Abstract
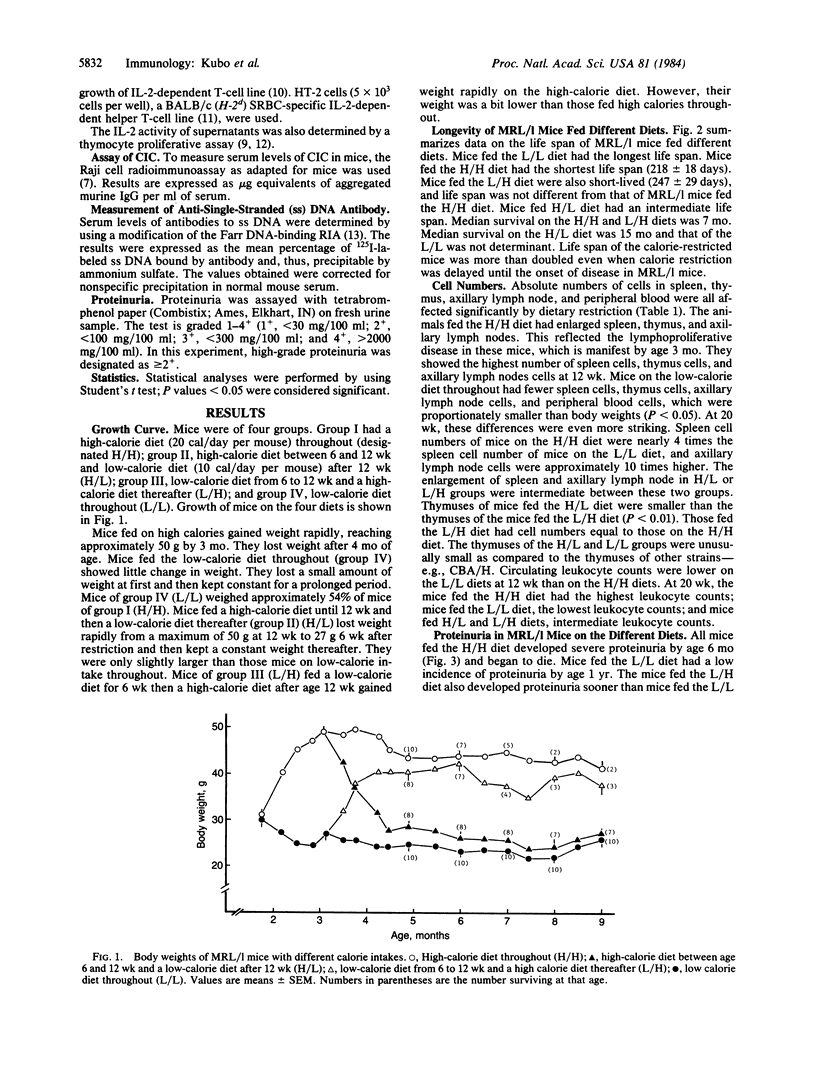
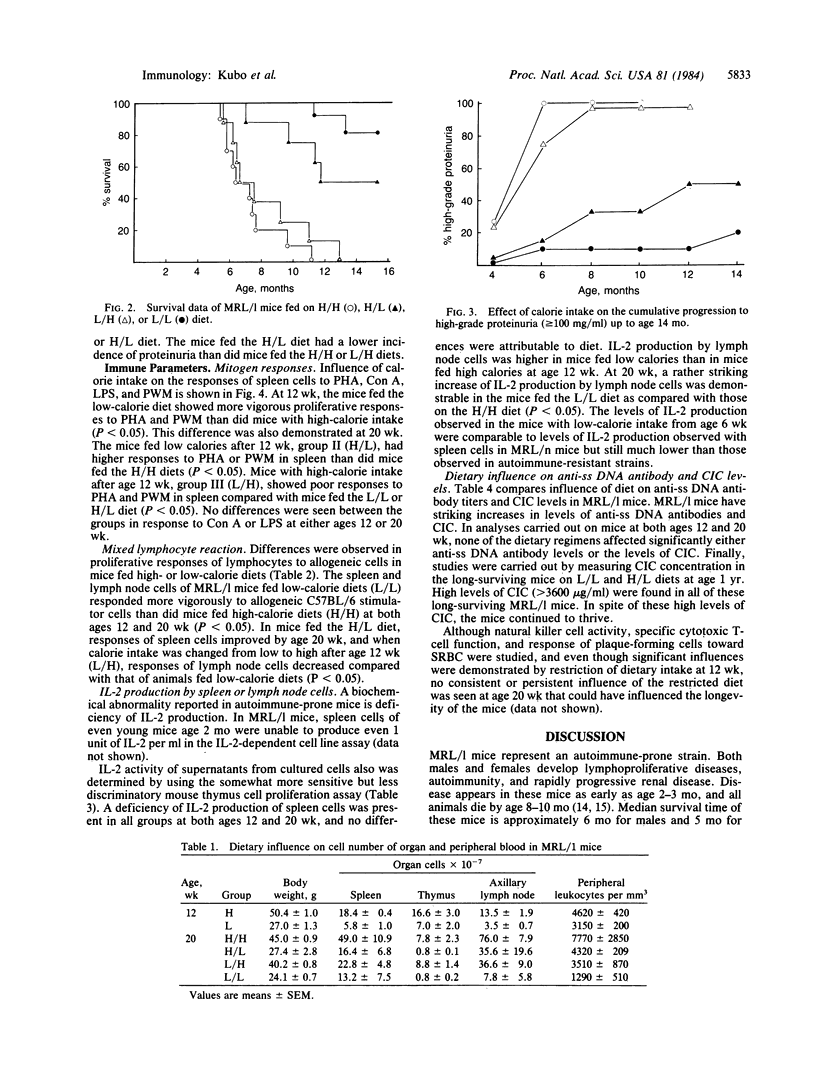
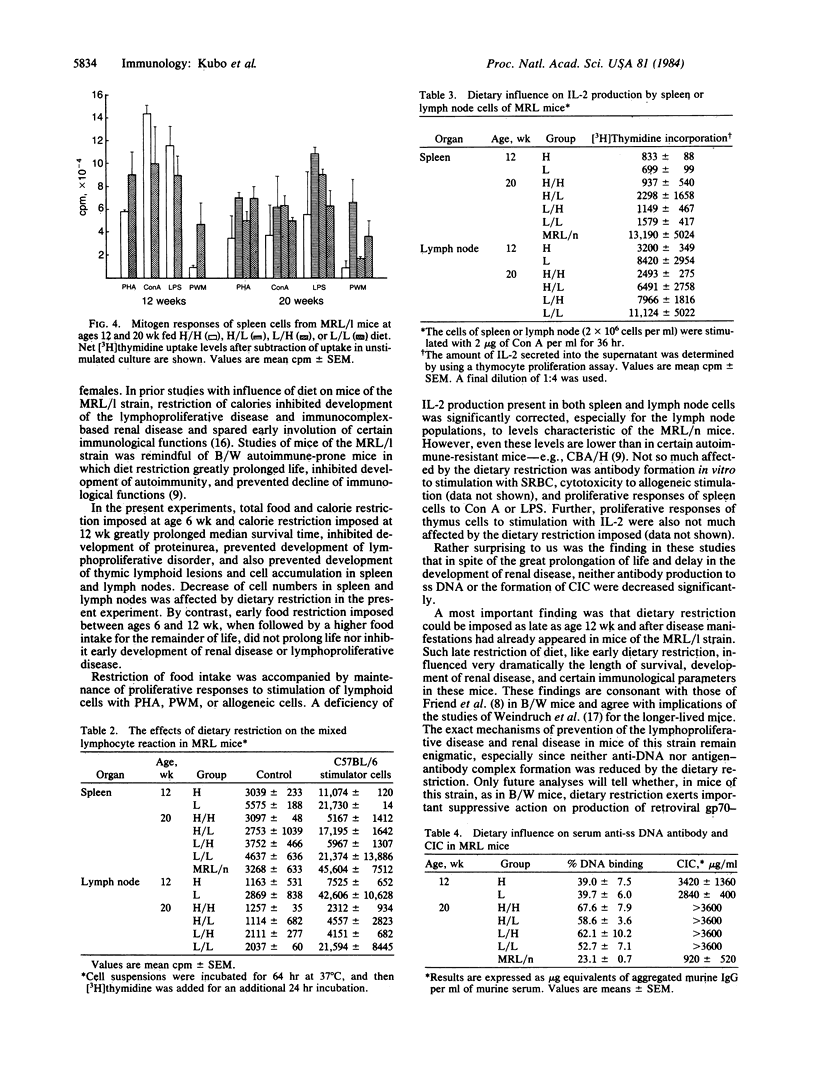
Reduced food intake doubles and even triples the life span of (NZB X NZW)F1 (B/W) mice and greatly influences of food intake while keeping vitamin and mineral intake constant in mice of the MRL/Mp-lpr/lpr (MRL/l) strain. Restriction of food intake greatly prolongs life. This influence also was seen when dietary restriction was imposed later in life. Dietary restriction inhibited development of lymphoproliferative disease and greatly decreased the numbers of cells in thymus, lymph nodes, and spleen. It also delayed development of glomerulonephritis and maintained certain immunological responses. Proliferative responses to phytohemagglutinin, pokeweed mitogen, or allogeneic spleen cells were maintained in the mice fed a low-calorie diet from 6 wk. Imposing diet at 12 wk had a lesser influence than earlier restriction. These dietary influences did not depress formation of anti-DNA antibodies or circulating immunocomplexes. MRL/l mice show an apparently extremely low production of interleukin 2, and dietary restriction increased the capacity of lymph node cells but not spleen cells to produce this immunomodulator.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adelman N. E., Watling D. L., McDevitt H. O. Treatment of (NZB x NZW)F1 disease with anti-I-A monoclonal antibodies. J Exp Med. 1983 Oct 1;158(4):1350–1355. doi: 10.1084/jem.158.4.1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman A., Theofilopoulos A. N., Weiner R., Katz D. H., Dixon F. J. Analysis of T cell function in autoimmune murine strains. Defects in production and responsiveness to interleukin 2. J Exp Med. 1981 Sep 1;154(3):791–808. doi: 10.1084/jem.154.3.