Abstract
The umbilical cord blood derived endothelial progenitor cells (EPCs) contribute to vascular regeneration in experimental models of ischaemia. However, their ability to participate in cardiovascular tissue restoration has not been elucidated yet. We employed a novel coculture system to investigate whether human EPCs have the capacity to integrate into living and ischaemic cardiac tissue, and participate to neovascularization. EPCs were cocultured with either living or ischaemic murine embryonic ventricular slices, in the presence or absence of a pro-angiogenic growth factor cocktail consisting of VEGF, IGF-1, EGF and bFGF. Tracking of EPCs within the cocultures was performed by cell transfection with green fluorescent protein or by immunostaining performed with anti-human vWF, CD31, nuclei and mitochondria antibodies. EPCs generated vascular tube-like structures in direct contact with the living ventricular slices. Furthermore, the pro-angiogenic growth factor cocktail reduced significantly tubes formation. Coculture of EPCs with the living ventricular slices in a transwell system did not lead to vascular tube-like structures formation, demonstrating that the direct contact is necessary and that the soluble factors secreted by the living slices were not sufficient for their induction. No vascular tubes were formed when EPCs were cocultured with ischaemic ventricular slices, even in the presence of the pro-angiogenic cocktail. In conclusion, EPCs form vascular tube-like structures in contact with living cardiac tissue and the direct cell-to-cell interaction is a prerequisite for their induction. Understanding the cardiac niche and micro-environmental interactions that regulate EPCs integration and neovascularization are essential for applying these cells to cardiovascular regeneration.
Keywords: endothelial progenitors, umbilical cord blood, ventricular slices, vascular tube-like structures, cardiac ischaemia, cellular integration
Introduction
Regenerative therapy represents an innovative treatment strategy that could address a large spectrum of chronic degenerative disorders. Validation of the cardiovascular regenerative potential of adult stem and progenitor cells is a prerequisite step towards advancing cell therapy to patients suffering from ischaemic heart failure associated with myocardial infarction or congenital heart diseases [1]; although still in its infancy, this challenge holds great promise for the future.
The umbilical cord blood (UCB) have been successfully applied for two decades to treat a variety of haematological disorders, and cord blood banks could provide now HLA-matched grafts for the majority of patients [2]. Evidence exists that small populations of pluripotent stem cells are present in the UCB [3–5], which are endowed with superior plasticity properties than bone marrow-derived stem cells [6]. Several groups addressed the use of UCB-derived stem cells for in vitro generation of endothelial progenitor cells (EPCs) [7–10]. Studies performed in animal models of haematopoietic cell transplantation or vascular ischaemia have shown that transplanted UCB-derived stem/progenitor cells could participate to neovascularization [11–14], which is a prerequisite for improvement of ischaemic heart function [12, 13, 15, 16]. However, the idea that UCB cells can be used for cardiac replacement therapy upon differentiation into cardiomyocytes [17–19] has been controversial, as these cells failed to form contractile tissue in vivo [20–22]. Furthermore, in a myocardial infarction setting, transplanted UCB-derived mononuclear cells acted through paracrine mechanisms to modify remodelling rather than myocyte regeneration [21]. Therefore, development of UCB-derived stem cell therapies relying on neovascularization rather than myocyte differentiation, to improve impaired cardiac function following cardiomyocyte loss, deserves further attention.
Stem and progenitor cell integration and maturation are essential to achieve cardiovascular regeneration. Current knowledge of the mechanisms of adult stem cell integration into the cardiac tissue, mechanical coupling and physiological reconstitution is still incomplete. The investigations performed at the level of cell cultures have generated valuable data, but of limited relevance to predict the effects of in vivo transplantation [23]. The multi-cellular in vitro models, such as monolayers of dissociated cardiomyocytes, used to study cellular integration into the cardiac tissue, do not preserve the in vivo structures [24]; furthermore, the aggressive dissociation methods required to prepare isolated cell suspensions often affect the function of membrane ion channels and receptors with deterministic roles in cellular integration. Alternatively, both clinical and animal studies are too complex to reveal the mechanisms underlying the outcomes of cell transplantation [25]. Therefore, our group developed a novel multi-cellular model of living ventricular tissue slices with morphologically conserved structures, preserved cell-to-cell interactions and stable electrophysiological characteristics. Such ventricular tissue slices, derived from either embryonic [26], neonatal [27] or adult [28] murine hearts, have been essential for studies of functional cross-talk between different cell types within the heart, at both cellular and molecular levels. Moreover, stem cell integration studies into injured cardiac tissue have been facilitated by the use of ischaemic ventricular slices irreversibly damaged by oxygen and glucose deprivation (OGD) [29]. However, more studies have to address the improvement of post-transplantation functional integration of stem cells recommended for cell replacement therapy. So far there are no data on the use of the above in vitro transplantation models for the study of human adult stem/progenitor cells integration, differentiation, maturation and survival into the cardiac tissue. To shed light on the potential of human UCB-derived EPCs for clinical cell replacement therapy, we proposed to elucidate their cardiac integration and neovascularization capacity by employing both living and ischaemic embryonic murine ventricular slices. The results obtained will add knowledge to the biology of human adult progenitor cells and may contribute to optimization of cell therapy protocols.
Materials and methods
Isolation and characterization of EPCs
Human UCB samples were collected at term delivery from the Department of Obstetrics and Gynecology, Polizu Hospital, Bucharest, Romania. The samples were obtained upon written informed consent and complied with European Union (EU) and national legislation regarding human samples collection, manipulation and personal data protection. All samples were tested for the absence of HIV1/2, HBV, HCV and HTLV and processed up to 5 hrs from collection. Mononuclear cells were separated from UCB on Ficoll Histopaque (1.077 g/ml; Sigma Aldrich, St. Louis, MO, USA) by density gradient centrifugation (400 ×g, 30 min., 25°C), collected from the interface, and washed twice by centrifugation in PBS (300 ×g, 7 min., 25°C). Freshly isolated mononuclear cells were immediately seeded onto tissue culture dishes pre-coated with 2 μg/cm2 fibronectin (Sigma Aldrich) and kept in endothelial cell growth medium (MV2; Promocell, Heidelberg, Germany), supplemented with 15% foetal bovine serum (FBS), 40 ng/ml vascular endothelial growth factor (VEGF), 100 μg/ml endothelial cell growth supplement, 100 U/ml penicillin, 100 μg/ml streptomycin and 50 μg/ml neomycin (all from Sigma-Aldrich). Cell cultures were maintained at 37°C with 5% CO2 and 21% O2 in a humidified atmosphere. On day 1 after plating, the non-adherent cells were removed and fresh medium was added. To maintain optimal culture conditions, medium was changed twice a week. Cells from passages 4–6 were used for the proposed experiments (see Fig. 1, describing the experimental design).
Fig 1.
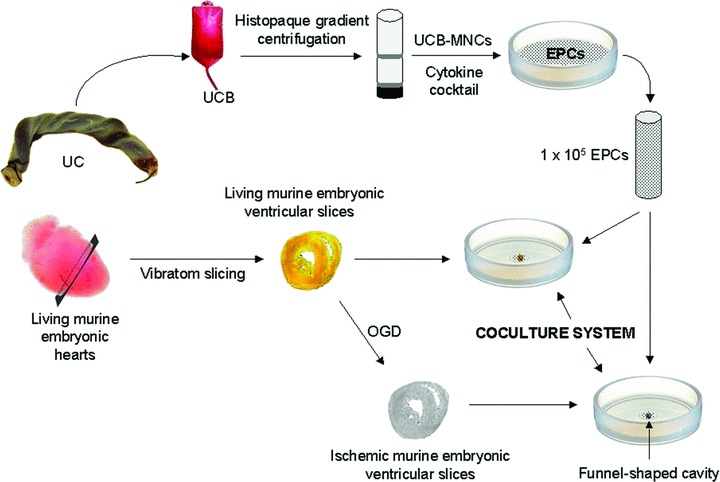
Schematic representation of the experimental design consisting of coculture of EPCs with either living or ischaemic murine embryonic ventricular slices. EPCs: endothelial progenitor cells; MNCs: mononuclear cells; OGD: oxygen and glucose deprivation; UC: umbilical cord; UCB: umbilical cord blood.
Morphological characterization of cells was performed by light microscopy, using an inverted epifluorescence microscope (Eclipse TE300; Nikon, Tokyo, Japan) and a digital camera system for imaging (Digital Net Camera DN100; Nikon), as well as by transmission electron microscopy (EM 400; Philips, FEI Company, Eindhoven, The Netherlands).
