Abstract
In exocrine glands, secretory proteins synthesized in the rough endoplasmic reticulum (RER) exhibit vectorial transport from ER through a succession of membrane-bounded components such as Golgi complex, condensing vacuoles and secretory granules. The secretory granules migrate to particular locations within the cell close to the apical membrane prior to the release of their contents into the acinar lumen. Currently, to release intragranular contents, secretory granules have been demonstrated to transiently dock and fuse at ‘porosome’, a permanent cup-shaped structures at the cell membranes. Then swelling of secretory granules occurs to allow explusion of intragranular contents. In this process, water and ion fluxes in the granule membrane appear to contribute to maintain secretory granule integrity and morphology via osmoregulation in secretory granules. Aquaporins (AQPs) are a family of small, hydrophobic, integral membrane proteins, which function as channels to permeate water and small solutes. The AQPs reside constitutively at the plasma membrane in most cell types. However, recent studies have demonstrated that the AQPs are present in secretory granules in exocrine glands, synaptic vesicles and intracellular vesicles in liver and kidney, implying that AQPs in secretory granules and vesicles are involved in their volume regulation. This paper reviews the possible role of AQPs on secretory granules, especially in exocrine glands, in secretory function.
Keywords: aquaporin, ion channel, granule swelling, secretory granules, porosome
Introduction
Aquaporins (AQPs) are a family of small, hydrophobic, integral membrane proteins of about 270 amino acids. In 1991, AQP1, formerly called CHIP28, was cloned as a first water channel and bio-physically characterized [1, 2]. Sequence analysis of AQP1 demonstrated that AQP protein subunits consist of six α-helix transmembrane domains with inverted symmetry between the first three and last three domains ([3]; Fig. 1). The two loops between transmembrane helices 2-3 and 5-6 contain the signature amino acid sequence motifs of the AQPs, asparagine-proline-alanine (NPA) sequences. This predicted topology led to the ‘hourglass model’ of AQP structure, in which these two NPA-containing loops connect in the centre of the lipid bilayer and form a hydrophilic pore for water transfer through the lipid bilayer ([4]; Fig. 1). The hourglass of AQP structure has been confirmed using electron and X-ray crystal analysis of AQP1 [5, 6].
1.
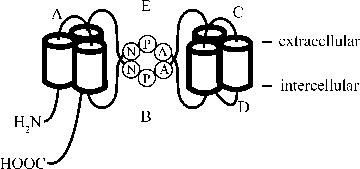
Hourglass model of aquaporins (AQP1) structure. AQP1 protein subunits consist of six α-helix transmembrane domains with inverted symmetry between the first three and last three domains. The two loops between transmembrane helices 2–3 (loop B) and 5-6 (loop E) contain the signature amino acid sequence motifs of the AQPs, asparagine-proline-alanine (NPA) sequences.
Biochemical and freeze fracture studies have indicated that AQP1 assembles in tetramer in the plasma membrane and each monomer contains a separate pore [4, 7]. The water selectivity of AQP1 has been suggested to be due to both the size-exclusion effect of the pore [8], and the orientation of asparagines in the NPA motifs providing necessary hydrogen-bonding interactions to isolate water molecule and avoid the passage of protons through the pore [9]. However, a direct barrier across the pore, centered around the NPA region, has also been suggested to form the major impediment to the proton passage [10, 11].
Subsequent studies have shown that AQP1 belongs to a large family of water-transporting proteins. Other AQPs have been cloned and at least 13 AQPs (AQP0 to AQP12) have so far been identified in mammals [12, 13]. They are expressed in various epithelia and endothelia and function as channels to permeate not only water but also small solutes. AQP1, AQP2, AQP4, AQP5 and AQP8 are primarily water selective, whereas AQP3, AQP7, AQP9 and AQP10 also transport glycerol, which are called ‘aquaglyc-eroporins' [14, 15]. Although AQP1 has been considered to be a water-selective channel as described above, AQP1 has also been reported to transport cations [16], gases such as carbon dioxide [17], and ammonia [18]. AQP7 and AQP9 have been reported to transport heavy metal salts such as arsenate [19], and AQP6 has been demonstrated to transport chloride at low pH [20]. It is most likely that AQPs function as channels that permeate water or small solutes.
