissue regeneration. In our study, we observed that FPCs had fine growth condition in joint collagen–Matrigel culture system. Gross observation (Fig. 2) and histological examination (Fig. 4) demonstrated that tissue sheets were in a fine 3D culture condition. After 7 and 14 days of culture, haematoxylin and eosin staining demonstrated that reconstructed tissue sheets had better activity. The nuclei of cells were integral. Cytoplasm could be stained by eosin (Fig. 4B and C). We also detected pan-CK, vimentin and SpC positive cells by immunohistochemistry, especially in 7- and 14-day cultured reconstructed tissue sheets (Fig. 5A–F). However, it is known that the Matrigel will be unlikely to be permitted for clinical use because of several reasons, i.e. immunogenicity and many unknown protein compositions. The engineered lung tissue constructed by using collagen/Matrigel as scaffold in this study cannot be used for clinical. It could serve as in vitro experimental models for drug screening, lung development studying, and the study of lung injury mechanism, etc.
The airway branches from terminal bronchiole to respiratory bronchiole, alveolar duct and alveolar sac by grade in lung. The alveolar duct and alveolar sac have a few common channels. Alveolar sac is the common opening for a number of pulmonary alveoli. Pulmonary alveoli are places for gas exchanges. Respiratory bronchioles, alveolar duct and alveolar sac are attached to pulmonary alveoli. These structures are the ministry of lung breathing. In a human being, the diameter of alveolar is about 200–250 μm in a vesicle-like shape. In prior researches, collagen [13–19], PLGA or PLLA [20] and Gelfoam [22] used as scaffold to construct alveolus-like structures have made marked progress. Yet a technical obstacle looms large. Scaffold materials had poor pore homogeneity and could not form intact alveoli structures because of the shortfall in processing methods. PLLA and PLGA [20] did not support the survival of distal lung epithelial cells, despite the presence of tissue-specific growth factors. In our study, TEM showed lamellar bodies of AE2 cells in the reconstructed tissues with APA microcapsules whereas RT-PCR identified SpC gene.
The APA microencapsulated material is characterized by its good compatibility and stability with mechanical load capacity. Furthermore, the processing technology of microcapsule has improved in 50 yrs of development. Researchers can effectively control the size of the microcapsules, micro-cyst wall permeability, shape, mechanical strength, etc. More importantly, we could effect on the character of alveolus-like structure by changing the physical microstructure of microcapsules. The electrostatic field of Encapsular generated by the larger then unit volume sodium alginate prepared more calcium alginate gel beads. The particle size was much smaller. The speed of forward pump was faster. The greater amount of sodium alginate delivered in a unit time, calcium alginate gel beads prepared by the greater volume of that size became larger. The smaller the needle used in the preparation of microcapsules used in diameter syringe, the smaller the particle size of microcapsules. With the increase of alginate concentration, microcapsules film thickness increased. Increase in thickness may also be attributed to the increase of anion through the reaction with poly-lysine. The poly-lysine concentration was appropriate. The role of reacting time is important because the parameters will directly affect the permeability of microcapsules. Therefore, such material may be the alveolar space occupying scaffold in the reconstructed process from a bionic standpoint. It is easy to form an alveolus-like structure. In this study, we also made use of the APA microcapsules, whose average diameter was about 200 μm to form an alveolar-like structure as a scaffold apart from the first joint use of collagen–Matrigel. Seeding cells grew well surrounding APA microcapsules in sheets (Fig. 3). In 7- and 14-day cultured reconstructed tissue sheets, haematoxylin and eosin staining showed that reconstructed tissue sheets had alveolus-like structures (Fig. 4B and C). From these aspects, APA microcapsules are proved not only to have space occupying effect, but also to possess the potential to be used as seeding cell growth scaffold to form alveolar-like structure. By controlling the size and shape of microcapsules as well as the structure of microcapsules wall, we can also effectively control the microstructure of the reconstructed alveoli. However, the degradation of APA microcapsules is difficult. They will affect the usage as the bionic lung tissue scaffold. At the meantime, as long as APA microcapsules are not strong enough and their fragments fall off from the scaffold, the defects will accelerate the breakdown of the scaffold and the dropped microcapsules pieces might cause blocking. With the improvement of processing methods, microcapsules will have a wide range of applications as a kind of recycled materials in lung regeneration.
