Abstract
Transplantation of mesenchymal stem cells (MSCs) derived from adult bone marrow has been proposed as a potential therapeutic approach for post-infarction left ventricular (LV) dysfunction. However, age-related functional decline of stem cells has restricted their clinical benefits after transplantation into the infarcted myocardium. The limitations imposed on patient cells could be addressed by genetic modification of stem cells. This study was designed to improve our understanding of genetic modification of human bone marrow derived mesenchymal stem cells (hMSCs) by polyethylenimine (PEI, branched with Mw 25 kD), one of non-viral vectors that show promise in stem cell genetic modification, in the context of cardiac regeneration for patients. We optimized the PEI-mediated reporter gene transfection into hMSCs, evaluated whether transfection efficiency is associated with gender or age of the cell donors, analysed the influence of cell cycle on transfection and investigated the transfer of therapeutic vascular endothelial growth factor gene (VEGF). hMSCs were isolated from patients with cardiovascular disease aged from 41 to 85 years. Optimization of gene delivery to hMSCs was carried out based on the particle size of the PEI/DNA complexes, N/P ratio of complexes, DNA dosage and cell viability. The highest efficiency with the cell viability near 60% was achieved at N/P ratio 2 and 6.0 μg DNA/cm2. The average transfection efficiency for all tested samples, middle-age group (<65 years), old-age group (>65 years), female group and male group was 4.32%, 3.85%, 4.52%, 4.14% and 4.38%, respectively. The transfection efficiency did not show any correlation either with the age or the gender of the donors. Statistically, there were two subpopulations in the donors; and transfection efficiency in each subpopulation was linearly related to the cell percentage in S phase. No significant phenotypic differences were observed between these two subpopulations. Furthermore, PEI-mediated therapeutic gene VEGF transfer could significantly enhance the expression level.
Keywords: human bone marrow derived mesenchymal stem cells, gene delivery, polyethylenimine, non-viral vector, cardiovascular disease
Introduction
Transplantation of mesenchymal stem cells (MSCs) derived from adult bone marrow has been proposed as a potential therapeutic approach for post-infarction left ventricular (LV) dysfunction. MSCs can protect cardiomyocytes by expressing genes encoding factors that are matrix-mediating, anti-apoptotic and angio-/arteriogenic, as interleukin (IL)-6, leukaemia inhibitory factor (LIF) and vascular endothelial growth factor (VEGF) family [1], prompt angiogenesis in injured area by secreting angiogenetic and vasculogenetic products, particularly VEGF, hepatocyte growth factors, adrenomedullin, placental growth factor and IL-6 [1]. MSCs were found to incorporate into vascular vessel walls of growing neovasculature during cardiac tissue regeneration [2] and to transmigrate over the endothelial barrier of capillaries, disrupting tight junctions between endothelial cells and developing tight cell–cell contacts with the endothelial cells on its way [3]. Li et al. [4] transplanted Bcl-2 modified MSCs into the infarcted heart in a rat model and found that some of the MSCs appeared to display endothelial cell-like phenotype. They also confirmed that a very few number of MSCs colocalized with cardiac Troponin T (cTnT) by the 3D reconstruction of the tissue acquired by a confocal microscopy, suggesting the fusion of the transplanted MSCs with host cells or the differentiation of the transplanted cells into cardiomyocytes. Intramyocardial transplantation of MSCs led to initial enhancement of coronary blood flow and subsequent improvement of cardiac performance 8 weeks after transient cardiac ischemia in pigs [5]. Clinical studies revealed a reduction of perfusion defect along with improved overall LV function after intracoronary autologous MSCs administration [6].
However, the beneficial effect regarding functional recovery after ischemia in patients was much less effective than predicted by preclinical studies. Notably, most preclinical studies were performed on healthy young animals, whereas most patients enrolled in clinical trials were older patients with multiple comorbidities [7].
Diminished stem cell functions with age have been extensively documented in previous studies [8–14]. Aging leads to a reduction of telomere length of bone marrow derived cells especially in patients with cardiovascular diseases [15–17], reduces cell survival to ischemic injury, incurs less stem cell release of VEGF and basic fibroblast growth factor (bFGF) in response to ischemia [7]. Fortunately the aging-related decline in cardiac and vascular regenerative capacity of stem cells could be addressed by pre-activation of donor cells with genetic modification before implantation. Indeed, genetic manipulation of rat MSCs with prosurvival kinase Akt [18], anti-apoptotic Bcl-2 [4] and genetic modification of EPCs with human telomerase reverse transcriptase [19], and VEGF [20] have induced an increase in stem cell survival, enhanced regenerative properties of stem cells in neovascularization and shown functional improvement in disease models.
However, multiple issues have to be addressed before this technology can be used in patients. In that silencing of virally encoded genes in cells is not always complete. It is highly essential to identify alternative genetic modification techniques such as non-viral vector gene transfer for delivering therapeutic genes into the hMSCs without integrating viruses, thereby lowering the risk of malignant transformation [21, 22]. Actually, genetic engineering of human stem cells with VEGF by biodegradable polymeric nanoparticles showed enhanced angiogenesis in a mouse ischemic hindlimb model [23].
In this study, we used the polyethylenimine (PEI; 25 kD), a widely used non-viral gene carrier with high delivery efficiency [22, 24–27], to deliver the gene into the bone marrow derived human mesenchymal stem cells (hMSCs). The aim was to optimize the transfection conditions, study the feasibility of non-viral gene delivery into hMSCs derived from patients and evaluate whether transfection efficiency is associated with gender or age of the cell donors. Here, we showed that there was no correlation between the transfection efficiency and the age or gender of the donors; the most interesting finding was that statistically there were two subpopulations in the donors; and the transfection efficiency in each subpopulation was linearly related to the cell percentage in S phase. There were no significant phenotypic differences between these two subpopulations. Furthermore, PEI-mediated therapeutic gene VEGF transfer could significantly enhance the expression level.