The expression of cell surface molecules on EPCs was assessed by flow cytometry [FACS Calibur (BD Biosciences, Heidelberg, Germany) and MoFlo FACS (Dako, Glostrup, Denmark)] performed with monoclonal mouse anti-human fluorescein isothiocyanate (FITC)-conjugated or phycoerythrin (PE)-conjugated CD9, CD31, CD34, CD54, CD105, CD144, CD146 (Beckman Coulter, Krefeld, Germany), CD14, CD45, CD117 (Dako) and CD133 (MACS, Miltenyi Biotec GmbH, Bergisch-Gladbach, Germany) antibodies. Accutase-detached cells were washed in PBS containing 2% FBS and incubated for 30 min. at 4°C with the appropriate diluted FITC-/PE-conjugated antibodies. For negative controls, the cells were stained with the corresponding isotype-matched Igs (Beckman Coulter and Dako). Flow cytometry data were analysed using the CellQuest Pro software (BD Biosciences) and Summit 4.0 software (Dako).
Molecular characterization of EPCs was performed using total RNA extracted with the GenElute™ Mammalian Total RNA Miniprep Kit (Sigma Aldrich). First-strand cDNA synthesis was performed on 1 μg of total RNA; cDNA samples were thereafter amplified in a thermocycler (Bio-Rad Laboratories, Hercules, CA, USA) for 35 cycles (94°C for 45 sec., 60°C for 45 sec. and 72°C for 45 sec.) with specific oligonucleotide primers to assess CD31, CD34, CD133, CD144, VEGF receptor (VEGFR) 1, VEGFR2, von Willebrand factor (vWF), GATA2, GATA3, GATA4, C-X-C chemokine receptor type 4 (CXCR4), Tie-2 and GAPDH, as endogenous control, mRNA expression. The oligonucleotide primer sequences (Metabion, Martinsried, Germany) are shown in Table S1.
The functional characterization of isolated EPCs was performed using the vascular tube formation and acetylated low-density lipoprotein (ac-LDL) uptake assays. For vascular tube formation assay, 50 μl of Matrigel Basement Membrane Matrix (BD Biosciences) were added into a 48-well plate and allowed to solidify at 37°C for 30 min.; 5 ×104 EPCs were suspended in 100 μl culture medium and plated onto Matrigel layer. After 24 hrs, the medium was removed and the formation of vascular tube-like structures was assessed with an inverted microscope (Eclipse TS100; Nikon) and a digital camera system for imaging (Digital SLR Camera D300; Nikon). For ac-LDL uptake assay, EPCs were seeded in 24-well plates at a density of 5 ×104 cells/well in MV2 medium, on glass cover slips. When cells reached ∼80% confluency, the cultures were serum-deprived overnight in Iscove’s Modified Dulbecco’s Medium (IMDM, Sigma Aldrich) supplemented with 2% lipoprotein deficient serum from human plasma (Sigma Aldrich) and then the medium was replaced with IMDM supplemented with 100 μg/ml human ac-LDL. After 24 hrs, cells on cover slips were stained with Nile red (Sigma Aldrich) and examined by fluorescence microscopy performed with an inverted microscope (Eclipse TS100; Nikon) and a digital camera system for imaging (Digital SLR Camera D300; Nikon).
Green fluorescent protein (GFP) transfection of EPCs
Transient GFP transfection of EPCs was performed to facilitate cell tracking upon coculture with living murine embryonic ventricular slices. Transfection of cultured EPCs, at 90% confluency, was employed with a HUVEC Nucleofactor Kit (VPB-1002; Lonza Group Ltd., Switzerland), in line with the manufacturer’s protocol. Immediately after transfection, EPCs were functionally tested for the formation of vascular-like structures, performed with the same methodology as described earlier for the characterization of the isolated EPCs.
Preparation of embryonic ventricular slices
All animal experiments were carried out according to EU legislation in force that sets the legal framework for the use of animals in scientific research, upon approval by the Ethics Committee of the Institute for Neurophysiology, University of Cologne, Cologne, Germany. Mice were superovulated, time-mated and the embryos harvested between days 16.5 and 18.5 of gestation. Isolated hearts were processed as previously reported [26], kept in ice-cold (4°C) Ca2+-free Tyrode’s solution under continuous oxygenation, then embedded in 4% low-melting agarose (Roth, Karlsruhe, Germany). Embedded ventricles were sectioned into 300-μm-thick slices along the short axis with a vibratome (Leica VT1000S, Leica Microsystems, Germany). Ventricular slices were kept for 30 min. in Tyrode’s solution supplemented with 0.9 mmol/l Ca2+, and then transferred in IMDM for 1 hour in an incubator under normoxic conditions (37°C, 5% CO2, 21% O2). To mimic myocardial infarction in vitro [29], OGD was induced by incubating 300-μm-thick ventricular slices, washed with phosphate-buffered saline (PBS), in a custom-made steel hypoxia chamber filled with Tryode’s solution, in which D-glucose was substituted for an equimolar concentration of 10 mmol/l 2-deoxyglucose. The hypoxia chamber was placed in a water-bath maintained at 37°C and oxygen tension was reduced by constant bubbling with pure nitrogen to induce hypoxia. Because O2 concentration and saturation are important parameters for the inducement of cardiac ischaemia, we measured these parameters (OxyScan graphics, UMS-GmbH & Co. KG, Meiningen, Germany) into the ischaemic chamber during the first 4 hrs of OGD, following a procedure previously described [30]. After 48 hrs of OGD, slices were washed twice in PBS, transferred into IMDM supplemented with 20% FBS, and stored in the incubator under normoxic conditions until coculturing with EPCs.
Generation of cocultures
Generation of cocultures in a funnel-shaped cavity-well system.
Custom-made wells containing a small funnel-shaped cavity at their bottom, to prevent EPCs from being washed away from the ventricular slices, were used for cocultures. EPCs (1 ×105) were added onto the top of either living or ischaemic ventricular slices placed into the funnel-shaped cavities of the coculture wells (see Fig. 1, describing the experimental design). Cocultures were maintained in the incubator under normoxic conditions between 5 and 14 days in IMDM supplemented with 20% FBS, 100 U/ml penicillin, 100 μg/ml streptomycin and 50 μg/ml neomycin. To test the sensitivity of vascular tube-like structures formation to pro-angiogenic factors, in half of the cocultures, a growth factor cocktail consisting of 40 ng/ml VEGF, 20 ng/ml insulin-like growth factor 1 (IGF-1), 10 ng/ml epidermal growth factor and 10 ng/ml basic fibroblast growth factor (bFGF) was added to the culture medium at the initiation of the cocultures, and then twice a week at medium change. The control consisted of both living and ischaemic ventricular slices exposed to the same culture conditions, in the absence of EPCs.
Generation of cocultures in a transwell system.
To test whether vascular tube-like structures formation requires the presence of soluble factors secreted by the living myocardium/matrix and/or a direct cell-to-cell interaction between the EPCs and the ventricular slice, 1 × 105 EPCs were cultured in IMDM supplemented with 20% FBS, 100 U/ml penicillin, 100 μg/ml streptomycin and 50 μg/ml neomycin, in the lower chamber of a 24-well plate transwell system (Sigma Aldrich), with the living ventricular slice placed into the upper chamber represented by a 3-μm pore size insert.
The coculture experiments were performed on three separate occasions, with triplicate samples per each experimental group. At the completion of the experiments, ventricular slice preparations were washed twice with PBS, fixed in 100% ice-chilled methanol for 20 min., embedded in O.C.T compound (Tissue-Tek®, Torrance, CA, USA), and kept at −70°C until further processing.
Evaluation of cocultures
Cocultures were evaluated daily by light and fluorescence microscopy. Pictures and movies were taken using an inverted epifluorescence microscope (Axiovert 10; Carl Zeiss MicroImaging GmbH, Germany) and a digital camera system for imaging (Digital Interface, DFW-x700; Sony Electronics Inc., Köln, Germany), to document the formation and extent of vascular tube-like structures, as well as the presence of beating exhibited by living ventricular slices. Although we did not assess the number of tube-like structures formed per sample or the number of beatings per sample, these phenomena were recorded at the level of each sample only as occurring events.
