Abstract
Autophagy defines the lifespan of eukaryotic organisms by ensuring cellular survival through regulated bulk clearance of proteins, organelles and membranes. Pathophysiological consequences of improper autophagy give rise to a variety of age-related human diseases such as cancer and neurodegeneration. Rational therapeutic implementation of autophagy modulation remains problematic, as fundamental molecular details such as the generation of autophagosomes, unique double-membrane vesicles formed to permit the process of autophagy, are insufficiently understood. Here, freeze-fracture replica immunolabelling reveals WD-repeat protein interacting with phosphoinositides 1 and 2 (WIPI-1 and WIPI-2) as membrane components of autophagosomes and the plasma membrane (PM). In addition, WIPI-1 is also present in membranes of the endoplasmic reticulum (ER) and WIPI-2 was further detected in membranes close to the Golgi cisternae. Our results identify WIPI-1 and WIPI-2 as novel protein components of autophagosomes, and of membrane sites from which autophagosomes might originate (ER, PM, Golgi area). Hence therapeutic modulation of autophagy could involve approaches that functionally target human WIPI proteins.
Keywords: WIPI-1, WIPI-2, Atg18, PtdIns(3)P, autophagy, freeze-fracture electron microscopy
Autophagy is an intracellular lysosomal bulk degradation process initiated by the generation of double-membraned autophagosomes (APs) that sequester cytoplasmic material and that fuse with lysosomes to autolysosomes for final degradation [1]. Models of AP formation anticipate one of three situations: (i) de novo membrane synthesis, (ii) AP formation from pre-existing intracellular membranes or (iii) pre-existing membranes providing a template on which APs form de novo [2–6]. Autophagosomal membranes were found to be predominantly composed of lipids with minimized protein content, as demonstrated by early freeze-fracture electron microscopy [7]. The notion that autophagosomal membranes are profoundly enriched in phosphatidylinositol 3-phosphate (PtdIns(3)P) [8] is of key interest because autophagy is positively regulated by the activation of the phosphatidylinositol 3-kinase class III complex 1, also including the tumour suppressor protein Beclin 1, that generates PtdIns(3)P [9].
Previously, we identified the human WIPI gene family that is aberrantly expressed in a variety of human cancer types [10]. We found that the WIPI protein family represents an ancient PtdIns(3)P/PtdIns(3,5)P2-binding β-propeller protein family that includes WIPI-1 and WIPI-2 [10], both of which evolved from the yeast ancestral autophagy protein Atg18 [10, 11]. We characterized WIPI-1 as a PtdIns(3)P-effector protein functioning in the process of autophagy in human cancer cells [10, 12–13]. Upon the induction of autophagy and the generation of PtdIns(3)P, both WIPI-1 and WIPI-2 specifically bind PtdIns(3)P and localize at initial autophagosomal membranes (phagophore) that are positive for further autophagy proteins, such as microtubule-associated protein 1 light chain 3 (LC3) and Atg16 [10–14]. To clarify the precise localization of WIPI-1 and WIPI-2 upon the induction of autophagy we conducted freeze-fracture immuno-electron microscopy (immuno-EM) because this method allows the employment of native, unfixed cells and, depending on the fracture, either of the two monolayers of a bilayer membrane is preserved [15].
By immuno-EM of generated freeze-fracture replicas we specifically detected membrane-bound WIPI-1 (Fig. 1A) in nutrient-starved (6 hrs) human tumour cells, either endogenous WIPI-1 in G361 cells (Fig. 1B) or WIPI-1 fused to the green fluorescent protein (GFP-WIPI-1) in stably transfected U2OS cells (Fig. 1C, E–G). Here, WIPI-1 specifically localized at APs (Fig. 1A), characterized by the unique lipid-rich, smooth double membrane (Fig. 1B, C, E, G). Strikingly, WIPI-1 localized at both monolayers of both the outer (OM) and the inner (IM) autophagosomal membrane, and appeared often enriched in the inner autophagosomal membrane of exogenous GFP-WIPI-1 (Fig. 1C) or endogenous WIPI-1 (data not shown), as previously shown for PtdIns(3)P in yeast APs [8]. Please note that we marked the P- and E-faces of the IM and the OM according to the P-face of the OM facing the cytoplasm (Fig. 1A). However, as the membrane origin of APs is unknown, this classification might be temporary and might change once the precise origin of the autophagosomal membrane is identified. The calculated approximate diameter of WIPI-1 positive APs ranged between 0.5 and 2.5 μm with an average of about 1 μm (Fig. 1D), typical for APs. Some WIPI-1 positive AP fractures displayed their contents, e.g. lipid droplets (Fig. 1E). In addition, upon autophagy induction WIPI-1 prominently localized at the P-face of both the plasma membrane (PM) and the endoplasmic reticulum (ER), particular at the nuclear membrane (NM) (Fig. 1F). Further, we also found WIPI-1 to localize at vesicles with higher membrane protein content then APs, some of them (6 out of 15) were also positive for lysosomal-associated membrane protein 1 (LAMP-1) (Fig. 1G), suggesting that these vesicles might represent autolysosomes.