Materials and methods
Chemicals and reagents
Branched PEI with weight average molecular weights (Mw) of 25,000 Dalton, MTT (3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide) and ethidium bromide were purchased from Sigma-Aldrich (St. Louis, MO, USA). Dimethyl sulphoxide (DMSO) with high purity (≥99.5%) was purchased from Carl Roth (Karlsruhe, Germany). Ficoll-Paque PLUS was purchased from GE Healthcare (Chalfont St. Giles, HP8 4SP, UK).
Amplification and purification of plasmid DNA
Plasmid enhanced green fluorescent protein (pEGFP-N3) (Clonetech, Palo Alto, CA, USA) and pcEP4-VEGF165 (Invitrogen, Carlsbad, CA, USA) were transformed into Escherichia coli DH5α strain and amplified in lysogeny broth (LB) medium at 37°C by shaking overnight at 200 rpm. Purification and isolation of the amplified plasmid was carried out with plasmid DNA purification kit (Macherey-Nagel, Diiren, Germany) according to the given protocol. The concentration and purity of the plasmid were determined by measuring the ultraviolet (UV) absorbance at 260 and 280 nm with a spectrophotometer (Thermo Electron, Waltham, MA, USA). Finally, the purified plasmid was stored in aliquots at −20°C prior to use.
PEI/DNA complexes preparation and characterization
The PEI/DNA complexes were prepared as we described previously [22, 28, 29]. Plasmid DNA was diluted to ≤0.1 mg/ml with 5% glucose. Then, PEI with appropriate concentration (normally ≤0.75 mM primary amine) in 5% glucose was added drop-wise into DNA solution and the mixture was immediately vortexed for 30 sec. and incubated at room temperature for 30 min. The ratio of PEI nitrogen in primary amine/DNA phosphate (N/P ratio) was calculated by taking into account that 1 μg DNA contains 3 nmol of phosphate and that 43 ng PEI (1 nmol of C2H5N repeat units) holds 0.25 nmol of primary amine nitrogen.
The retardation of DNA by PEI was studied by gel electrophoresis. PEI/DNA complexes mixed with loading buffer was loaded onto the ethidium bromide containing gel (1.5% agarose) and the electrophoretic mobility of the sample was measured at 100 V in tris/borate/ethylenediaminetetraacetic acid (TBE) buffer at room temperature. The DNA bands were visualized under an UV illuminator (Gel Doc 2000 system, Bio-Rad, Hercules, CA, USA). PEI/DNA complex size and ζ potential were measured with ZetaPALS analyser (Brookhaven Instruments Corporation, Holtsville, NY, USA) at 25°C by diluting the complex solution with 5% glucose to the final DNA concentration of 0.025 mg/ml for characterization.
hMSCs isolation, culture and characterization
The study conforms to the Declaration of Helsinki and cell donors gave their informed written consent to use their bone marrow for experimental purposes. hMSCs were isolated from bone marrow samples. Firstly, bone marrow samples were diluted with serum-free Roswell Park Memorial Institute (RPMI)1640 medium (PAA Laboratories, Coelbe, Germany) and shook 30 min. at room temperature for complete mixing. Then, the mixtures were filtered with 30 μM cell strainer to remove the clusters, layered on Ficoll-Paque PLUS and centrifuged at 1000 × g for 10 min. at room temperature. The separated monocytes layer above Ficoll after centrifugation was carefully collected and washed three times with 1× PBS/EDTA [phosphate-buffered solution/ethylenediaminetetraacetic acid (2 mM)]. Finally, the cells were resuspended in MSC growth medium (Lonza, Walkersville, MD, USA) and cultured in a humidified atmosphere of 5% CO2 in air at 37°C. The growth medium was changed regularly during the culture.
hMSCs were analysed for purity and epitope expression using fluorescence-activated cell sorter (FACS) analysis. The cells were blocked with FcR Blocking Reagent (human) (Miltenyi Biotec, Bergisch Gladbach, Germany) and incubated for 30 min. at 4°C with corresponding antibodies: CD29, CD44, CD45, CD73 (BD Biosciences, Heidelberg, Germany) and CD105 (AbD Serotec, Düsseldorf, Germany). Suggested mouse isotype antibodies were used as control. After incubation, the cells were washed with 1 × PBS/EDTA (2 mM) and analysed using a LSR II Flow Cytometer (Becton Dickinson, Heidelberg, Germany). Dead cells were excluded using dead cell stain kit (LIVE/DEAD®, Invitrogen). Data analysis was performed with BD FACSDiva™ software (BD Biosciences). Histograms of cell number versus logarithmic fluorescence intensity were recorded for at least 10,000 cells per sample. The cell morphology was observed under microscope (Axiovert40 CFL, Carl Zeiss, Goettingen, Germany).
MTT cytotoxicity assay
hMSCs were seeded into the 96-well plates with the density of 4.27 × 103 cells/well. After 40 hrs of culture at 37°C, 20 μl MTT (5 mg/ml in 1× PBS) was added into every well. After 4 hrs of incubation at 37°C, the medium was removed and the purple crystals were dissolved in 100 μl DMSO. The absorbance was measured at a test wavelength of 550 nm and a reference wavelength of 655 nm using a microplate reader (Bio-Rad, Model 680). The results were expressed as the percentage of viability compared with control cells, which were cultured without complex treatment. Cell viability was calculated using the equation below:
In vitro transfection
For the optimization of the transfection conditions, hMSCs were seeded into 6-well plates at the density of 4.18 × 104 cells/per well. The medium volume for every plate was 5 ml. After 24 hrs of culture, the PEI/DNA complexes were added into the cell culture medium. After 24 hrs of transfection, the cells were observed by fluorescence microscopy and the transfection efficiency was analysed with FACSCalibur™ flow cytometer (Becton Dickinson). Briefly, the culture medium was removed and the cells were washed three times by 1× PBS. Then, the cells were harvested by incubating with trypsin-EDTA and washed twice with 1× PBS. For every FACS-running at least 10,000 cells were counted. The untransfected cells from the same donors were used as control. The data were analysed with ‘FlowJo’ software and the gate for control was set as ≤1%. After that, all of the hMSCs from different resources were transfected using the optimal conditions with the same protocol.