Immunocytochemistry, immunohistochemistry and histochemistry techniques
For immunocytochemistry, EPCs were cultured on glass cover slips, washed twice with PBS, dried at room temperature, and fixed for 20 min. with ice-chilled 100% methanol. For immunohistochemistry, 8-μm-thick cryosections of the slice preparations, resulted from cocultures carried out in the funnel-shaped cavity wells, were prepared (3050S, Leica Microsystems GmbH, Wetzlar, Germany). Cell and tissue preparations were treated for 30 min., at room temperature, with blocking buffer, consisting of 5% bovine serum albumin in PBS, then incubated overnight, at 4°C, with the following primary antibodies: monoclonal IgG1 mouse anti-human CD31 (1:200; BD Biosciences), polyclonal rabbit anti-human vWF (1:200; Sigma Aldrich), and monoclonal IgG1 mouse anti-human E-selectin, nuclei and mitochondria (1:40, 1:200 and 1:100, respectively; Millipore, Billerica, MA, USA). The slides were washed three times with PBS, for 10 min., and incubated for 2 hrs at room temperature, in the dark, with the secondary antibodies: goat-anti-mouse Alexa Fluor 555 and goat-anti-rabbit polylonal Alexa Fluor 488 (1:2000 and 1:1000, respectively; Sigma Aldrich); cell nuclei were stained with Hoechst (1:1000; Sigma Aldrich). The slides were then washed three times with PBS, for 10 min., in the dark, and mounted in Vectashield mounting media for fluorescence (Vector Laboratories, Burlingame, CA, USA). In addition, histochemistry was performed on living ventricular slice preparations, directly in funnel dishes; ventricular preparations were stained with Calcein AM (1:2000; Molecular Probes, Eugene, OR, USA) and Hoechst (1:1000; Sigma Aldrich) for 30 min. at 37°C, and briefly washed twice with PBS. Cell and tissue preparations were examined under an inverted epifluorescence microscope with an incorporated digital camera system for imaging and a module for optical sectioning (Axiovert 200M ApoTome, Carl Zeiss MicroImaging GmbH); picture acquisition was performed with the AxioVision 4.6 software (Carl Zeiss MicroImaging GmbH).
Dye injection into vascular tube-like structures
To investigate whether the tube-like structures formed in living ventricular slice preparations have a lumen, the gap junction impermeable fluorescent dye tetramethylrhodamine (TMR) dextran (Mr 10 000; Molecular Probes, Eugene, OR, USA) was injected by iontophoresis via the recording electrode, following a protocol previously established by our group [31]. For these experiments, electrodes were filled with 1% TMR dextran dissolved in 0.2 mol/l KCl. Before the extraction of the electrode from the tube-like structure, a depolarizing direct current was applied (2–10 nA, 5–8 min.). During and after injection, TMR fluorescence within vascular tube-like structures was examined with a fluorescence microscope (Axiovert 200; Carl Zeiss MicroImaging GmbH) with an incorporated camera system for imaging (Digital Interface, DFW-x710; Sony Electronics Inc.).
Data analysis
Statistical analysis of the experimental data recorded daily, by visual inspection of the cocultures, was performed using the SPSS software (Version 10; SPSS Inc., Chicago, IL, USA). To evaluate the association of either vascular tube-like structure formation or beating of the ventricular slice (dependent variables) with the coculture regimen (with or without growth factors, independent variable), univariate logistic regression analysis with calculation of odd ratios (O.R.) and 95% confidence intervals (C.I.) was performed. Multi-variate logistic regression was performed to evaluate the impacts of the coculture regimen, day of coculture and presence of beating (independent variables) on the formation of vascular tube-like structures (dependent variable). Both independent and dependent variables were dichotomous. Statistical significance was assigned for a P-value less than 0.05.
Results
Characterization of EPCs
After ∼10 days of stimulation with endothelial-differentiation growth factors, cultures of adherent UCB-derived mononuclear cells commence to form colonies with epithelial-like cobblestone morphology [Fig. 2A(a and b)]. Transmission electron microscopy examination revealed the presence of typical: rough endoplasmic reticulum; cisternae of Golgi complex with numerous adjacent secretory vesicles, indicating an active, secretory phenotype of these cells; mitochondria; dense (lysosomal) inclusion bodies, and the characteristic morphological markers of endothelial cells: the Weibel-Palade bodies and numerous plasmalemmal vesicles (caveolae). The latter were particularly well observed in sections obtained from en face preparations that revealed the presence of numerous caveolae open to the plasmalemma or within the cytoplasm, sometimes appearing as in clusters [Fig. 2B(a–e)]. The isolated cells had some typical functional characteristics of endothelial cells. When cultured on Matrigel for 24 hrs, isolated EPCs formed tube-like structures [Fig. S1A(a, b)] and upon incubation with ac-LDL, these cells incorporated the tracer [Fig. S1B(a, b)]. Evaluation of endothelial cell markers by flow cytometry indicated that the cells were markedly positive for CD9, CD31, CD54, CD105, CD144 and CD146, low positive for CD34 and CD133 and negative for CD14, CD45 and CD117 markers (Fig. S2). Immunocytochemical staining revealed the presence of the endothelial cell markers CD31, vWF and E-selectin [Fig. S3(C, E and F), respectively]; furthermore, the cells stained with anti-human nuclei [Fig. S3(A.1, A.2 and A.3] and mitochondria (Fig. S3B) antibodies used to track human cells upon coculture with the murine ventricular slices. Reverse transcription polymerase chain reaction showed mRNA expression for: VWF, CD31, CD133, CD144 and CD34 that control EPCs proliferation, differentiation and survival; VEGFR1 with role in stem cell recruitment and angiogenesis [32]; chemokine receptors VEGFR2, Tie-2 and CXCR4; transcription factors that regulate cell fate determination such as GATA2 that promotes angiogenesis [33], GATA3, which regulates critical steps of differentiation during embryonic development [34] and GATA4 that regulates genes involved in embryogenesis and myocardial differentiation and function [35] (Fig. S4).
Fig 2.
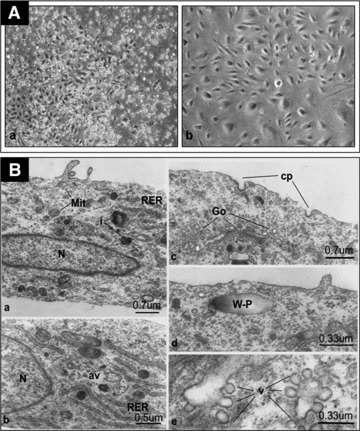
(A) Adherence of epithelial-like cells derived from UCB-mononuclear cells under growth factors stimulation. (a) Primary cell culture after 2 weeks in culture (×4); (b) Cells at passage 5 after 6 weeks in culture (×10), Nikon Eclipse TE300 and Nikon Digital Net Camera DN100. (B) EPCs in culture show general characteristics of endothelial cells as depicted by transmission electron microscopy, Philips EM 400. N: nucleus; Mit: mitochondria; RER: rough endoplasmic reticulum; v: plasmalemmal vesicles (caveolae); cp: coated pits; i: inclusions (lysosomal); av: autophagic vacuole; Go: Golgi apparatus; W-P: Weibel-Palade body.
Transfection of EPCs
To track and monitor the interaction between the EPCs and ventricular slices, the isolated cells were transfected with GFP before coculture. Quantification of the efficiency of cell transfection was 75%, as indicated by flow cytometry (data not shown). To investigate whether the transfection affected the functional properties of EPCs to form vascular tube-like structures, an in vitro vascular tubes formation assay on Matrigel was performed. We have found that, within 24 hrs of incubation, the GFP-transfected EPCs were able to assemble into organized capillary-like networks, indicating that the transfection did not affect their angiogenic capability (Fig. S5).
Interaction between EPCs and ventricular slices
Microscopy evaluation of the vibratome-generated sections of embryonic ventricles showed that the majority of slices were vital and contracted spontaneously. In contrast, the ventricular sections obtained after the OGD procedure resulted in non-contractile, non-vital slices, reproducing the pathological setting of in vivo myocardial infarction [29]. O2 concentration and saturation into the ischaemic chamber started decreasing after 10 min. of OGD. After 120 min. of OGD, O2 concentration and O2 saturation stabilized at 0.11 ± 0.02 mg/l and 1.71 ± 0.25%, respectively (Fig. S6).