The AQPs reside constitutively at the plasma membrane in most cell types. However, recent studies have demonstrated that the AQPs are present in zymogen granules in rat pancreatic acinar cells [21], secretory granules in rat parotid acinar cells [22] and Brunner's gland [23], cytoplasmic vesicles in human parotid aci-nar cells [24] and mouse liver [25], synaptic vesicles from rat brain [26], and intracellular vesicles in rat kidney [27]. These observations imply that AQPs in secretory granules and vesicles are involved in their volume regulation.
In various exocrine and endocrine cells, secretory proteins are synthesized in rough endoplasmic reticulum (RER). Then the proteins are vectorially transport from RER through a succession of membrane-bounded compartments including the Golgi complex, condensing vacuoles, and secretory granules and vesicles. The secretory granule or vesicle is formed from the condensing vacuole, which buds off the trans face of the Golgi complex. In the condensing vacuole, secretory proteins are present as dilute form. In a subsequent packing process, the proteins are condensed. During this process, it is conceivable that transport of ions and water through secretory granule membrane is necessary for the protein condensation, although there is not yet complete agreement on the basic principles involved [28–30].
The secretory granules and vesicles move to particular locations within the cell close to the plasma membrane prior to the release of their contents to the outside. Since the secreteory vesicles have been suggested to fuse with the plasma membrane by using electron microscopy [31], it has been considered that cell secretion involves the fusion of membrane-bound granules and vesicles at the plasma membrane at the secretory sites, and the release of intra-granular and intravesicular contents to the extracellular sites. Currently, by using atomic force microscopy, it has been demonstrated that secretory granules and vesicles transiently dock and fuse at ‘porosome’, a permanent cup-shaped structures at the cell membranes, and swell to allow explusion of their contents [32–36]. Therefore, secretion involves the fusion of the granule or vesicle membrane with porosome in the plasma membrane, followed by the release of their contents outside. Secretion without stimulation is referred to as ‘constitutive secretion’, but cell secretion following a stimulus is termed ‘regulatory secretion’.
During the secretory process, regulation of secretory granule or vesicle volume is important, in which contribution of various ion channels has been demonstrated [21, 26, 37–39]. This paper reviews the possible role of AQPs on secretory granules and vesicles in secretory function, especially in exocrine glands.
Role of AQPs in cell secretion
AQP5 is a water-selective channel protein widely expressed in exocrine glands [40–42]. In rat duodenal Brunner's gland, vasoac-tive intestinal polypeptide (VIP) has been reported to increase the flow rate as well as bicarbonate and protein output [43]. Parvin et al. [23] have demonstrated that AQP5 localizes in the secretory granule membrane and the apical membrane in rat Brunner's gland by immunohistochemistry and electron microscopic immunohisto-chemistry, and that the AQP5 level in the apical membrane is increased by VIP stimulation. This observation suggests that the AQP5 translocates from the secretory granule membrane to the apical membrane on secretion provoked by VIP. In the rat parotid gland, AQP5 is localized in the apical membrane including the intracellular canaliculi of acinar cells [41]. In rat parotid acinar cells, AQP5 has also been reported to traslocate from the intracel-lular vesicles to the apical membrane in vitro in response to stimulation with muscarinic agonists, which induce water secretion and feeble release of the digestive enzyme amylase [44]. However, the immunofluorescence and immunoelectron microscopic studies demonstrated that AQP5 was predominantly localized in the apical plasma membrane in the mouse parotid and submandibu-lar glands after stimulation or inhibition of secretion in vivo, indicating that no changes in the subcellular localization of AQP5 occurs [45]. On the other hand, it has been reported that immunostained AQP5 was scattered as clusters in the submem-branous area of the acinar cells in the rat injected with the β-ago-nist isoproterenol, which provokes amylase secretion in parotid acinar cells [42]. In the mouse parotid gland, AQP5 was localized along the apical membrane and its small invaginations formed by fusion of secretory granules after isoproterenol-administration [46]. The fluidity of the primary secretion has been proposed to be important for the release of intragranular contents from membrane-bound secretory granules in pancreas [47] and parotid gland [48]. It is most likely that components of granule membrane such as ion channels might insert into the apical membrane during fusion process at porosome, and subsequently salt and water would flush out the stored macromolecules into the acinar lumen and provide for an appropriate amount of fluid to be secreted with the proteins [47, 48].