Cell cycle analysis
The cell cycle analysis was performed to investigate the effect of cell cycle especially S phase on the transfection efficiency. The cells were stained with propidium iodide (PI) according to the standard protocol followed by the FACS detection. The data were analysed using the ‘Cell Cycle’ platform of the ‘FlowJo’ software.
Human VEGF165 transfection
hMSCs from eight patients were transfected with effector gene (human VEGF165). The cells from each patient were divided into 3 groups: negative control (without any treatment), naked plasmid (6 μg VEGF165/cm2) transfected group and PEI/DNA (N/P ratio 2, 6 μg VEGF165/cm2) transfected group. Cells were pre-seeded in 24-well plates. The medium was replaced with fresh medium (1 ml/well) before transfection. After 24 hrs of transfection, the culture medium was collected and stored at −20°C. The VEGF protein content in the culture medium was quantified using a human VEGF ELISA kit (QuantiGlo, R&D Systems, Wiesbaden-Nordenstadt, Germany) following the given manual. The total protein level in the cell extracts was measured with a BCA protein assay kit (Thermo Fisher Scientific, Bonn, Germany) and was used as reference.
Statistical analysis
Statistical analysis was performed with the statistic program package SPSS Version 15.0. A significance level (Sig.) <0.05 was considered to be statistically significant.
Results
PEI/DNA complexes characterization
DNA immobilization by PEI was studied by electrophoresis. As shown in Figure 1A, with the increase of N/P ratio, the DNA retardation increased. At the absence of PEI (N/P ratio = 0), the naked DNA showed a broad and bright band under the UV illuminator. However, with the gradually increased N/P ratio, the DNA band became narrower and weaker. Finally, the DNA band disappeared at N/P ratio 1 indicating that the DNA was completely retarded.
Fig 1.
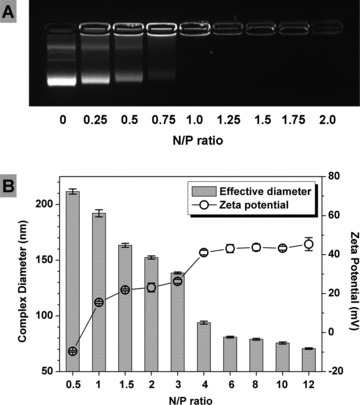
Characterization of PEI/DNA complexes. Plasmid DNA was increasingly retarded by PEI with the increase of N/P ratio, until the complete retardation at N/P ratio 1 (A). PEI/DNA complex size was reduced, whereas ζ potential was increased with the increase of N/P ratio from 0.5 to 12 (B).
The size and surface charge of complexes are important factors which affect the transfection efficiency [30]. Figure 1B indicated the decreasing trend of complex size and the increasing trend of surface charge with the increase of N/P ratio. The average complex size was 211.6 nm at N/P ratio 0.5 and significantly decreased to 93.9 nm at N/P ratio 4. After that, the complex size became close to constant from N/P ratio 6 (80.9 nm) to N/P ratio 12 (70.6 nm). Correspondingly, the average ζ potential increased from −9.61 mV to 41.03 mV with the increase of N/P ratio from 0.5 to 4. After that, it increased gently from N/P ratio 6 (43.07 mV) to N/P ratio 12 (45.35 mV).
hMSCs characterization
The immunophenotyping of cell surface antigens was examined by flow cytometry. As shown in Figure 2A, the cells showed positive expression of MSC markers CD29, CD44, CD73, CD105, and negative expression of haematopoietic stem cell markers CD45. Although there were some differences in the morphology of the cells isolated from different donors, the cells displayed a homogenous elongated spindle-shaped population and maintained a similar shape during the subsequent passages (Fig. 2B).
Fig 2.
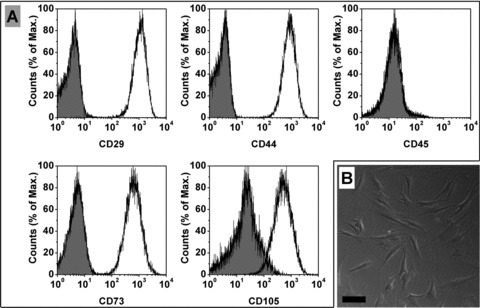
Representative result of the phenotypic characterization of hMSCs (A). The cells showed positive expression of MSC markers CD29, CD44, CD73, CD105 and negative expression of haematopoietic stem cell markers CD45. The hMSCs had a spindle-shaped morphology (B) (Bar = 100 μM).
MTT cytotoxicity assay
The cytotoxicity induced by PEI/DNA complexes was studied by MTT assay (Fig. 3). Both of N/P ratio and DNA dosage were varied. The cell viability decreased with the increase of the complex dosage when the N/P ratio was fixed. At lower N/P ratio, PEI/DNA complexes did not show high toxicity even though the DNA dosage was relatively large. The cell viability exceeded 100% at N/P ratio 1 and DNA dosage of 7 μg DNA/cm2. When the N/P ratio increased to 2, it still kept more than 50% even at high DNA dosage (58.95 ± 10.24% at 6 μg DNA/cm2 and 59.82 ± 6.95% at 7 μg DNA/cm2). However, when the N/P ratio exceeded 2, cell viability with more than 50% could be achieved only at lower DNA dosage (0.5 or 1 μg DNA/cm2). On the other side, if the DNA dosage was fixed, the cell viability showed the decreasing trend with the increase of N/P ratio. Summarily, the cell viability was affected by both of the N/P ratio and DNA dosage.