To evaluate the EPCs integration and capacity to form vascular tube-like structures, 1 ×105 EPCs were loaded onto the surface of either living or ischaemic ventricular slices, with or without growth factors, and assessed in culture for up to 6 (ischaemic slices) and 14 (living slices) days. Within the first two days of coculture with living ventricular slices, the EPCs proliferated uniformly (as assessed morphologically) on the whole surface of the funnel-shaped cavity of the coculture wells. During this time, no vascular tube-like structure formation was observed either in the presence or in the absence of growth factors; furthermore, ventricular slice beating was detected only in a small fraction of living samples. Starting from day 3 of coculture, EPCs appeared as embedded within the living ventricular slices and in surface fissures and cavities of the slices. At this time, even rigorous washing could not detach the EPCs from the ventricular slices. By day 3 of coculture, vascular tube-like structures were also detected on the living ventricular slices (Fig. 3A, Movie S1). They grew direct proportionally with day of coculture (Movies S2 and S3), and by day 5 of coculture, some of them organized into vascular network-like structures (Movie S4). The percentage of coculture preparations that exhibited formation of vascular tube-like structures and/or beating behavior under specific culture conditions (either with or without growth factors) is shown in Figure 4. Interestingly, within the following 3 days of coculture, samples receiving growth factor supplementation (n = 9) displayed a lower incidence of vascular tube-like structures formation compared to control coculture preparations not receiving growth factors (P < 0.001, O.R. = 0.213, 95%, C.I. = 0.126–0.357). A similar trend was also observed for the presence of beating, growth factor supplementation resulting in a lower incidence of beating slices compared to control coculture preparations not receiving growth factors (P = 0.005, O.R. = 0.229, 95% C.I. = 0.082–0.637). Multi-variable logistic regression analysis indicated that growth factor supplementation and day of coculture, but not the presence of beating, are significant and independent positive predictors for vascular tube-like structures formation (data not shown). After adjustment for day of coculture and growth factor supplementation, on day 5 of coculture slice beating became a significant independent positive predictor for the vascular tube-like structures formation, too (P = 0.017, O.R. = 3.240, 95% C.I. = 1.235–8.501). In contrast with the living slices, in the presence of ischaemic ventricular slices EPCs failed to proliferate and fill the cavities of the ventricular slices (as assessed morphologically); furthermore, no vascular tube-like structures were detected in any ischaemic ventricular slice preparations, neither in the presence nor in the absence of growth factor supplementation (Fig. 3C). Moreover, EPCs failed to form vascular tube-like structures when cocultured with living ventricular slices in a transwell system (Fig. 3D).
Fig 3.
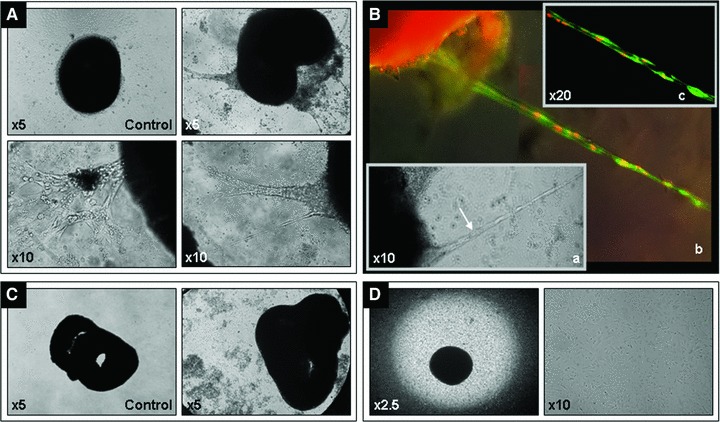
Representative images for cocultures of living and ischaemic murine embryonic ventricular slices with human EPCs – day 6 of coculture. Vascular tube-like structures formation in living ventricular slice preparations as compared to control-murine living ventricular slices only (A); tube-like structures formed in living ventricular slice preparations (B.a) were stained with Hoechst (nuclei, red, false colour) and Calcein AM (living cells, green); merged image (B.b); optical sectioning was applied to generate a multi-focus image (B.c). No vascular tube-like structures formation was detected in ischaemic ventricular slice preparations (C) or in living ventricular slice preparations cultured in a transwell system (D); Carl Zeiss Axiovert 10 microscope and Sony Electronics Digital Interface DFW-x700 camera system.
Fig 4.
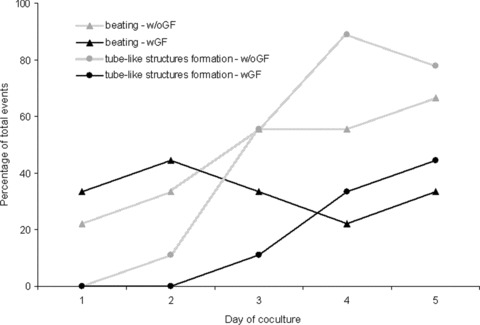
Dynamics of living cocultures. A higher number of living ventricular slices cocultured with EPCs in the absence of growth factors (w/oGF) were beating and showed vascular tube-like structures formation, as compared to preparations cocultured in the presence of growth factors (wGF).
To facilitate the assessment of vascular tube-like structures formation and cell integration, GFP-transfected EPCs were cocultured with living ventricular slices. By tracking of GFP-labelled cells we documented EPCs integration into the living slices starting on day 1 of coculture (Movie S5), as well as EPCs-associated vascular tube-like structures formation inside and outside of the ventricular slices (Fig. 5).
Fig 5.
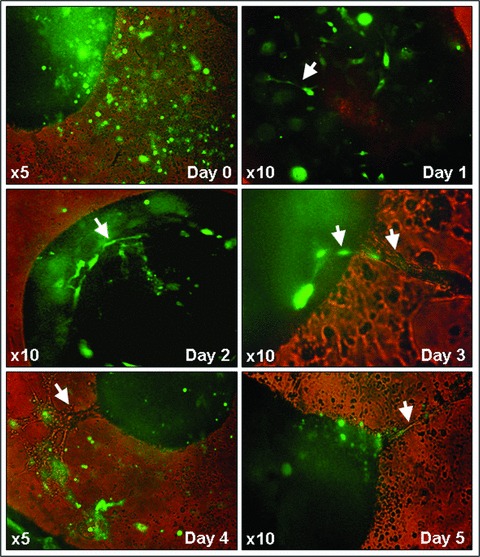
Representative images showing the dynamics of GFP-labelled EPCs integration and vascular tube-like structures formation in living murine embryonic ventricular slice preparations – day 1 to day 5 of coculture. Carl Zeiss Axiovert 10 microscope and Sony Electronics Digital Interface DFW-x700 camera system.
Immunohistochemical staining with anti-vWF and anti-nuclei antibodies, highly specific for human antigens, of serial ventricular sections generated from living cocultures, performed as early as day 6 of coculture, showed that EPCs became organized into vascular tube-like structures surrounding the ventricular slices and infiltrated into the depth of the living tissue (Fig. 6A–F). Cell integration into the living ventricular slices was documented as late as day 14 of coculture (Fig. 6G). The results were confirmed by experiments in which antibodies to human CD31 were employed, showing that EPCs formed capillary network-like structures on the surface of and into the ventricular slices (Fig. 6H and I). In addition, histochemical assessment of vascular tube-like structures, performed directly on living slice preparations, with Calcein AM and Hoechst allowed for further evaluation of tube-like structures morphology (Fig. 3B, Movie S6). Furthermore, injection of TMR-labelled dextran into these tube-like structures, showed the transport of the dye through the structures, indicating the existence of a lumen (Movies S7 and S8).
Fig 6.
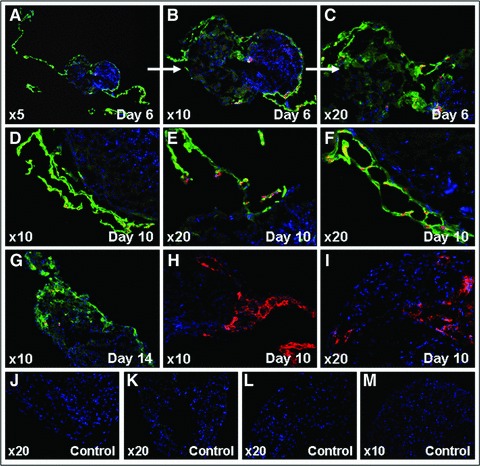
Immunohistochemical analyses showing EPCs integration and vascular tube-like structures formation in living murine embryonic ventricular slice preparations. Human vWF and human nuclei expression – day 6 (A–C), day 10 (D–F), and day 14 (G) of coculture; polyclonal rabbit anti-human vWF-Alexa Fluor 488 (green), monoclonal IgG1 mouse anti-human nuclei-Alexa Fluor 555 (red), and Hoechst (nuclei, blue); negative controls-secondary antibodies only (J) and murine ventricular slice only (K). Human CD31 expression-day 10 of coculture (H, I); monoclonal IgG1 mouse anti-human CD31-Alexa Fluor 555 (red) and Hoechst (nuclei, blue); negative controls-secondary antibodies only (L) and murine ventricular slice only (M). Carl Zeiss Axiovert 200M ApoTome and AxioVision 4.6 software.