Fig 1.
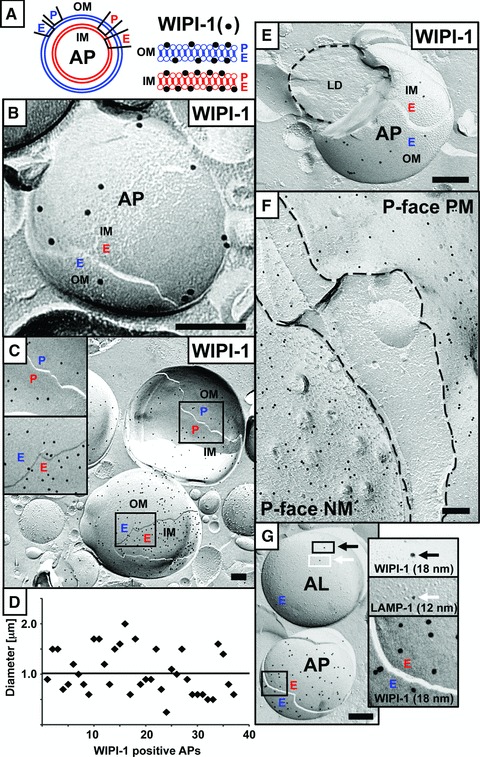
Human G361 (malignant melanoma cell line) and U2OS cells (osteosarcoma cell line) (both from ATCC) were cultured in DMEM, 10% foetal calf serum, 100 U/ml penicillin/100 μg/ml streptomycin, 5 μg/ml plasmocin (Invivogen, Toulouse, France) at 37°C, 5% CO2. Stable GFP-WIPI-1 U2OS clones were selected by using 0.6 mg/ml G418 (Invitrogen, Darmstadt, Germany). In this study, a GFP-WIPI-1 U2OS clone was used that expresses similar GFP-WIPI-1 protein levels when compared to endogenous WIPI-1 in G361 cells (data not shown). Autophagy was induced by nutrient starvation using EBSS (Sigma-Aldrich, Taufkirchen, Germany) for 6 hrs. Freeze fracture immuno-EM images using unfixed (B) human G361 (anti-WIPI-1 antiserum [10], goat anti-rabbit 18 nm gold complexes; Jackson Immunoresearch, Neumarket, UK) or (C) stable GFP-WIPI-1 U2OS cells (anti-GFP antiserum/ab290; Abcam, Cambridge, UK; goat anti-rabbit 18 nm gold complexes; Jackson Immunoreserach) identified the localization of WIPI-1 in both monolayers of the inner (IM) and of the outer (OM) autophagosomal membrane (A). Monolayers were termed P- and E-face according to the P-face of the OM facing the cytoplasm (A–C, E, G); this terminology might become adapted once the membrane origin of APs is identified. Approximate diameters of WIPI-1 positive APs were determined (D). Engulfment of a lipid droplet (LD) by a GFP-WIPI-1+ AP (E). GFP-WIPI-1 was also found to localize at the P-face of the ER/NM and of the PM (F). Co-labelling of GFP-WIPI-1 (anti-GFP antiserum/ab290; Abcam; goat anti-rabbit 18 nm gold complexes; Jackson Immunoresearch) and endogenous LAMP-1 (anti-LAMP-1 antibody/ sc-5570; Santa Cruz, Heidelberg, Germany; goat antimouse 12 nm gold complexes; Jackson Immunoresearch) provides evidence that WIPI-1 is also present on autolysosomes (G). Scale bars 200 nm.