Fig 3.
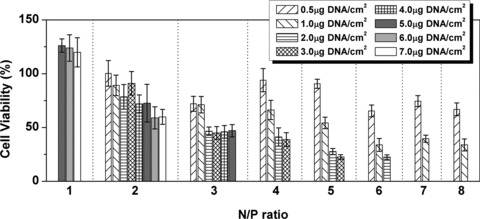
The cell viability decreased with the increase of N/P ratio and/or DNA dosage. The result was given as average ± S.D. (n = 8).
In vitro transfection optimization
To optimize the transfection conditions, three hMSC samples were used for gene delivery assay with various N/P ratio and DNA dosage. The representative results from FACS analysis (Fig. 4A) showed that both N/P ratio and DNA dosage could influence the gene delivery efficiency. At N/P ratio 1, although the efficiency increased with the increase of DNA dosage, it was still very low even at high DNA dosage (5.98% at 8.0 μg DNA/cm2). When the N/P ratio increased to 2, the efficiency was enhanced and showed a maximum peak (24.24%) at 6.0 μg DNA/cm2. After that, the efficiency showed a decreasing trend with the increase of N/P ratio. The FACS analysis histograms at N/P ratio 2 with various DNA dosages are shown in Figure 4B. The green fluorescent protein (GFP) expression of transfected cells at the optimal conditions are shown in Figure 4C–E. Hence, all of the transfection were carried out with the optimal conditions, i.e. N/P ratio 2 and 6.0 μg DNA/cm2, in the following experiments.
Fig 4.
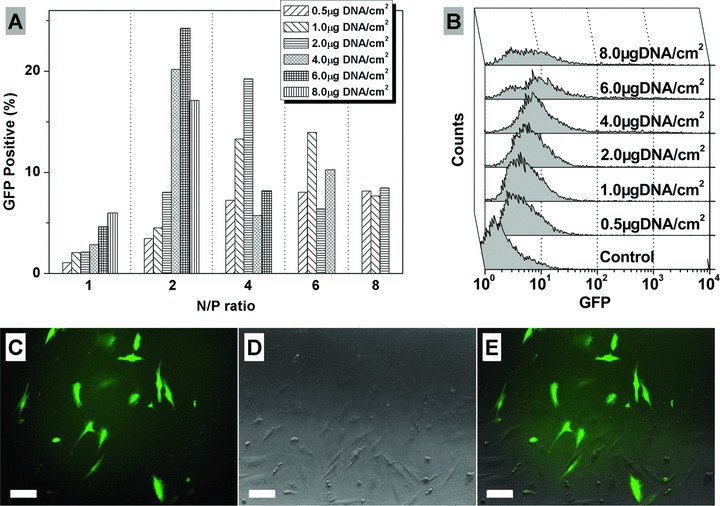
Representative transfection efficiencies of hMSCs (donor age 67, male) with various N/P ratio and DNA dosage (A), and the histograms of FACS analysis at N/P ratio 2 and various DNA dosage (B). (C–E): Representative GFP expression of transfected hMSCs under optimal transfection conditions. Fluorescence image (C), phase-contrast image (D) and merged (E) (Bar = 100 μM).
Effect of cell resource on transfection efficiency
Figure 5A revealed the effect of cell resource on transfection. The cells used were from 30 donors, including 23 male and 7 female. The average age of these donors was 67.6 years old and the average transfection efficiency was 4.32%. The Spearman’s rank correlation coefficient (rs) between transfection efficiency and donors’ age was not significant (rs(GFP: Age)= 0.042, Sig. = 0.824), which indicated that the transfection efficiency was not correlated with donors’ age. Furthermore, to evaluate the transfection efficiency difference between middle- and old-age people, we divided the donors into two groups. Independent-samples t-test revealed there was no significant transfection efficiency difference (Sig. = 0.634) between the under 65 years old group (n = 9, average age = 55.2 years, average GFP+= 3.85%) and over 65 years old group (n = 21, average age = 72.9 years, average GFP+= 4.52%), as shown in Figure 5B. Moreover, we did not find the significant transfection efficiency difference (Sig. = 0.874) between the female (n = 7, average age = 66.7 years, average GFP+= 4.14%) and male (n = 23, average age = 67.8 years, average GFP+= 4.38%) groups via independent-samples t-test (Fig. 5C).
Fig 5.
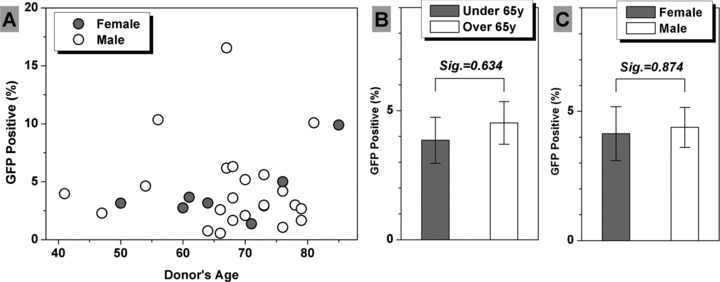
The effect of cell resource on transfection efficiency. No correlation was observed between transfection efficiency and donors’ age according to Spearman’s rank correlation analysis (A). Independent-samples t-test revealed there was no significant difference of transfection efficiency between middle-age (n = 9) and old-age (n = 21) groups (B), and between female (n = 7) and male (n = 23) groups (C). The result was expressed as average ± S.E.M. (standard error of the mean).