Moreover, to improve the quality of lung tissue sheets and facilitate the exchange of nutrition, we used a self-made casting mould during reconstruction in this study. The mould was used to provide static stretch for constructed tissues and prevent the collagen–Matrigel from gradual contraction during the growth of cells to avoid sheets thickening [40]. It could facilitate the survival of the cells at the centre of the reconstructed tissues. By this mould, the viability of cells and the quality of the reconstructed tissues would be improved. It was reported that static stretch had important effects on the characters of cells like muscle [41], alveolar epithelial [42] or renal proximal tubule cells [43]. Stretch also invokes a cascade of events that ultimately results in the cellular growth and tissue regeneration [44]. Therefore, with better nutrition exchange and static stretch, the seeding cells survived. Zimmerman et al. has exerted mechanical stretch in the reconstruction of engineered cardiac tissue [45]. Yet so far, no report is found on the use of static stretch in reconstructing distal lung tissues. Because the mechanical environment plays important role in the development, growth and maintenance of internal organs, static stretch shall be used widely in organ tissue engineering [46].
In fact, the reconstructed tissue in vitro is different form native tissue in vivo. In our study, the results of immunohistochemistry showed that pan-CK-positive cells and SpC-positive cells decreased in 21-day-cultured lung tissue sheets (Fig. 5G and I). No vimentin-positive results in 21-day-cultured lung tissue sheets (Fig. 5H). We could not detect the expression of SpC gene in 21-day-cultured lung tissue sheets with or without microcapsules. We presume that reconstructed lung tissue sheets failed to acquire sufficient nutrition from our culture system when sheets cultured for a long time. We therefore believe that 3D culture condition must be improved in future studies. The vascularization of reconstructed tissue will help to resolve these drawbacks in tissue engineering [47–49]. So the function of vascularization on the reconstructed alveolus-like structure needs to be studied in the future, too.
The alveolus-like structure is promising in respiratory system, developmental biology and regenerative medicine as a research model in vitro. It may also be used to replace the damaged distal lung tissues of patients in the future.
Acknowledgments
This work was supported by Nature Science Foundation of China (No. 50773093).
Conflict of interest
The authors declare no conflicts of interest.
References
- 1.American Lung Association. Fact sheet: chronic obstructive pulmonary disease (COPD) [Internet][accessed January 30, 2008] . Available from: http://www.lungusa.org/site/pp.asp?c5dvLUK9O0E&b535020.
- 2.Nichols JE, Cortiella J. Engineering of a complex organ: progress toward development of a tissue-engineered lung. Proc Am Thorac S. 2008;15:723–30. doi: 10.1513/pats.200802-022AW. [DOI] [PubMed] [Google Scholar]
- 3.Junqueira LC, Carneiro J, Kelley RO. Basic histology. Danbury, CT: Appleton and Lange; 1989. pp. 342–8. [Google Scholar]
- 4.Poelma DL, Zimmermann LJ, Scholten HH, et al. In vivo and in vitro uptake of surfactant lipids by alveolar type II cells and macrophages. Am J Physiol. Lung Cell Mol Physiol. 2002;283:L648–54. doi: 10.1152/ajplung.00478.2001. [DOI] [PubMed] [Google Scholar]