Individual secretory events can be visualized with sulforho-damine B (SRB), a fluorescent fluid-phase polar tracer, as the formation of docked-granule profiles at apical membrane using two-photon excitation microscopy, since SRB remaining in the luminal region rapidly diffuses into granules fused with the plasma membrane [49]. When secretory events in rat parotid acini were investigated using the two-photon microscopy, the β-agonist isoproterenol provoked cell secretion, docked-granule profiles, at apical region as Figure 2 shows (Sugiya, Nemoto & Kasai, in preparation). However, the profiles formed disappeared soon. This observation suggests that SRB diffused into granules fused with the plasma membrane may subsequently be diluted by water rapidly, and implies function of AQPs in secretory granule membrane.
2.
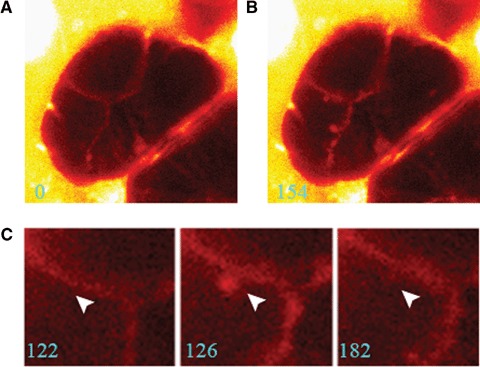
Cell secretion provoked by isoproterenol in rat parotid acinar cells. In sulforhodamine B (SRB) fluorescence image, there was no docked-granule profile before stimulation (A). When the cells were stimulated by isoproterenol for 154 sec., docked-granule profiles appeared at apical region (B). During stimulation with isopro-terenol (122–182 sec.), a docked-granule profile formed and subsequently disappeared as indicated by an arrowhead (C).
Role of AQPs in secretory granule swelling
In cell secretion process, role of secretory granule swelling has been investigated. In beige mouse mast cells, membrane fusion has been demonstrated to precede secretory granule swelling during cell secretion by the studies with electrophysiological membrane capacitance measurements [50, 51], which proved that osmotic swelling is not required for fusion. It has been inferred that secretory granule swelling is necessary to stabilize and widen the fusion pore and is caused by movement small solutes through the fusion pore into the granule matrix.
On the contrary, secretory granule swelling has been proposed to be prerequisite for secretory granule fusion with plasma membrane [52–56]. On the hypothesis, it has been considered that swelling of secretory granules results in a build-up of pressure for allowing expulsion of intragranular contents and the extent of secretory granule swelling dictates the amount of intragranular contents expelled during secretion.
In rat pancreas, AQP1 had been demonstrated to be localized at zymogen granules, the membrane-bound secretory vesicles [21]. The amino and carboxyl domains of AQP1 have suggested to localize at the luminal side of zymogen granule membrane, because immunoreacted signal of AQP1 was detected by immunoblot analysis when anti-AQP1 antibody to the carboxyl terminus of AQP1 was introduced into to the zymogen granules permeabilized by streptolysin-O, no immunoreacted signal was detectable in the sample of intact zymogen granules pre-exposed to the anti-AQP1 antibody, and immunogold-labelling was observed at the inner side of membrane of streptolysin-O-per-meabilized zymogen granules on the immunoelectron microscopy using the anti-AQP1 antibody [21]. This topology is curious, because the hydropathy analysis suggests that AQPs have six putative helical domain, and the studies utilizing epitope tagging, AQP-reporter chimeras, and site-specific antibodies confirmed this fundamentally topological organization and indicated that the amino and carboxyl termini are cytoplasmically oriented [57].