Effect of S phase on transfection efficiency
Previous works have revealed that the cells in S phase achieved significant higher transfection efficiency than the cells in G1 phase [31, 32]. The effect of S phase on the transfection efficiency was investigated in this study (Fig. 6A). Interestingly statistic analysis found that there were two subpopulations in donors; in each of these two subpopulations transfection efficiency was linearly related to the cell percentage in S phase. Pearson correlation coefficient between transfection efficiency and the cell percentage in S phase (Pearson r) was analysed, which was 0.969 for subpopulation 1 (P1) (n = 6, Sig. < 0.01) and 0.822 for subpopulation 2 (P2) (n = 6, Sig. < 0.05). Furthermore, non-parametric Mann-Whitney test revealed that the GFP+ percentages of P1 and P2 were from two different distributions (Asymp. Sig. < 0.01); but the cell percentages in S phase of P1 and P2 were not (Asymp. Sig. > 0.10). A representative histogram of cell cycle analysis via PI staining and FACS detection is shown in Figure 6B. In addition, phenotypic characterization was performed with cells of these two subpopulations and there was no significant difference observed on the surface markers: CD29, CD44, CD45, CD73 and CD105 (Fig. 6C).
Fig 6.
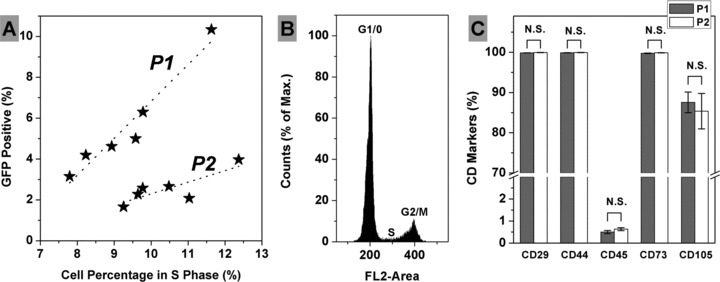
Association between transfection efficiency and the cell percentage in S phase. Two subpopulations were found and linear regression indicated the trend towards the correlation between transfection efficiency and the cell percentage in S phase in each subpopulation (A). A representative histogram of cell cycle analysis via PI staining and FACS detection (B). Phenotypic characterization showed no significant difference between these two subpopulations (C) [Average ± S.E.M, n = 4 (P1) and n = 3 (P2)].
Human VEGF165 Transfection
To investigate whether PEI could mediate the delivery of therapeutic gene into hMSC, the cells were transfected with human VEGF165 gene (Fig. 7). Both naked plasmid DNA and PEI/DNA complex led to a significant expression of VEGF165 compared with untransfected cells. However, the utilization of PEI resulted in a significant higher transgene expression than naked plasmid DNA.
Fig 7.
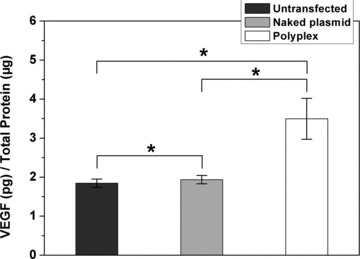
VEGF165 expression level of untransfected cells (1.84 ± 0.11 pg/μg total protein), naked plasmid DNA transfected cells (1.94 ± 0.11 pg/μg total protein) and PEI/DNA transfected cells (3.49 ± 0.52 pg/μg total protein). The result was given as average ± S.E.M. (standard error of the mean). (n = 8, *P < 0.05 by paired-samples t-test).
Similar to the transfer of GFP reporter gene, the VEGF165 transfection mediated by PEI presented large variation of efficiency in cells from different patients. Among the eight patients, the highest VEGF165 expression was 5.79 pg/μg total cell protein, which showed 4.54-fold enhancement compared to untransfected cells (donor age 70, male); whereas the lowest VEGF165 expression was 1.86 pg/μg total cell protein, which only showed 1.18-fold enhancement compared to untransfected cells (donor age 73, female). Further calculation based on cell number showed that VEGF expression levels ranged from 35 ng/106 cells/day to 230 ng/106 cells/day.
Discussion
The current study optimized the PEI-mediated reporter gene transfection into hMSCs derived from patients based on the particle size of the PEI/DNA complexes, N/P ratio of complexes, DNA dosage and cell viability, and investigated the influence of cell cycle on transfection. The transfection efficiency did not show any correlation either with the age or the gender of the donors. Statistically, there were two subpopulations in the donors; and transfection efficiency in each subpopulation was linearly related to the cell percentage in S phase. No significant phenotypic differences were observed between these two subpopulations. Furthermore, the PEI-mediated transfer of therapeutic gene VEGF could significantly enhance the expression level.
We tried to determine whether the transfection efficiency in hMSCs is related to the donors’ age or gender. No statistical significance was observed showing that the transfection efficiency did not have any correlation either with the age or the gender of the donors. Also the individual differences in the transfection efficiency were relatively large. The most evident contrast came from the two samples, which were from the donors with the same age (67 years old) and gender (male). The transfection efficiencies of these two samples were 16.55% and 6.17%, respectively. It is not clear which specific differences in donors lead to the individual difference in the transfection of hMSCs. Further studies are clearly needed to study the inherent differences from the patients, which induce the individual difference in the transfection of hMSCs.