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauphinée M. J., Kipper S. B., Wofsy D., Talal N. Interleukin 2 deficiency is a common feature of autoimmune mice. J Immunol. 1981 Dec;127(6):2483–2487. [PubMed] [Google Scholar]
- Fernandes G., Friend P., Yunis E. J., Good R. A. Influence of dietary restriction on immunologic function and renal disease in (NZB x NZW) F1 mice. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1500–1504. doi: 10.1073/pnas.75.3.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes G., Yunis E. J., Good R. A. Influence of diet on survival of mice. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1279–1283. doi: 10.1073/pnas.73.4.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes G., Yunis E. J., Good R. A. Influence of protein restriction on immune functions in NZB mice. J Immunol. 1976 Mar;116(3):782–790. [PubMed] [Google Scholar]
- Fernandes G., Yunis E. J., Jose D. G., Good R. A. Dietary influence on antinuclear antibodies and cell-mediated immunity in NZB mice. Int Arch Allergy Appl Immunol. 1973;44(6):770–782. doi: 10.1159/000230981. [DOI] [PubMed] [Google Scholar]
- Fernandes G., Yunis E. J., Smith J., Good R. A. Dietary influence on breeding behavior, hemolytic anemia, and longevity in NZB mice. Proc Soc Exp Biol Med. 1972 Apr;139(4):1189–1196. doi: 10.3181/00379727-139-36327. [DOI] [PubMed] [Google Scholar]