The contribution of AQP1 to zymogen granule swelling was studied [21]. Fusion of zymogen granules with plasma membrane has previously been reported to be facilitated by activation of the trimeric GTP-binding protein Giα3 in zymogen granule membrane by in vitro fusion assay [58, 59]. Subsequently, the guanosine 5’-triphosphate (GTP)-binding proteins has been shown to contribute to secretory granule swelling using atomic force microscopy [37], which provides three-dimensional data with the structure and dynamics of single biomolecule, living cells and organelles including secretory granules [60]. These observations suggest that zymogen granule swelling is an important prerequisite for zymogen granule fusion with plasma membrane. Cho et al. [21] have been demonstrated that the GTP-mediated increase in granule volume and water entry into granules determined using atomic force microscopy and tritiated water, respectively, were inhibited by HgCl2, an inhibitor of AQP1 [2], or by insertion of anti-AQP1 antibody into the zymogen granules. Therefore, it is most likely that AQP1 contributes to zymogen granule fusion with plasma membrane and expulsion of granule contents during secretion in pancreatic acinar cells.
In synaptosomes and synaptic vesicles from rat brain, Kelly et al. [39] examined whether synaptic vesicle swelling is similarly regulated to the zymogen granules in exocrine pancreas using atomic force microscopy. Since size of synaptic vesicles increased in the presence of GTP and the Go/Gi stimulator mastoparan, an amphiphilic tetradecapeptide from wasp venom, synaptic vesicle swelling via activation of heterotrimeric GTP-binding protein has been demonstrated [39]. Subsequently, water channels, AQP1 and AQP6, associated with synaptic vesicles isolated from rat brain were detected [26]. Since Goα was also detected as the major heterotrimeric GTP-binding protein in synaptic vesicles, Jeremic et al. [26] examined the role of AQPs in synaptic vesicle swelling via the activation of Go. The effects on synaptic vesicle size of Go protein stimulators, GTP and mastoparan, were examined using various approaches, such as photon-correlation spectroscopy, right-angle light scattering and atomic force microscopy. Consequently swelling of synaptic vesicles rapidly occurred in response to GTP and mastoparan. However, in the presence of the AQP inhibitor HgCl2, the stimulatory effect of mastoparan on synaptic vesicle swelling was significantly abrogated [26]. These observations strongly suggest that synaptic vesicle swelling is caused by a Go-regulated, AQP-mediated water entry in secretory vesicles and involved in neurotransmitter expulsion, as suggested in pancreatic zymogen granules.
In secretory granules isolated from the rat salivary parotid gland, AQP5 has been demonstrated to localize on the membrane of secretory granules by immunoblot analysis and immunoelectron microscopy [22]. On the immunoelectron microscopy using anti-AQP5 antibody to the carboxyl terminus of AQP5, the immunogold particles were detected at the outside of secretory granule membrane. To study the function of AQP5 in the parotid secretory granules, Matsuki et al. [22] utilized a quantitative in vitro assay involving rapid osmotic swelling and end-point measurements of granular osmotic lysis, which has been used for the investigation of ion conductance in secretory granules [61]. In this assay system, it has been demonstrated that anti-AQP5 antibody induces secretory granule swelling and lysis in iso-osmotic KCl solution, suggesting that inhibition of AQP5 function causes secretory granule swelling and lysis. In secretory granules of the rat parotid gland, expression of the heterotrimeric GTP-binding protein Gsα and Gsα-regulated Cl− conductance have been reported [62, 63]. However, AQP5 regulation by GTP-binding proteins in the secretory granule is still unknown.
Role of AQPs in ion permeation
On the basis of studies using atomic force microscopy in pancreatic acinar cells and isolated zymogen granules, Jena et al. [37] proposed that K+ and Cl– channels in the granule membrane need to induce granule swelling during secretion to prevent collapse of zymogen granules. Ion fluxes through K+ and Cl− channels in the granule membrane and osmotic swelling thus appear to contribute to maintain granule integrity and morphology in secretory function.