Brunner et al. [31] used TfPEI (a conjugate with molar ratio of approximately three transferring molecules linked to PEI800 Da) to mediate GFP reporter gene expression in K562 cells. They also used branched PEI25 kD and a small amount of TfPEI25 kD (human transferrin coupled to branched PEI25 kD, representing 5% of the total PEI used for complex formation) to investigate the luciferase reporter gene expression in HeLa cells [32]. Both studies showed that the transfection efficiency was strongly dependent on the cell cycle stage at the time of transfection, and the higher transfection efficiency appeared during S or G2/M phase. We tried to verify whether the cell cycle dependence finding based on the cell line studies from Professor Wagner’s group [31, 32] are applicable to hMSCs as well. If all the donors were assumed from one population for statistic analysis, no correlation between the transfection efficiency and the cell percentage in S phase was found. It seems that the cell cycle dependence is not applicable to hMSCs. However, Pearson correlation analysis revealed that there were two subpopulations; in each subpopulation the transfection efficiency was linearly related to the cell percentage in S phase. Non-parametric Mann-Whitney test further confirmed that the transfection efficiencies in these two subpopulations were from two different distributions, but the cell percentages in S phase were not. We screened the immunophenotyping surface antigens of cells from these two subpopulations by FACS analysis; however, we did not observed significant differences in the cell surface antigens between these two subpopulations. It is still not clear which internal factors of donors lead to the two subpopulations. Further studies are needed to investigate the correlation between the cell cycle and the transfection efficiency in hMSCs.
The importance of this study is related to the clinical trials that administrate human MSCs to the patients with ischemic cardiovascular diseases for regenerating cardiac functions. Age-related decline in cardiac and vascular regenerative capacity of stem cells indicate that cardiac regeneration is a highly complex process involving a series of coordinated key events including cell homing, engraftment, survival, paracrine factor release and differentiation that need to be exploited for cardiac repair and functional recovery [33, 34]. Hence, genetic modification of stem cell may be an important strategy with virus vectors in the preclinical identification of genes responsible for the key events in stem cell based cardiac regeneration. Indeed, genetic manipulation of rat MSCs with Akt [18], heme oxygenase (HO)-1 [35] and stromal cell-derived factor (SDF)-1 [36] by virus vectors have induced an increase in stem cell survival within the infarct zone and shown improved cardiac function. As virus vector may not completely silence its encoded genes in cells, it may induce malignant transformation of stem cells and immune response after cell transplantation. Hence, it is indispensable to deliver therapeutic genes into the MSCs with non-viral vector in clinical application.
Although we got some transfections with relative high efficiency (>15%), not all samples showed such high efficiency. Among the 30 samples we tested, only 8 samples had efficiency more than 5%, and only 2 had efficiency more than 10%. The average transfection efficiency for all of samples was 4.32%.
Ozawa et al. implanted VEGF-transduced myoblasts into muscles of adult mice and found that a very low level of VEGF (∼5 ng/106 cells/day) was able to yield homogeneous normal capillary-like vessels while aberrant vasculature was induced at VEGF levels above 70 ng/106 cells/day [37]. Our data suggested that PEI-mediated therapeutic gene VEGF transfer into hMSCs could produce expression levels ranging from 35 ng/106 cells/day to 230 ng/106 cells/day. This provides the probability of achieving therapeutic efficacy by implanting modified cells with lower number than unmodified cells. Thus, the duration of ex vivo expansion of hMSCs could be shortened, which could reduce the risk of long-term culture such as spontaneous immortalization and malignant transformation [38–42].
However, the transfection efficiency still needs to be enhanced to improve therapeutic efficacy by transplantation of non-viral vector modified hMSCs. It may be critical to develop nuclear targeting strategies for hMSCs transfection. Hence, intracellular trafficking of PEI/DNA complexes is highly valued to elucidate the cellular mechanism of non-viral vector mediated transfection in hMSCs.
Conclusion
In summary, the optimization of PEI mediated gene delivery into hMSCs derived from patients has been carried out. The transfection efficiency did not show any correlation either with the age or the gender of the donors. Statistically, there were two subpopulations in the patients; and transfection efficiency in each subpopulation was linearly related to the cell percentage in S phase. No significant phenotypic differences were observed between these two subpopulations. Furthermore, PEI-mediated therapeutic gene VEGF transfer could significantly enhance the expression level.
Acknowledgments
This work was supported by Sonderforschungsbereich/Transregio 37 (B5, B2 and A4); German Federal Ministry of Education and Research, BioChancePlus program (0313191); The German Helmholtz Association, Mecklenburg-Vorpommern, German Federal Ministry of Education and Research, German Research Foundation (Nachwuchsgruppe Regenerative Medizin Regulation der Stammzellmigration 0402710); Förderkennzeichen 0312138 A (Ministry of Education (Germany, Berlin)); V220-630-08-TFMV-F/S-035 (Ministry of Economy (Mecklenburg-West Pommerania, Schwerin)); Marie Curie International Research Staff Exchange Scheme (IRSES, FP7-PEOPLE-2009-IRSES) and the Reference and Translation Center for Cardiac Stem Cell Therapy (RTC). The authors thank Ms. Margit Fritsche for her technical assistance.
Conflict of interest
The authors confirm that there are no conflicts of interest.