- Friend P. S., Fernandes G., Good R. A., Michael A. F., Yunis E. J. Dietary restrictions early and late: effects on the nephropathy of the NZB X NZW mouse. Lab Invest. 1978 Jun;38(6):629–632. [PubMed] [Google Scholar]
- Gabrielsen A. E., Lubert A. S., Olsen C. T. Suppression of murine lupus erythematosus by dactinomycin. Nature. 1976 Dec 2;264(5585):439–440. doi: 10.1038/264439a0. [DOI] [PubMed] [Google Scholar]
- Gillis S., Ferm M. M., Ou W., Smith K. A. T cell growth factor: parameters of production and a quantitative microassay for activity. J Immunol. 1978 Jun;120(6):2027–2032. [PubMed] [Google Scholar]
- Hang L., Theofilopoulos A. N., Balderas R. S., Francis S. J., Dixon F. J. The effect of thymectomy on lupus-prone mice. J Immunol. 1984 Apr;132(4):1809–1813. [PubMed] [Google Scholar]
- Hefeneider S. H., Conlon P. J., Dower S. K., Henney C. S., Gillis S. Limiting dilution analysis of interleukin 2 and colony-stimulating factor producer cells in normal and autoimmune mice. J Immunol. 1984 Apr;132(4):1863–1868. [PubMed] [Google Scholar]
- Izui S., Fernandes G., Hara I., McConahey P. J., Jensen F. C., Dixon F. J., Good R. A. Low-calorie diet selectively reduces expression of retroviral envelope glycoprotein gp70 in sera of NZB x NZW F1 hybrid mice. J Exp Med. 1981 Oct 1;154(4):1116–1124. doi: 10.1084/jem.154.4.1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izui S., McConahey P. J., Theofilopoulos A. N., Dixon F. J. Association of circulating retroviral gp70-anti-gp70 immune complexes with murine systemic lupus erythematosus. J Exp Med. 1979 May 1;149(5):1099–1116. doi: 10.1084/jem.149.5.