Using generated stable U2OS cell lines that express either of the WIPI-2 isoforms WIPI-2B or WIPI-2D (Fig. 2) we found that WIPI-2 also localizes in all monolayers of double-membraned APs (Fig. 2B, C) and at the PM (Fig. 2D–F) specifically upon nutrient starvation. In contrast to WIPI-1, WIPI-2 did not localize prominently to the ER/NM although some WIPI-2D could be detected at the NM (Fig. 2F). In addition, WIPI-2D was also found in membranes close to the Golgi cisternae (Fig. 2G).
Fig 2.
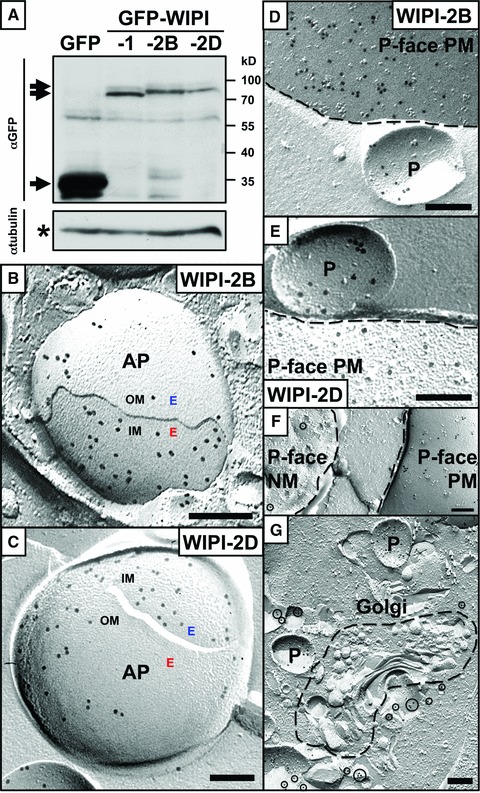
U2OS cells (osteosarcoma cell line; ATCC) were cultured in DMEM, 10% foetal calf serum, 100 U/ml penicillin/100 μg/ml streptomycin, 5 μg/ml plasmocin (Invivogen) at 37°C, 5% CO2 and stably transfected GFP-WIPI-2B or GFP-WIPI-2D U2OS cell clones were selected by using 0.6 mg/ml G418 (Invitrogen). Comparable levels of expressed GFP-WIPI proteins are demonstrated by anti-GFP enhanced chemiluminescence (ECL) detection of total protein extracts (A). Protein expression of GFP-WIPI-1, -2B and -2D is shown, demonstrating relatively low expression when compared to a generated control GFP cell clone (A). Freeze fracture immuno-EM images of stable GFP-WIPI-2B (B) or GFP-WIPI-2D (C) U2OS cells upon nutrient starvation (6 hrs) identified a prominent localization of WIPI-2 in both the inner (IM) and of the outer (OM) autophagosomal membrane (AP) (B, C), and at the PM (D, E). GFP-WIPI-2B (D) and GFP-WIPI-2D (E) harbouring vesicles (P: P-face), may be premature APs, were identified close to the PM, suggesting that these vesicles might have originated from the PM. In contrast to the prominent PM localization, some WIPI-2D was also detected at the ER/NM (F) and near the Golgi cisternae (G). Antibodies: anti-GFP antiserum/ab290; Abcam; goat anti-rabbit 18 nm gold complexes; Jackson Immunoresearch. Scale bars 200 nm.
Here we provide high-resolution imaging analyses which display both membranes of the double-membraned AP. Thereby, the first detailed membranous localization of WIPI-1 and WIPI-2 is achieved, allowing us to distinguish between all monolayers of both the inner and outer autophagosomal membrane and to demonstrate that all monolayers harbour PtdIns(3)P-bound WIPI proteins. Because we further identified specific membranes in which WIPI-1 and WIPI-2 accumulate upon autophagy induction, our data suggest that these pre-existing membranes (PM, ER, Golgi area) provide the source for WIPI-1 positive APs.
Acknowledgments
We kindly thank Katharina Hentschel, Christina Köppler and Karin Schlattmann for technical assistance. This work was supported by grants to T.P.-C. from the Federal Ministry for Education and Science (BMBF, BioProfile), Germany; Ministry of Science, Research and Arts Baden-Wuerttemberg (Landesstiftung) and the German Research Foundation (DFG, SFB 773).