- 5.Boos AM, Loew JS, Deschler G, et al. Directly auto-transplanted mesenchymal stem cells induce bone formation in a ceramic bone substitute in an ectopic sheep model. J Cell Mol Med. 2010 doi: 10.1111/j.1582-4934.2010.01131.x. 10.1111/j.1582-4934.2010.01131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang HB, Zhou J, Liu ZQ, et al. Injectable cardiac tissue engineering for the treatment of myocardial infarction. J Cell Mol Med. 2010;14:1044–1055. doi: 10.1111/j.1582-4934.2010.01046.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang H, Lau SF, Heng BF, et al. Generation of easily accessible human kidney tubules on two-dimensional surfaces in vitro. J Cell Mol Med. 2010 doi: 10.1111/j.1582-4934.2010.01113.x. 10.1111/j.1582-4934.2010.01113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gaetani R, Rizzitelli G, Chimenti I, et al. Cardiospheres and tissue engineering for myocardial regeneration: potential for clinical application. J Cell Mol Med. 2010;14:1071–7. doi: 10.1111/j.1582-4934.2010.01078.x. 10.1111/j.1582-4934.2010.01078.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Möllmann H, Nef HM, Voss S, et al. Stem cell-mediated natural tissue engineering. J Cell Mol Med. 2009 doi: 10.1111/j.1582-4934.2009.00972.x. 10.1111/j.1582-4934.2009.00972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hutmacher DW, Horch RE, Loessner D, et al. Translating tissue engineering technology platforms into cancer research. J Cell Mol Med. 2009;13:1417–27. doi: 10.1111/j.1582-4934.2009.00853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Polykandriotis E, Euler S, Arkudas A, et al. Regression and persistence: remodelling in a tissue engineered axial vascular assembly. J Cell Mol Med. 2009;13:4166–75. doi: 10.1111/j.1582-4934.2009.00828.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Petersen ThomasH, Calle ElizabethA, Zhao Liping, et al. Tissue-engineered lungs for in vivo implantation. Science. 2010;329:538–41. doi: 10.1126/science.1189345. .doi: 10.1126/science.1189345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blau H, Guzowski DE, Siddiqi ZA, et al. Fetal type 2 pneumocytes form alveolar-like structures and maintain long term differentiation on extracellular matrix. J Cell Physiol. 1988;136:203–14. doi: 10.1002/jcp.1041360202. [DOI] [PubMed] [Google Scholar]
- 14.Chen P, Marsilio E, Goldstein RH, et al. Formation of lung alveolar-like structures in collagen-glycosaminoglycan scaffolds in vitro. Tissue Eng. 2005;11:1436–48. doi: 10.1089/ten.2005.11.1436. [DOI] [PubMed] [Google Scholar]
- 15.Mondrinos MJ, Koutzaki S, Lelkes PI, et al. A tissue engineered model of fetal distal lung tissue. Am J Physiol Lung Cell Mol Physiol. 2007;293:L639–50. doi: 10.1152/ajplung.00403.2006. [DOI] [PubMed] [Google Scholar]
- 16.Sugihara H, Toda S, Miyabara S, et al. Reconstruction of alveolus-like structure from alveolar type II epithelial cells in three-dimensional collagen gel matrix culture. Am J Pathol. 1993;142:783–92. [PMC free article] [PubMed] [Google Scholar]
- 17.Chakir J, Pagé N, Hamid Q, et al. Bronchial mucosa produced by tissue engineering: a new tool to study cellular interactions in asthma. J Allergy Clin Immunol. 2001;107:36–40. doi: 10.1067/mai.2001.111929. [DOI] [PubMed] [Google Scholar]
- 18.McAteer JA, Cavanagh TJ, Evan AP. Submersion culture of the intact fetal lung. In Vitro. 1983;19:210–8. doi: 10.1007/BF02618061. [DOI] [PubMed] [Google Scholar]