Conversely, immunohistochemical staining of serial ischaemic ventricular sections with anti-human vWF and mitochondria antibodies, performed on day 6 of coculture, showed that EPCs attached to the ischaemic ventricular slices, integrated into the depth of the tissue, but failed to form any vascular tube-like structures (Fig. 7).
Fig 7.
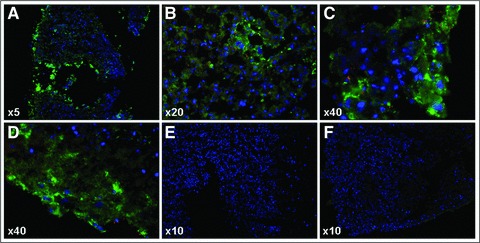
Immunohistochemical analyses for human vWF and human mitochondria expression, showing modest integration of EPCs into the ischaemic embryonic murine ventricular tissue and lack of vascular tube-like structures formation – day 6 of coculture (A–D); polyconal rabbit anti-human vWF-Alexa Fluor 488 (green), monoclonal IgG1mouse anti-human mitochondria-Alexa Fluor 555 (red), and Hoechst (nuclei, blue); negative controls-secondary antibodies only (E) and murine ventricular slice only (F). Carl Zeiss Axiovert 200M ApoTome and AxioVision 4.6 software.
Discussion
Our results demonstrate that human EPCs derived from UCB mononuclear cells had the capacity to integrate and form vascular tube-like structures when in direct contact with living murine embryonic ventricular slices. In contact with ischaemic murine ventricular slices, EPCs integrated but failed to form vascular tube-like structures.
EPCs integration and the formation of vascular-like structures in the ventricular slice preparations were assessed by GFP transfection, immunohistochemistry and histochemistry. Only a partial contribution of the GFP-positive EPCs to the vascular tube-like structures could be documented because GFP transfection was achieved with ∼75% efficiency, it was transient, and the vascular tubes formed around day 3 of coculture. The experiments were therefore completed by histochemistry and immunohistochemistry employing highly specific anti-human antibodies, which proved the formation, the extent and the human origin of the vascular tube-like structures generated by EPCs upon contact with murine living ventricular slices. In addition, injection of TMR dextran dye directly into the tube-like structures indicated the existence of a lumen by showing the transport of the dye through the structures. Because of its high molecular weight and size, dextran cannot be transferred between cells over gap junctions [36]. Hence, we concluded that by injecting the fluorescent dye-labelled dextran, we stained tube-like structures presenting a lumen and not just single cells.
Although the paracrine factors secreted by EPCs were shown to be the major contributors to angiogenesis and tissue neovascularization [37, 38], our data bring evidence that they are not the sole players. Although there is proof that hypoxia augmented growth factors secretion by EPCs [39], in our study, there was absolute lack of tube-like structures formation when ischaemic slices were employed in the coculture system, regardless of direct contact of EPCs with the ischaemic cardiac tissue or pro-angiogenic growth factor supplementation. This indicates that even if hypoxic conditions enhance growth factors secretion by EPCs, yet there was no vessel-like structures formation because of the lack of viable cell-to-cell interactions between EPCs and the cardiac tissue; these interactions may be compulsory for the release of essential trophic factors that promote neovascularization [40]. It has been well documented that chronic exposure to hypoxia and oxidative stress inhibited the ability of UCB-derived EPCs to form vascular tubes in vitro in the absence of supporting stromal/fibroblastic cells [41, 42]; furthermore, human dermal fibroblasts, capable of secreting essential growth factors and extracellular matrix components, provided a niche for vascular tubes development in vitro [43]. Taken together, these observations suggest that direct living cell-to-cell interactions and secretion of essential soluble factors by the living myocardium/cardiac matrix are necessary to promote vascular tubes formation within the cardiac niche.
In the randomized clinical trials of intracoronary bone marrow-derived mononuclear cell therapy employed for the treatment of acute myocardial infarction [44, 45], the modest and transient clinical benefits, most likely exerted by paracrine mechanisms [46], might be caused by the failure of the injected cells to integrate and repopulate the ischaemic tissue; this may be due to the lack of living extracellular matrix components and/or engraftment-promoting factors in which the infarcted tissue is deficient. Because of the limited success of both clinical and pre-clinical studies that used single cell types to induce cardiac regeneration [12, 13, 44, 45], recent efforts have been focused on coculturing approaches to induce synergistic tissue-repair effects. In this regard, coculturing of UCB-derived cells with other cell types, such as umbilical cord-derived myofibroblasts [47] or cells isolated from brown adipose tissue [48], resulted in improved cardiac regeneration effects. Therefore, the in vitro cardiac transplantation models used in this study could be further utilized for the investigation of synergistic cardiovascular repair effects induced by mixed cell types [42, 43, 47–49], including EPCs, for optimization of cell therapy approaches.
Our data also indicated that supplementation of coculture medium with growth factors impairs the vascular tube-like structure formation in living coculture preparations. We assume that some of the growth factors contained in the supplement may have induced anti-angiogenic effects. It has been demonstrated that the growth factors used in this study stimulated differentiation and proliferation of EPCs, and induced angiogenic effects [50, 51]; however, the high growth factor concentrations that had been used might have induced undesired anti-angiogenic effects through release of anti-angiogenic factors. This hypothesis is supported by the fact that much lower concentrations of these growth factors were used by other groups in the composition of EPCs differentiation medium for studies addressing the assessment of EPCs angiogenic function [52, 53]. Although VEGF has been primarily identified as an endothelial cell specific growth factor stimulating angiogenesis [54], IGF-1 supplementation might have up-regulated the expression of anti-angiogenic isoforms of VEGF [55]. Furthermore, the anti-angiogenic factors thrombospondin (TSP)-1 [56] and pigment epithelium-derived factor [37], secreted by EPCs, have been associated with their dysfunction and delayed re-endothelialization. It has been reported that early preservation of the myocardial mass is an important target for therapy of myocardial infarction, not only to prevent mechanical dysfunction, but also to maintain proper secretory function [38]. Since we have demonstrated that cellular interaction between EPCs and the cardiac tissue was needed for vascular tube-like structures formation, we hereby further hypothesize that growth factors supplementation may have interfered with the innate signalling pathways of the candidate paracrine factors secreted by EPCs and cardiomyocytes, which modulate vascular tube-like structures formation. It has been shown that EPCs interaction with the cardiomyocytes led to paracrine secretion of transforming growth factor-β [40], which induces anti-angiogenic factors, such as TSP-1, a key negative regulator of EPCs function [57]. Furthermore, exogenous growth factors supplementation in the presence of EPCs paracrine growth factors secretion, up-regulated growth factor release by the host cardiac tissue [38]. Therefore, in our experiments, upon growth factor supplementation, the coculture milieu might have been markedly modified, thus negatively influencing vascular tube-like structures formation.
Our observation that beating ventricular slices are an independent positive predictor for vascular tube-like structures formation brings further indication that muscle contraction, through mechanical forces acting on the tissue, may be an important contributor to angiogenesis [58–61]. It has been well documented that mechanical stretch can induce signals that modulate endothelial cell invasion [62] and vascular remodelling [61], mainly through matrix metalloproteinases and VEGF expression [58–60, 63–65]. In our model, the addition of growth factors may have interfered with the innate process of shear stress-inducing vasculogenesis. It has been illustrated that bFGF, which controls intracellular Ca2+ homeostasis, induced negative inotropic effects on cardiomyocytes in a concentration-dependent manner [66, 67]. Despite of these interesting findings, it has been difficult to establish experiments that quantitatively impart and/or control contraction stimuli and relate them to the in vivo angiogenic/vasculogenic outcome [68]. Therefore, our in vitro transplantation model, which provides a well-controlled, reproducible experimental setting, could answer essential questions on how cardiac contraction induces vasculogenesis and how EPCs-induced vasculogenesis is controlled to accommodate the demands within active cardiac muscle.