Conflict of interest
The authors confirm that there are no conflicts of interest.
References
- 1.Mizushima N, Levine B, Cuervo AM, et al. Autophagy fights disease through cellular self-digestion. Nature. 2008;451:1069–75. doi: 10.1038/nature06639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reggiori F. Membrane origin for autophagy. Curr Top Dev Biol. 2006;74:1–30. doi: 10.1016/S0070-2153(06)74001-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Axe EL, Walker SA, Manifa M, et al. Autophagosome formation from membrane compartments enriched in phosphatidylinositol 3-phosphate and dynamically connected to the endoplasmic reticulum. J Cell Biol. 2008;182:685–701. doi: 10.1083/jcb.200803137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hayashi-Nishino M, Fujita N, Noda T, et al. A subdomain of the endoplasmic reticulum forms a cradle for autophagosome formation. Nat Cell Biol. 2009;11:1433–7. doi: 10.1038/ncb1991. [DOI] [PubMed] [Google Scholar]
- 5.Hailey DW, Rambold AS, Satpute-Krishnan P, et al. Mitochondria supply membranes for autophagosome biogenesis during starvation. Cell. 2010;141:656–67. doi: 10.1016/j.cell.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ravikumar B, Moreau K, Jahreiss L, et al. Plasma membrane contributes to the formation of pre-autophagosomal structures. Nat Cell Biol. 2010;12:747–57. doi: 10.1038/ncb2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fengsrud M, Erichsen ES, Berg TO, et al. Ultrastructural characterization of the delimiting membranes of isolated autophagosomes and amphisomes by freeze-fracture electron microscopy. Eur J Cell Biol. 2000;79:871–82. doi: 10.1078/0171-9335-00125. [DOI] [PubMed] [Google Scholar]
- 8.Obara K, Noda T, Niimi K, et al. Transport of phosphatidylinositol 3-phosphate into the vacuole via autophagic membranes in Saccharomyces cerevisiae. Genes Cells. 2008;13:537–47. doi: 10.1111/j.1365-2443.2008.01188.x. [DOI] [PubMed] [Google Scholar]
- 9.Pattingre S, Espert L, Biard-Piechaczyk M, et al. Regulation of macroautophagy by mTOR and Beclin 1 complexes. Biochimie. 2008;90:313–23. doi: 10.1016/j.biochi.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 10.Proikas-Cezanne T, Waddell S, Gaugel A, et al. WIPI-1alpha (WIPI49), a member of the novel 7-bladed WIPI protein family, is aberrantly expressed in human cancer and is linked to starvation-induced autophagy. Oncogene. 2004;23:9314–25. doi: 10.1038/sj.onc.1208331. [DOI] [PubMed] [Google Scholar]
- 11.Polson HE, de Lartigue J, Ridgen DJ, et al. Mammalian Atg18 (WIPI2) localizes to omegasome-anchored phagophores and positively regulates LC3 lipidation. Autophagy. 2010;6:506–22. doi: 10.4161/auto.6.4.11863. [DOI] [PubMed] [Google Scholar]
- 12.Proikas-Cezanne T, Ruckerbauer S, Stierhof YD, et al. Human WIPI-1 puncta-formation: a novel assay to assess mammalian autophagy. FEBS Lett. 2007;581:3396–404. doi: 10.1016/j.febslet.2007.06.040. [DOI] [PubMed] [Google Scholar]
- 13.Vergne I, Roberts E, Elmaoued RA, et al. Control of autophagy initiation by phosphoinositide 3-phosphatase jumpy. EMBO J. 2009;28:2244–58. doi: 10.1038/emboj.2009.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Itakura E, Mizushima N. Characterization of autophagosome formation site by a hierarchical analysis of mammalian Atg proteins. Autophagy. 2010;6:764–76. doi: 10.4161/auto.6.6.12709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robenek H, Buers I, Hofnagel O, et al. GFP-tagged proteins visualized by freeze-fracture immuno-electron microscopy: a new tool in cellular and molecular medicine. J Cell Mol Med. 2009;13:1381–90. doi: 10.1111/j.1582-4934.2008.00407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