- 19.Hermanns MI, Unger RE, Kehe K, et al. Lung epithelial cell lines in coculture with human pulmonary microvascular endothelial cells: development of an alveolo-capillary barrier in vitro. Lab Invest. 2004;84:736–52. doi: 10.1038/labinvest.3700081. [DOI] [PubMed] [Google Scholar]
- 20.Mondrinos MJ, Koutzaki S, Jiwanmall E, et al. Engineering three-dimensional pulmonary tissue constructs. Tissue Eng. 2006;12:717–28. doi: 10.1089/ten.2006.12.717. [DOI] [PubMed] [Google Scholar]
- 21.Mondrinos MJ, Koutzaki SH, Poblete HM, et al. In vivo pulmonary tissue engineering: contribution of donor-derived endothelial cells to construct vascularization. Tissue Eng. Part A. 2008;14:361–8. doi: 10.1089/tea.2007.0041. [DOI] [PubMed] [Google Scholar]
- 22.Andrade CF, Wong AP, Waddell TK, et al. Cell-based tissue engineering for lung regeneration. Am J Physiol Lung Cell Mol Physiol. 2007;292:510–8. doi: 10.1152/ajplung.00175.2006. [DOI] [PubMed] [Google Scholar]
- 23.Shigemura N, Okumura M, Mizuno S, et al. Lung tissue engineering technique with adipose stromal cells improves surgical outcome for pulmonary emphysema. Am J Respir Crit Care Med. 2006;174:1199–205. doi: 10.1164/rccm.200603-406OC. [DOI] [PubMed] [Google Scholar]
- 24.Cortiella J, Nichols JE, Kojima K, et al. Tissue-engineered lung: an in vivo and in vitro comparison of polyglycolic acid and pluronic F-127hydrogel/somatic lung progenitor cell constructs to support tissue growth. Tissue Eng. 2006;12:1213–25. doi: 10.1089/ten.2006.12.1213. [DOI] [PubMed] [Google Scholar]
- 25.Liu Y, Wang S, Krouse J, et al. Rapid aqueous photo-polymerization route to polymer and polymercomposite hydrogel 3D inverted colloidal crystal scaffolds. J Biomed Mater Res A. 2007;83:1–9. doi: 10.1002/jbm.a.31199. [DOI] [PubMed] [Google Scholar]
- 26.Chang TM. Semipermeable microcapsules. Science. 1964;146:524–5. doi: 10.1126/science.146.3643.524. [DOI] [PubMed] [Google Scholar]
- 27.Lanza RP, Chick WL. Transplantation of encapsulated cells and tissues. Surgery. 1997;121:1–9. doi: 10.1016/s0039-6060(97)90175-6. [DOI] [PubMed] [Google Scholar]
- 28.Clayton HA, James RF, London NJ. Islet microencapsulation: a review. Acta Diabetol. 1993;30:181–9. doi: 10.1007/BF00569928. [DOI] [PubMed] [Google Scholar]
- 29.Guo XM, Zhao YS, Chang HX, et al. Creation of engineered cardiac tissue in vitro from mouse embryonic stem cells. Circulation. 2006;113:2229–37. doi: 10.1161/CIRCULATIONAHA.105.583039. [DOI] [PubMed] [Google Scholar]
- 30.Lü S, Li Y, Liu S, et al. Engineered heart tissue graft derived from somatic-cell-nuclear-transferred embryonic stem cells improve myocardial performance in infarcted rat heart. J Cell Mol Med. 2010 doi: 10.1111/j.1582-4934.2010.01112.x. 10.1111/j.1582-4934.2010.01112.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lü SH, Wang HB, Liu H, et al. Reconstruction of engineered uterine tissues containing smooth muscle layer in collagen/Matrigel scaffold in vitro. Tissue Eng Part A. 2009;15:1611–8. doi: 10.1089/ten.tea.2008.0187. [DOI] [PubMed] [Google Scholar]
- 32.Wang HB, Lü SH, Lin QX, et al. Reconstruction of endometrium in vitro via rabbit uterine endometrial cells expanded by sex steroid. Fertil Steril. 2010;93:2385–95. doi: 10.1016/j.fertnstert.2009.01.091. [DOI] [PubMed] [Google Scholar]
- 33.Li HB, Jiang H, Wang CY, et al. Comparison of two types of alginate microcapsules on stability and biocompatibility in vitro and in vivo. Biomed Mater. 2006;1:42–7. doi: 10.1088/1748-6041/1/1/007. [DOI] [PubMed] [Google Scholar]