Tissue-targeted cell therapies relying on ligand–receptor interactions have been proposed during the recent years. The chemokine receptors identified on EPCs (i.e. CXCR4, VEGFR2, Tie-2) may play important roles in EPCs integration into the ventricular tissue, in response to specific ligands secreted by the cardiac tissue. Thus, our in vitro transplantation models could be used to investigate and establish signalling pathways suggested as having a role in EPCs migration and integration into the cardiac tissue. Furthermore, the vasculogenesis model generated in this study could serve for alternative drug testing of pro-angiogenic inhibitors suggested for cancer therapy. Nevertheless, the embryonic cardiac slices might be also useful to elucidate the mechanisms of vascular tubes formation by UCB-derived EPCs, in response to the angiogenic program dictated by the embryonic tissue, which possesses unique properties to influence survival and differentiation [69].
Human UCB is easy and inexpensive to collect and cryopreserve for potential therapeutic use. The accessibility of EPCs isolation could even enable the use of vascular engineered grafts immediately after birth for the treatment of congenital cardiovascular diseases, since a UCB sample can be harvested prenatally, by ultrasound guided cordocentesis [47]. Our data bring further evidence that the UCB-derived EPCs may be a valuable cell source to induce neovascularization in the setting of cardiovascular repair. Therefore, the investigation in standardized, well-controlled settings of EPCs interactions with the cardiac niche, which may regulate their integration and vascular tubes formation, deserves special consideration.
Acknowledgments
This work was supported by the EU Framework Programme 7 (FP7)-Marie Curie international reintegration grant no. 224888/2008–2010 (ML); Romanian Ministry of Education and Research reintegration and cooperation PNCDI-II grants no. 2/2008–2010 (M.L.) and no. 31042/2007–2010 (H.M.), respectively; EU FP7-Capacity grant no. 245691/2010–2013; Romanian Ministry of Education and Research/German Federal Ministry of Education and Research (BMBF) bilateral cooperation grant no. 347/2009–2010 (M.L.) and BMBF grant no. 01GN0947 (M.K.). We are grateful to Dr. Anca Sima for the kind gift of ac-LDL, Dr. Elena Constantinescu for transmission electron microscopy, Drs. Alina Constantin and Constantina Heltianu for assistance in statistical data analysis, and Moritz Haustein for oxygen saturation measurements.
Conflict of interest
The authors confirm that there are no conflicts of interest.
Supporting Information
Functional testing of EPCs.
Assessment of surface markers on EPCs by flow-cytometry.
Immunofluorescence analyses on EPCs.
mRNA expression of endothelial markers determined in EPCs as compared to human umbilical cord vascular endothelial cells (HUVECs)-positive control.
GFP transfection of EPCs and maintenance of their capacity to form tube-like structures in Matrigel.
Assessment of O2 concentration andO2 saturation into the ischaemic chamber during the first 4 hrs of inducement of oxygen-glucose deprivation.
Sequences of the oligonucleotide primers used for reverse transcription polymerase chain reaction.
Initiation of vascular tube-like structuresformation on day 3 of coculture of human EPCs with living murineembryonic ventricular tissue (×10).
Assessment of vascular tube-like structuresdevelop-ment over a 5-day period, following coculture of human EPCswith living murine embryonic ventricular tissue (×5).
High magnification (×20) of a vasculartube-like structure generated following coculture of human EPCswith living murine embryonic ventricular tissue-day 5 ofcoculture.
Vascular network-like structure generatedfollowing coculture of human EPCs with living murine embryonicventricular tissue-day 5 of coculture (×10).
Integration of GFP-labelled human EPCs intoliving murine embryonic ventricular slice preparations startingfrom day 1 of coculture (×10).
Tube-like structure formed in a livingventricular slice preparation-day 6 of coculture, stained withHoechst (nuclei, red, false colour) and Calcein AM (living cells,green); optical sectioning was applied to generate a multi-focusmovie (×20).
Tetramethylrhodamine dextran injection byiontophoresis via a sharp electrode into a tube-like structure, while the living ventricular slice was beating-day 6 of coculture.
Tetramethyrhodamine dextrane injection byiontophoresis via a sharp electrode into a tube like-structure formed following coculture of EPCs with living ventricular tissue-day 6 of coculture.
References
- 1.Pillekamp F, Khalil M, Emmel M, et al. Stem cells in pediatric heart failure. Minerva Cardioangiol. 2008;56:335–48. [PubMed] [Google Scholar]
- 2.Ballen K. Challenges in umbilical cord blood stem cell banking for stem cell reviews and reports. Stem Cell Rev. 2010;6:8–14. doi: 10.1007/s12015-009-9105-x. [DOI] [PubMed] [Google Scholar]
- 3.Erices A, Conget P, Minguell JJ. Mesenchymal progenitor cells in human umbilical cord blood. Br J Haematol. 2000;109:235–42. doi: 10.1046/j.1365-2141.2000.01986.x. [DOI] [PubMed] [Google Scholar]
- 4.Mayani H, Lansdorp PM. Biology of human umbilical cord blood-derived hematopoietic stem/progenitor cells. Stem Cells. 1998;16:153–65. doi: 10.1002/stem.160153. [DOI] [PubMed] [Google Scholar]
- 5.Kogler G, Sensken S, Airey JA, et al. A new human somatic stem cell from placental cord blood with intrinsic pluripotent differentiation potential. J Exp Med. 2004;200:123–35. doi: 10.1084/jem.20040440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldstein G, Toren A, Nagler A. Human umbilical cord blood biology, transplantation and plasticity. Curr Med Chem. 2006;13:1249–59. doi: 10.2174/092986706776872998. [DOI] [PubMed] [Google Scholar]
- 7.Hristov M, Erl W, Weber PC. Endothelial progenitor cells: mobilization, differentiation, and homing. Arterioscler Thromb Vasc Biol. 2003;23:1185–9. doi: 10.1161/01.ATV.0000073832.49290.B5. [DOI] [PubMed] [Google Scholar]
- 8.Wu KH, Zhou B, Lu SH, et al. In vitro and in vivo differentiation of human umbilical cord derived stem cells into endothelial cells. J Cell Biochem. 2007;100:608–16. doi: 10.1002/jcb.21078. [DOI] [PubMed] [Google Scholar]
- 9.Ingram DA, Mead LE, Tanaka H, et al. Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood. 2004;104:2752–60. doi: 10.1182/blood-2004-04-1396. [DOI] [PubMed] [Google Scholar]
- 10.Murohara T. Cord blood-derived early outgrowth endothelial progenitor cells. Microvasc Res. 2010;79:174–7. doi: 10.1016/j.mvr.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 11.Droetto S, Viale A, Primo L, et al. Vasculogenic potential of long term repopulating cord blood progenitors. FASEB J. 2004;18:1273–5. doi: 10.1096/fj.03-1444fje. [DOI] [PubMed] [Google Scholar]
- 12.Botta R, Gao E, Stassi G, et al. Heart infarct in NOD-SCID mice: therapeutic vasculogenesis by transplantation of human CD34+ cells and low dose CD34+KDR+ cells. FASEB J. 2004;18:1392–4. doi: 10.1096/fj.03-0879fje. [DOI] [PubMed] [Google Scholar]
- 13.Ott I, Keller U, Knoedler M, et al. Endothelial-like cells expanded from CD34+ blood cells improve left ventricular function after experimental myocardial infarction. FASEB J. 2005;19:992–4. doi: 10.1096/fj.04-3219fje. [DOI] [PubMed] [Google Scholar]
- 14.Yang C, Zhang ZH, Li ZJ, et al. Enhancement of neovascularization with cord blood CD133+ cell-derived endothelial progenitor cell transplantation. Thromb Haemost. 2004;91:1202–12. doi: 10.1160/TH03-06-0378. [DOI] [PubMed] [Google Scholar]
- 15.Kocher AA, Schuster MD, Szabolcs MJ, et al. Neovascularization of ischaemic myocardium by human bone-marrow-derived angioblasts prevents cardiomyocyte apoptosis, reduces remodeling and improves cardiac function. Nat Med. 2001;7:430–6. doi: 10.1038/86498. [DOI] [PubMed] [Google Scholar]