When the molecular mechanism of swelling of pancreatic zymogen granules was studied, detergent-solubilized zymogen granules co-isolate the inwardly rectifying K+ channel IRK-8 and the chloride channel CLC-2, in addition to other proteins such as AQP1, Giα3 and phospholipase A2 after immunoprecipitation with a monoclonal AQP1 antibody [64]. Exposure of zymogen granules to either the K+-channel blocker glyburide or the phospholipase A2 inhibitor ONO-RS-082 blocked GTP-induced zymogen granule swelling. In liposomes reconstituted with the AQP1 immunoisolated complex from solubilized zymogen granules, swelling in response to GTP occurred, but the GTP effect was abolished by glyburide or ONO-RS-082. In the planar lipid membranes reconstituted with the immunoisolate complex, conductance corresponding to the passage of K+ was decreased by glyburide or an anti-AQP1 antibody. These observations suggest that Giα3-phospholipase A2 -mediated pathway and K+ channels are involved in AQP1 regulation [64]. It has been reported that AQP1 expressed in Xenopus oocytes conducted cations [65] and the water permeability function was inhibited by the K+ channel blocker, tetraethyl ammonium [66]. When AQP1 purified from Saccharomyces was reconstituted into planar lipid bilayers, cyclic GMP-induced ion conductance was detected, although the ion channel number was exceedingly low [67]. The amount of similarity between K+ channels and AQP1 has been demonstrated [68]. These observations imply that AQP1 is involved in K+ flux in zymogen granules directly or indirectly, although crystallography studies have been demonstrated that AQP1 is a water-specific channel [6].
Matsuki et al. [22] have also demonstrated that the anti-AQP5 antibody-induced granule lysis is inhibited in the absence of Cl− or in the presence of 4,4-diisothiocyanostilbene-2,2’-disulfonic acid, an anion channel blocker in the reaction mixture. There is no evidence that AQP5 acts as ion channels in the AQP5-expressing oocytes [65, 69]. The presence of Cl− conductance in the secretory granule membrane of the rat parotid gland has been demonstrated [48, 70]. In airway secretory glands, the expression of cystic fibrosis transmembrane conductance regulator (CFTR), a cAMP-activated Cl− channel, has been reported [71], and CFTR has been suggested to be related to the ion content in the secretory granules and granule expansion [72]. As a consequence, we hypothesize that a balance of water permeation via AQP5 and Cl− conductance is necessary for secretory granule volume regulation. Currently, the hypothesis that AQPs function as osmotic and turgor sensors rather than water channels, the sensor hypothesis, has been advocated [73, 74]. Therefore, AQP5 appears to act as an osmotic sensor in the secretory granules of the parotid gland, although further studies with precise mechanisms of the relationship between AQP5 and the Cl− channels are necessary.
The function of most AQPs is well known to be inhibited by mercurial agents which bind the sulfhydryl (SH) group of cys-teines. In AQP5, a cysteine at residue 182 in loop E, a hydropho-bic loop (Fig. 1), is considered to be a mercury-sensitive domain [75], and corresponds to the known mercurial-inhibitory site [76]. Therefore, we examined the effect of HgCl2 on lysis of secretory granule in the parotid gland. Interestingly, HgCl2 clearly induced parotid secretory granule lysis [77]. The HgCl2-induced granule lysis was also completely blocked in the presence of ß-mercap-toethanol, a protective agent for SH groups, which has been demonstrated to restrain the effect of HgCl2 on AQP5 [78]. These observations were thought to support the view that the inhibition of AQP5 function causes secretory granule lysis.