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safai-Kutti S., Fernandes G., Wang Y., Safai B., Good R. A., Day N. K. Reduction of circulating immune complexes by calorie restriction in (NZB x NZW) F1 mice. Clin Immunol Immunopathol. 1980 Mar;15(3):293–300. doi: 10.1016/0090-1229(80)90041-0. [DOI] [PubMed] [Google Scholar]
- Steinberg A. D., Roths J. B., Murphy E. D., Steinberg R. T., Raveche E. S. Effects of thymectomy or androgen administration upon the autoimmune disease of MRL/Mp-lpr/lpr mice. J Immunol. 1980 Aug;125(2):871–873. [PubMed] [Google Scholar]
- Theofilopoulos A. N., Balderas R. S., Shawler D. L., Lee S., Dixon F. J. Influence of thymic genotype on the systemic lupus erythematosus-like disease and T cell proliferation of MRL/Mp-lpr/lpr mice. J Exp Med. 1981 Jun 1;153(6):1405–1414. doi: 10.1084/jem.153.6.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theofilopoulos A. N., Dixon F. J. Etiopathogenesis of murine SLE. Immunol Rev. 1981;55:179–216. doi: 10.1111/j.1600-065x.1981.tb00343.x. [DOI] [PubMed] [Google Scholar]
- Weindruch R., Walford R. L. Dietary restriction in mice beginning at 1 year of age: effect on life-span and spontaneous cancer incidence. Science. 1982 Mar 12;215(4538):1415–1418. doi: 10.1126/science.7063854. [DOI] [PubMed] [Google Scholar]
- Wold R. T., Young F. E., Tan E. M., Farr R. S. Deoxyribonucleic acid antibody: a method to detect its primary interaction with deoxyribonucleic acid. Science. 1968 Aug 23;161(3843):806–807. doi: 10.1126/science.161.3843.806. [DOI] [PubMed] [Google Scholar]