- 34.Mason RJ, Williams MC. Type II alveolar cell. Defender of the alveolus. Am Rev Respir Dis. 1977;115:81–91. doi: 10.1164/arrd.1977.115.S.81. [DOI] [PubMed] [Google Scholar]
- 35.Lane S, Rippon HJ, Bishop AE. Stem cells in lung repair and regeneration. Regen Med. 2007;2:407–15. doi: 10.2217/17460751.2.4.407. [DOI] [PubMed] [Google Scholar]
- 36.Loebinger MR, Aguilar S, Janes SM. Therapeutic potential of stem cells in lung disease: progress and pitfalls. Clin Sci (Lond) 2008;114:99–108. doi: 10.1042/CS20070073. [DOI] [PubMed] [Google Scholar]
- 37.Cen L, Liu W, Cui L, et al. Collagen tissue engineering: development of novel biomaterials and applications. Pediatr Res. 2008;63:492–6. doi: 10.1203/PDR.0b013e31816c5bc3. [DOI] [PubMed] [Google Scholar]
- 38.Zhou J, Zhang Y, Lin QX, et al. Embryoid bodies formation and differentiation from mouse embryonic stem cells in collagen/Matrigel scaffolds. J Genet Genomics. 2010;37:451–60. doi: 10.1016/S1673-8527(09)60064-3. [DOI] [PubMed] [Google Scholar]
- 39.Leung BM, Sefton MV. A modular approach to cardiac tissue engineering. Tissue Eng Part A. 2010;16:3207–18. doi: 10.1089/ten.tea.2009.0746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Occleston NL, Alexander RA, Mazure A, et al. Effects of single exposures to antiproliferative agents on ocular fibroblast-mediated collagen contraction. Invest Ophthalmol Vis Sci. 1994;35:3681–90. [PubMed] [Google Scholar]
- 41.De Filippo RE, Atala A. Stretch and growth: the molecular and physiologic influences of tissue expansion. Plast Reconstr Surg. 2002;109:2450–62. doi: 10.1097/00006534-200206000-00043. [DOI] [PubMed] [Google Scholar]
- 42.Cevallos M, Riha GM, Wang X, et al. Cyclic strain induces expression of specific smooth muscle cell markers in human endothelial cells. Differentiation. 2006;4:552–61. doi: 10.1111/j.1432-0436.2006.00089.x. [DOI] [PubMed] [Google Scholar]
- 43.Vlahakis NE, Schroeder MA, Limper AH, et al. Stretch induces cytokine release by alveolar epithelial cells in vitro. Am J Physiol. 1999;277:167–73. doi: 10.1152/ajplung.1999.277.1.L167. [DOI] [PubMed] [Google Scholar]
- 44.Sackin H. Stretch-activated ion channels. Kidney Int. 1995;48:1134–47. doi: 10.1038/ki.1995.397. [DOI] [PubMed] [Google Scholar]
- 45.Zimmermann WH, Schneiderbanger K, Schubert P, et al. Tissue engineering of a differentiated cardiac muscle construct. Circ Res. 2002;90:223–30. doi: 10.1161/hh0202.103644. [DOI] [PubMed] [Google Scholar]
- 46.Nichols JE, Niles JA, Cortiella J. Design and development of tissue engineered lung: progress and challenges. Organogenesis. 2009;5:57–61. doi: 10.4161/org.5.2.8564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bleiziffer O, Horch RE, Hammon M, et al. T17b murine embryonal endothelial progenitor cells can be induced towards both proliferation and differentiation in a fibrin matrix. J Cell Mol Med. 2009;13:926–35. doi: 10.1111/j.1582-4934.2008.00527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Arkudas A, Tjiawi J, Saumweber A, et al. Evaluation of blood vessel ingrowth in fibrin gel subject to type and concentration of growth factors. J Cell Mol Med. 2009;13:2864–74. doi: 10.1111/j.1582-4934.2008.00410.x. .doi: 10.1111/j.1582-4934.2008.00410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fiegel HC, Pryymachuk G, Rath S, et al. Foetal hepatocyte transplantation in a vascularized AV-Loop transplantation model in the rat. J Cell Mol Med. 2010;14:267–74. doi: 10.1111/j.1582-4934.2008.00369.x. .doi: 10.1111/j.1582-4934.2008.00369.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