- 16.Kawamoto A, Gwon HC, Iwaguro H, et al. Therapeutic potential of ex vivo expanded endothelial progenitor cells for myocardial ischemia. Circulation. 2001;103:634–7. doi: 10.1161/01.cir.103.5.634. [DOI] [PubMed] [Google Scholar]
- 17.Prat-Vidal C, Roura S, Farre J, et al. Umbilical cord blood-derived stem cells spontaneously express cardiomyogenic traits. Transplant Proc. 2007;39:2434–7. doi: 10.1016/j.transproceed.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 18.Nishiyama N, Miyoshi S, Hida N, et al. The significant cardiomyogenic potential of human umbilical cord blood-derived mesenchymal stem cells in vitro. Stem Cells. 2007;25:2017–24. doi: 10.1634/stemcells.2006-0662. [DOI] [PubMed] [Google Scholar]
- 19.Bonanno G, Mariotti A, Procoli A, et al. Human cord blood CD133+ cells immunoselected by a clinical-grade apparatus differentiate in vitro into endothelial- and cardiomyocyte-like cells. Transfusion. 2007;47:280–9. doi: 10.1111/j.1537-2995.2007.01104.x. [DOI] [PubMed] [Google Scholar]
- 20.Ma N, Ladilov Y, Kaminski A, et al. Umbilical cord blood cell transplantation for myocardial regeneration. Transplant Proc. 2006;38:771–3. doi: 10.1016/j.transproceed.2006.01.061. [DOI] [PubMed] [Google Scholar]
- 21.Rabald S, Marx G, Mix B, et al. Cord blood cell therapy alters LV remodeling and cytokine expression but does not improve heart function after myocardial infarction in rats. Cell Physiol Biochem. 2008;21:395–8. doi: 10.1159/000129632. [DOI] [PubMed] [Google Scholar]
- 22.Moelker AD, Baks T, Wever KM, et al. Intracoronary delivery of umbilical cord blood derived unrestricted somatic stem cells is not suitable to improve LV function after myocardial infarction in swine. J Mol Cell Cardiol. 2007;42:735–45. doi: 10.1016/j.yjmcc.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 23.Sys SU, De Keulenaer GW, Brutsaert DL. Reappraisal of the multicellular preparation for the in vitro physiopharmacological evaluation of myocardial performance. Adv Exp Med Biol. 1998;453:441–50. doi: 10.1007/978-1-4684-6039-1_49. [DOI] [PubMed] [Google Scholar]
- 24.Beeres SL, Atsma DE, van der Laarse A. Human adult bone marrow mesenchymal stem cells repair experimental conduction block in rat cardiomyocyte cultures. J Am Coll Cardiol. 2005;46:1943–52. doi: 10.1016/j.jacc.2005.07.055. [DOI] [PubMed] [Google Scholar]
- 25.Wollert KC, Meyer GP, Lotz J, et al. Intracoronary autologous bone-marrow cell transfer after myocardial infarction: the BOOST randomised controlled clinical trial. Lancet. 2004;364:141–8. doi: 10.1016/S0140-6736(04)16626-9. [DOI] [PubMed] [Google Scholar]
- 26.Pillekamp F, Reppel M, Dinkelacker V, et al. Establishment and characterization of a mouse embryonic heart slice preparation. Cell Physiol Biochem. 2005;16:127–32. doi: 10.1159/000087739. [DOI] [PubMed] [Google Scholar]
- 27.Pillekamp F, Halbach M, Reppel M, et al. Neonatal murine heart slices. A robust model to study ventricular isometric contractions. Cell Physiol Biochem. 2007;20:837–46. doi: 10.1159/000110443. [DOI] [PubMed] [Google Scholar]
- 28.Halbach M, Pillekamp F, Brockmeier K, et al. Ventricular slices of adult mouse hearts-a new multicellular in vitro model for electrophysiological studies. Cell Physiol Biochem. 2006;18:1–8. doi: 10.1159/000095132. [DOI] [PubMed] [Google Scholar]
- 29.Pillekamp F, Reppel M, Rubenchyk O, et al. Force measurements of human embryonic stem cell-derived cardiomyocytes in an in vitro transplantation model. Stem Cells. 2007;25:174–80. doi: 10.1634/stemcells.2006-0094. [DOI] [PubMed] [Google Scholar]
- 30.Zhang JG, Ghosh S, Ockleford CD, et al. Characterization of an in vitro model for the study of the short and prolonged effects of myocardial ischaemia and reperfusion in man. Clin Sci. (Lond.) 2000;99:443–53. [PubMed] [Google Scholar]
- 31.Halbach M, Pfannkuche K, Pillekamp F, et al. Electrophysiological maturation and integration of murine fetal cardiomyocytes after transplantation. Circ Res. 2007;101:484–92. doi: 10.1161/CIRCRESAHA.107.153643. [DOI] [PubMed] [Google Scholar]
- 32.Eriksson U, Alitalo K. VEGF receptor 1 stimulates stem-cell recruitment and new hope for angiogenesis therapies. Nat Med. 2002;8:775–7. doi: 10.1038/nm0802-775. [DOI] [PubMed] [Google Scholar]
- 33.Lugus JJ, Chung YS, Mills JC, et al. GATA2 functions at multiple steps in hemangioblast development and differentiation. Development. 2007;134:393–405. doi: 10.1242/dev.02731. [DOI] [PubMed] [Google Scholar]
- 34.Debacker C, Catala M, Labastie MC. Embryonic expression of the human GATA-3 gene. Mech Dev. 1999;85:183–7. doi: 10.1016/s0925-4773(99)00088-x. [DOI] [PubMed] [Google Scholar]
- 35.Jiang Y, Drysdale TA, Evans T. A role for GATA-4/5/6 in the regulation of Nkx2.5 expression with implications for patterning of the precardiac field. Dev Biol. 1999;216:57–71. doi: 10.1006/dbio.1999.9469. [DOI] [PubMed] [Google Scholar]
- 36.Simon AM, McWhorter AR, Chen H, et al. Decreased intercellular communication and connexin expression in mouse aortic endothelium during lipopolysaccharide-induced inflammation. J Vasc Res. 2004;41:323–33. doi: 10.1159/000079614. [DOI] [PubMed] [Google Scholar]
- 37.Jujo K, Ii M, Losordo DW. Endothelial progenitor cells in neovascularization of infarcted myocardium. J Mol Cell Cardiol. 2008;45:530–44. doi: 10.1016/j.yjmcc.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cho HJ, Lee N, Lee JY, et al. Role of host tissues for sustained humoral effects after endothelial progenitor cell transplantation into the ischemic heart. J Exp Med. 2007;204:3257–69. doi: 10.1084/jem.20070166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Di Santo S, Yang Z, Wyler von Ballmoos M, et al. Novel cell-free strategy for therapeutic angiogenesis: in vitro generated conditioned medium can replace progenitor cell transplantation. PLoS One. 2009;4:e5643–53. doi: 10.1371/journal.pone.0005643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Doyle B, Sorajja P, Hynes B, et al. Progenitor cell therapy in a porcine acute myocardial infarction model induces cardiac hypertrophy, mediated by paracrine secretion of cardiotrophic factors including TGFbeta1. Stem Cells Dev. 2008;17:941–51. doi: 10.1089/scd.2007.0214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ingram DA, Krier TR, Mead LE, et al. Clonogenic endothelial progenitor cells are sensitive to oxidative stress. Stem Cells. 2007;25:297–304. doi: 10.1634/stemcells.2006-0340. [DOI] [PubMed] [Google Scholar]
- 42.Kobayashi H, Shimizu T, Yamato M, et al. Fibroblast sheets co-cultured with endothelial progenitor cells improve cardiac function of infarcted hearts. J Artif Organs. 2008;11:141–7. doi: 10.1007/s10047-008-0421-8. [DOI] [PubMed] [Google Scholar]
- 43.Bishop ET, Bell GT, Bloor S, et al. An in vitro model of angiogenesis: basic features. Angiogenesis. 1999;3:335–44. doi: 10.1023/a:1026546219962. [DOI] [PubMed] [Google Scholar]
- 44.Schachinger V, Erbs S, Elsasser A, et al. Improved clinical outcome after intracoronary administration of bone-marrow-derived progenitor cells in acute myocardial infarction: final 1-year results of the REPAIR-AMI trial. Eur Heart J. 2006;27:2775–83. doi: 10.1093/eurheartj/ehl388. [DOI] [PubMed] [Google Scholar]
- 45.Lunde K, Solheim S, Aakhus S, et al. Intracoronary injection of mononuclear bone marrow cells in acute myocardial infarction. N Eng J Med. 2006;355:1199–209. doi: 10.1056/NEJMoa055706. [DOI] [PubMed] [Google Scholar]
- 46.Gnecchi M, He HM, Liang OD, et al. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat Med. 2005;11:367–8. doi: 10.1038/nm0405-367. [DOI] [PubMed] [Google Scholar]