References
- 1.Iso Y, Spees JL, Serrano C, et al. Multipotent human stromal cells improve cardiac function after myocardial infarction in mice without long-term engraftment. Biochem Biophys Res Commun. 2007;354:700–6. doi: 10.1016/j.bbrc.2007.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Silva GV, Litovsky S, Assad JA, et al. Mesenchymal stem cells differentiate into an endothelial phenotype, enhance vascular density, and improve heart function in a canine chronic ischemia model. Circulation. 2005;111:150–6. doi: 10.1161/01.CIR.0000151812.86142.45. [DOI] [PubMed] [Google Scholar]
- 3.Schmidt A, Ladage D, Steingen C, et al. Mesenchymal stem cells transmigrate over the endothelial barrier. Eur J Cell Biol. 2006;85:1179–88. doi: 10.1016/j.ejcb.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 4.Li W, Ma N, Ong LL, et al. Bcl-2 engineered MSCs inhibited apoptosis and improved heart function. Stem Cells. 2007;25:2118–27. doi: 10.1634/stemcells.2006-0771. [DOI] [PubMed] [Google Scholar]
- 5.Schuleri KH, Amado LC, Boyle AJ, et al. Early improvement in cardiac tissue perfusion due to mesenchymal stem cells. Am J Physiol Heart Circ Physiol. 2008;294:H2002–11. doi: 10.1152/ajpheart.00762.2007. [DOI] [PubMed] [Google Scholar]
- 6.Chen SL, Fang WW, Ye F, et al. Effect on left ventricular function of intracoronary transplantation of autologous bone marrow mesenchymal stem cell in patients with acute myocardial infarction. Am J Cardiol. 2004;94:92–5. doi: 10.1016/j.amjcard.2004.03.034. [DOI] [PubMed] [Google Scholar]
- 7.Zhuo YF, Li SH, Chen MS, et al. Aging impairs the angiogenic response to ischemic injury and the activity of implanted cells: combined consequences for cell therapy in older recipients. J Thorac Cardiov Sur. 2010;139:1286–94. doi: 10.1016/j.jtcvs.2009.08.052. [DOI] [PubMed] [Google Scholar]
- 8.Rivard A, Fabre JE, Silver M, et al. Age-dependent impairment of angiogenesis. Circulation. 1999;99:111–20. doi: 10.1161/01.cir.99.1.111. [DOI] [PubMed] [Google Scholar]
- 9.Edelberg JM, Tang L, Hattori K, et al. Young adult bone marrow-derived endothelial precursor cells restore aging-impaired cardiac angiogenic function. Circ Res. 2002;90:E89–93. doi: 10.1161/01.res.0000020861.20064.7e. [DOI] [PubMed] [Google Scholar]
- 10.Dimmeler S, Vasa-Nicotera M. Aging of progenitor cells: limitation for regenerative capacity. J Am Coll Cardiol. 2003;42:2081–2. doi: 10.1016/j.jacc.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 11.Rauscher FM, Goldschmidt-Clermont PJ, Davis BH, et al. Aging, progenitor cell exhaustion, and atherosclerosis. Circulation. 2003;108:457–63. doi: 10.1161/01.CIR.0000082924.75945.48. [DOI] [PubMed] [Google Scholar]
- 12.Scheubel RJ, Zorn H, Silber RE, et al. Age-dependent depression in circulating endothelial progenitor cells in patients undergoing coronary artery bypass grafting. J Am Coll Cardiol. 2003;42:2073–80. doi: 10.1016/j.jacc.2003.07.025. [DOI] [PubMed] [Google Scholar]
- 13.Heeschen C, Lehmann R, Honold J, et al. Profoundly reduced neovascularization capacity of bone marrow mononuclear cells derived from patients with chronic ischemic heart disease. Circulation. 2004;109:1615–22. doi: 10.1161/01.CIR.0000124476.32871.E3. [DOI] [PubMed] [Google Scholar]
- 14.Dimmeler S, Leri A. Aging and disease as modifiers of efficacy of cell therapy. Circ Res. 2008;102:1319–30. doi: 10.1161/CIRCRESAHA.108.175943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brouilette SW, Moore JS, McMahon AD, et al. Telomere length, risk of coronary heart disease, and statin treatment in the West of Scotland Primary Prevention Study: a nested case-control study. Lancet. 2007;369:107–14. doi: 10.1016/S0140-6736(07)60071-3. [DOI] [PubMed] [Google Scholar]
- 16.van der Harst P, van der Steege G, de Boer RA, et al. Telomere length of circulating leukocytes is decreased in patients with chronic heart failure. J Am Coll Cardiol. 2007;49:1459–64. doi: 10.1016/j.jacc.2007.01.027. [DOI] [PubMed] [Google Scholar]
- 17.Spyridopoulos I, Erben Y, Brummendorf TH, et al. Telomere gap between granulocytes and lymphocytes is a determinant for hematopoetic progenitor cell impairment in patients with previous myocardial infarction. Arterioscler Thromb Vasc Biol. 2008;28:968–74. doi: 10.1161/ATVBAHA.107.160846. [DOI] [PubMed] [Google Scholar]
- 18.Mangi AA, Noiseux N, Kong D, et al. Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infarcted hearts. Nat Med. 2003;9:1195–201. doi: 10.1038/nm912. [DOI] [PubMed] [Google Scholar]
- 19.Murasawa S, Llevadot J, Silver M, et al. Constitutive human telomerase reverse transcriptase expression enhances regenerative properties of endothelial progenitor cells. Circulation. 2002;106:1133–9. doi: 10.1161/01.cir.0000027584.85865.b4. [DOI] [PubMed] [Google Scholar]
- 20.Iwaguro H, Yamaguchi J, Kalka C, et al. Endothelial progenitor cell vascular endothelial growth factor gene transfer for vascular regeneration. Circulation. 2002;105:732–8. doi: 10.1161/hc0602.103673. [DOI] [PubMed] [Google Scholar]