- 47.Schmidt D, Mol A, Neuenschwander S, et al. Living patches engineered from human umbilical cord derived fibroblasts and endothelial progenitor cells. Eur J Cardiothorac Surg. 2005;27:795–800. doi: 10.1016/j.ejcts.2005.01.064. [DOI] [PubMed] [Google Scholar]
- 48.Yamada Y, Yokoyama S, Fukuda N, et al. A novel approach for myocardial regeneration with educated cord blood cells cocultured with cells from brown adipose tissue. Biochem Biophys Res Commun. 2007;353:182–8. doi: 10.1016/j.bbrc.2006.12.017. [DOI] [PubMed] [Google Scholar]
- 49.Sekine H, Shimizu T, Hobo K, et al. Endothelial cell coculture within tissue-engineered cardiomyocyte sheets enhances neovascularization and improves cardiac function of ischemic hearts. Circulation. 2008;118:S145–52. doi: 10.1161/CIRCULATIONAHA.107.757286. [DOI] [PubMed] [Google Scholar]
- 50.Reinisch A, Strunk D. Isolation and animal serum free expansion of human umbilical cord derived mesenchymal stromal cells (MSCs) and endothelial colony forming progenitor cells (ECFCs) J Vis Exp. 2009 doi: 10.3791/1525. 10.3791/1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schmidt D, Mol A, Odermatt B, et al. Engineering of biologically active living heart valve leaflets using human umbilical cord-derived progenitor cells. Tissue Eng. 2006;12:3223–32. doi: 10.1089/ten.2006.12.3223. [DOI] [PubMed] [Google Scholar]
- 52.Duan HX, Cheng LM, Wang J, et al. Angiogenic potential difference between two types of endothelial progenitor cells from human umbilical cord blood. Cell Biol Int. 2006;30:1018–27. doi: 10.1016/j.cellbi.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 53.Krenning G, Dankers PY, Drouven JW, et al. Endothelial progenitor cell dysfunction in patients with progressive chronic kidney disease. Am J Physiol Renal Physiol. 2009;296:F1314–22. doi: 10.1152/ajprenal.90755.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Byrne AM, Bouchier-Hayes DJ, Harmey JH. Angiogenic and cell survival functions of vascular endothelial growth factor (VEGF) J Cell Mol Med. 2005;9:777–94. doi: 10.1111/j.1582-4934.2005.tb00379.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nowak DG, Woolard J, Amin EM, et al. Expression of pro- and anti-angiogenic isoforms of VEGF is differentially regulated by splicing and growth factors. J Cell Sci. 2008;121:3487–95. doi: 10.1242/jcs.016410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ii M, Takenaka H, Asai J, et al. Endothelial progenitor thrombospondin-1 mediates diabetes-induced delay in reendothelialization following arterial injury. Circ Res. 2006;98:697–704. doi: 10.1161/01.RES.0000209948.50943.ea. [DOI] [PubMed] [Google Scholar]
- 57.Nakagawa T, Li JH, Garcia G, et al. TGF-beta induces proangiogenic and antiangiogenic factors via parallel but distinct Smad pathways. Kidney Int. 2004;66:605–13. doi: 10.1111/j.1523-1755.2004.00780.x. [DOI] [PubMed] [Google Scholar]
- 58.Haas TL, Milkiewicz M, Davis SJ, et al. Matrix metalloproteinase activity is required for activity-induced angiogenesis in rat skeletal muscle. Am J Physiol Heart Circ Physiol. 2000;279:H1540–7. doi: 10.1152/ajpheart.2000.279.4.H1540. [DOI] [PubMed] [Google Scholar]
- 59.Brown MD, Hudlicka O. Modulation of physiological angiogenesis in skeletal muscle by mechanical forces: involvement of VEGF and metalloproteinases. Angiogenesis. 2003;6:1–14. doi: 10.1023/a:1025809808697. [DOI] [PubMed] [Google Scholar]
- 60.Hanjaya-Putra D, Yee J, Ceci D, et al. Vascular endothelial growth factor and substrate mechanics regulate in vitro tubulogenesis of endothelial progenitor cells. J Cell Mol Med. 2010;14:2436–47. doi: 10.1111/j.1582-4934.2009.00981.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Egginton S, Zhou AL, Brown MD, et al. Unorthodox angiogenesis in skeletal muscle. Cardiovasc Res. 2001;49:634–46. doi: 10.1016/s0008-6363(00)00282-0. [DOI] [PubMed] [Google Scholar]
- 62.Kang H, Bayless KJ, Kaunas R. Fluid shear stress modulates endothelial cell invasion into three-dimensional collagen matrices. Am J Physiol Heart Circ Physiol. 2008;295:H2087–97. doi: 10.1152/ajpheart.00281.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yamaguchi S, Yamaguchi M, Yatsuyanagi E, et al. Cyclic strain stimulates early growth response gene product 1-mediated expression of membrane type 1 matrix metalloproteinase in endothelium. Lab Invest. 2002;82:949–56. doi: 10.1097/01.lab.0000020408.77307.e9. [DOI] [PubMed] [Google Scholar]
- 64.Zheng W, Seftor EA, Meininger CJ, et al. Mechanisms of coronary angiogenesis in response to stretch: role of VEGF and TGF-beta. Am J Physiol Heart Circ Physiol. 2001;280:H909–17. doi: 10.1152/ajpheart.2001.280.2.H909. [DOI] [PubMed] [Google Scholar]
- 65.Egginton S. Invited review: activity-induced angiogenesis. Pflugers Arch. 2009;457:963–977. doi: 10.1007/s00424-008-0563-9. [DOI] [PubMed] [Google Scholar]
- 66.Ishibashi Y, Urabe Y, Tsutsui H, et al. Negative inotropic effect of basic fibroblast growth factor on adult rat cardiac myocyte. Circulation. 1997;96:2501–4. doi: 10.1161/01.cir.96.8.2501. [DOI] [PubMed] [Google Scholar]
- 67.Padua RR, Merle PL, Doble BW, et al. FGF-2-induced negative inotropism and cardioprotection are inhibited by chelerythrine: involvement of sarcolemmal calcium-independent protein kinase C. J Mol Cell Cardiol. 1998;30:2695–709. doi: 10.1006/jmcc.1998.0832. [DOI] [PubMed] [Google Scholar]
- 68.Prior BM, Yang HT, Terjung RL. What makes vessels grow with exercise training. J Appl Physiol. 2004;97:1119–28. doi: 10.1152/japplphysiol.00035.2004. [DOI] [PubMed] [Google Scholar]
- 69.Eisenberg CA, Burch JB, Eisenberg LM. Bone marrow cells transdifferentiate to cardiomyocytes when introduced into the embryonic heart. Stem Cells. 2006;24:1236–45. doi: 10.1634/stemcells.2005-0128. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Functional testing of EPCs.
Assessment of surface markers on EPCs by flow-cytometry.
Immunofluorescence analyses on EPCs.
mRNA expression of endothelial markers determined in EPCs as compared to human umbilical cord vascular endothelial cells (HUVECs)-positive control.
GFP transfection of EPCs and maintenance of their capacity to form tube-like structures in Matrigel.
Assessment of O2 concentration andO2 saturation into the ischaemic chamber during the first 4 hrs of inducement of oxygen-glucose deprivation.
Sequences of the oligonucleotide primers used for reverse transcription polymerase chain reaction.
Initiation of vascular tube-like structuresformation on day 3 of coculture of human EPCs with living murineembryonic ventricular tissue (×10).
Assessment of vascular tube-like structuresdevelop-ment over a 5-day period, following coculture of human EPCswith living murine embryonic ventricular tissue (×5).
High magnification (×20) of a vasculartube-like structure generated following coculture of human EPCswith living murine embryonic ventricular tissue-day 5 ofcoculture.
Vascular network-like structure generatedfollowing coculture of human EPCs with living murine embryonicventricular tissue-day 5 of coculture (×10).
Integration of GFP-labelled human EPCs intoliving murine embryonic ventricular slice preparations startingfrom day 1 of coculture (×10).
Tube-like structure formed in a livingventricular slice preparation-day 6 of coculture, stained withHoechst (nuclei, red, false colour) and Calcein AM (living cells,green); optical sectioning was applied to generate a multi-focusmovie (×20).
Tetramethylrhodamine dextran injection byiontophoresis via a sharp electrode into a tube-like structure, while the living ventricular slice was beating-day 6 of coculture.
Tetramethyrhodamine dextrane injection byiontophoresis via a sharp electrode into a tube like-structure formed following coculture of EPCs with living ventricular tissue-day 6 of coculture.