- 21.Aslan H, Zilberman Y, Arbeli V, et al. Nucleofection-based ex vivo nonviral gene delivery to human stem cells as a platform for tissue regeneration. Tissue Eng. 2006;12:877–89. doi: 10.1089/ten.2006.12.877. [DOI] [PubMed] [Google Scholar]
- 22.Li W, Ma N, Ong LL, et al. Enhanced thoracic gene delivery by magnetic nanobead-mediated vector. J Gene Med. 2008;10:897–909. doi: 10.1002/jgm.1208. [DOI] [PubMed] [Google Scholar]
- 23.Yang F, Cho SW, Son SM, et al. Genetic engineering of human stem cells for enhanced angiogenesis using biodegradable polymeric nanoparticles. Proc Natl Acad Sci USA. 2010;107:3317–22. doi: 10.1073/pnas.0905432106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boussif O, Lezoualc’h F, Zanta MA, et al. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proc Natl Acad Sci USA. 1995;92:7297–301. doi: 10.1073/pnas.92.16.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Godbey WT, Wu KK, Mikos AG. Poly(ethylenimine) and its role in gene delivery. J Control Release. 1999;60:149–60. doi: 10.1016/s0168-3659(99)00090-5. [DOI] [PubMed] [Google Scholar]
- 26.Densmore CL, Orson FM, Xu B, et al. Aerosol delivery of robust polyethyleneimine-DNA complexes for gene therapy and genetic immunization. Mol Ther. 2000;1:180–8. doi: 10.1006/mthe.1999.0021. [DOI] [PubMed] [Google Scholar]
- 27.Lee JH, Ahn HH, Kim KS, et al. Polyethyleneimine-mediated gene delivery into rat pheochromocytoma PC-12 cells. J Tissue Eng Regen Med. 2008;2:288–95. doi: 10.1002/term.94. [DOI] [PubMed] [Google Scholar]
- 28.Wang W, Li W, Ong LL, et al. Localized and sustained SDF-1 gene release mediated by fibronectin films: a potential method for recruiting stem cells. Int J Artif Organs. 2009;32:141–9. doi: 10.1177/039139880903200304. [DOI] [PubMed] [Google Scholar]
- 29.Wang W, Li W, Ong LL, et al. Localized SDF-1alpha gene release mediated by collagen substrate induces CD117 stem cells homing. J Cell Mol Med. 2010;14:392–402. doi: 10.1111/j.1582-4934.2008.00624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prabha S, Zhou WZ, Panyam J, et al. Size-dependency of nanoparticle-mediated gene transfection: studies with fractionated nanoparticles. Int J Pharm. 2002;244:105–15. doi: 10.1016/s0378-5173(02)00315-0. [DOI] [PubMed] [Google Scholar]
- 31.Brunner S, Sauer T, Carotta S, et al. Cell cycle dependence of gene transfer by lipoplex, polyplex and recombinant adenovirus. Gene Ther. 2000;7:401–7. doi: 10.1038/sj.gt.3301102. [DOI] [PubMed] [Google Scholar]
- 32.Brunner S, Furtbauer E, Sauer T, et al. Overcoming the nuclear barrier: cell cycle independent nonviral gene transfer with linear polyethylenimine or electroporation. Mol Ther. 2002;5:80–6. doi: 10.1006/mthe.2001.0509. [DOI] [PubMed] [Google Scholar]
- 33.Dzau VJ, Gnecchi M, Pachori AS. Enhancing stem cell therapy through genetic modification. J Am Coll Cardiol. 2005;46:1351–3. doi: 10.1016/j.jacc.2005.07.023. [DOI] [PubMed] [Google Scholar]
- 34.Penn MS, Mangi AA. Genetic enhancement of stem cell engraftment, survival, and efficacy. Circ Res. 2008;102:1471–82. doi: 10.1161/CIRCRESAHA.108.175174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tang YL, Tang Y, Zhang YC, et al. Improved graft mesenchymal stem cell survival in ischemic heart with a hypoxia-regulated heme oxygenase-1 vector. J Am Coll Cardiol. 2005;46:1339–50. doi: 10.1016/j.jacc.2005.05.079. [DOI] [PubMed] [Google Scholar]
- 36.Zhang M, Mal N, Kiedrowski M, et al. SDF-1 expression by mesenchymal stem cells results in trophic support of cardiac myocytes after myocardial infarction. FASEB J. 2007;21:3197–207. doi: 10.1096/fj.06-6558com. [DOI] [PubMed] [Google Scholar]
- 37.Ozawa CR, Banfi A, Glazer NL, et al. Microenvironmental VEGF concentration, not total dose, determines a threshold between normal and aberrant angiogenesis. J Clin Invest. 2004;113:516–27. doi: 10.1172/JCI18420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou YF, Bosch-Marce M, Okuyama H, et al. Spontaneous transformation of cultured mouse bone marrow-derived stromal cells. Cancer Res. 2006;66:10849–54. doi: 10.1158/0008-5472.CAN-06-2146. [DOI] [PubMed] [Google Scholar]
- 39.Miura M, Miura Y, Padilla-Nash HM, et al. Accumulated chromosomal instability in murine bone marrow mesenchymal stem cells leads to malignant transformation. Stem Cells. 2006;24:1095–103. doi: 10.1634/stemcells.2005-0403. [DOI] [PubMed] [Google Scholar]
- 40.Tolar J, Nauta AJ, Osborn MJ, et al. Sarcoma derived from cultured mesenchymal stem cells. Stem Cells. 2007;25:371–9. doi: 10.1634/stemcells.2005-0620. [DOI] [PubMed] [Google Scholar]
- 41.Furlani D, Li W, Pittermann E, et al. A transformed cell population derived from cultured mesenchymal stem cells has no functional effect after transplantation into the injured heart. Cell Transplant. 2009;18:319–31. doi: 10.3727/096368909788534906. [DOI] [PubMed] [Google Scholar]
- 42.Rubio D, Garcia-Castro J, Martin MC, et al. Spontaneous human adult stem cell transformation. Cancer Res. 2005;65:3035–9. doi: 10.1158/0008-5472.CAN-04-4194. [DOI] [PubMed] [Google Scholar]