However, it has been demonstrated that AQP6 function is activated by HgCl2[79, 80]. AQP6 genes have been identified in rat and human kidneys with high homologies to AQP0 and AQP2 [81, 82]. Subsequently, AQP6 has been found to be localized in intercellular sites of acid-secreting α-intercalated cells from renal-collecting duct [27]. It has been considered that AQP6 functions not as a water channel but as an anion channel, because permeation by anions in response to acidic pH or Hg2+ activation in Xenopus Laevis oocytes expressing AQP6 was found [79] and Hg2+-activated ion conductance was verified by single-channel recordings of the oocytes [83]. In Xenopus Laevis oocytes expressing AQP6, Hg2+ has also been demonstrated to stimulate transport of glycerol and urea [80]. Currently, we found the presence of AQP6 associated with secretory granule membranes in the rat parotid acinar cells [84]. In the granule membrane isolated from the purified secretory granules, anti-AQP6 antibody specifically recognized 33 kDa band by western blotting, indicating the presence of AQP6 in secretory granule membrane of rat parotid acinar cells, as Figure 3 shows. To confirm the presence of AQP6 in parotid secretory granules, immunoelectron microscopy in ultrathin cryosection was carried out (Fig. 4). Close observation of immunogold labelling of secretory granules demonstartrates gold labelling both the inner as well as outer leaflet of the granule membrane. In HEK239 cells, when a green fluorescence protein (GFP) tag was added to the N-terminus of AQP6, GFP-AQP6 was redirected to the plasma membranes [85]. In HEK239 cells expressing GFP-tagged AQP6, AQP6 has also been demonstrated to function as an anion channel with the halide permeability sequence: NO3− > I− >> Br− > Cl− >> F−[85], strongly supporting that AQP6 functions as anion channel in mammalian cells. Therefore, it is most likely that AQP6 functions as a Cl− channel and contributes to regulation of osmoregulation in secretory granules of the parotid gland, although further studies need to elucidate AQP6 function in secretory granule. In synaptic vesicles, HgCl2 inhibited the GTP-induced vesicle swelling, despite of the presence of AQP6 [26]. Therefore, role of AQP6 in the synaptic vesicle swelling is unknown.
3.
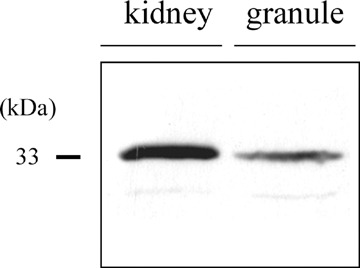
Localization of AQP6 in rat parotid secretory granule membrane. Protein expression of AQP6 in parotid secretory granule membrane (granule) was detected by western blotting using anti-AQP6 antibody. In granule membrane, 33 kDa band of AQP6 was detected. Kidney medul-lae epithelial cells (kidney) were used as a positive control.
4.
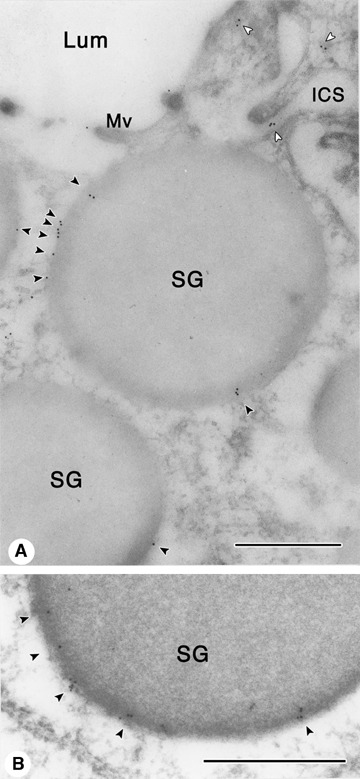
Localization of AQP6 in rat parotid secretory granules. Immunoelectron microscopy in ultrathin cryosection was carried out. Anti-AQP6 antibody was labelled with 10 nm colloidal gold-conjugated secondary antibody. (A) Colloidal gold particles were observed in parotid secretory granules as indicated (solid arrowheads) and in other spots (open arrowheads). (B) Colloidal gold particles were observed both in outer and inner leaflets of secretory granule membrane. SG, secretory granule; Lum, lumen; Mv, microvilli; ICS, intercellular canali-culi. Bar, 0.5 μm.
Conclusions
Secretion of macromolecules in exocrine and endocrine cells occurs through docking and fusion of secretory granule membrane at porosome in plasma membrane and the subsequent discharge of secretory granule contents [32–36]. It has been suggested that secretory granule swelling is involved in the exocytotic process. A specific set of ion channels in secretory granule membrane has been proposed to contribute to the secretory granule swelling [21, 26, 37–39]. AQPs in secretory granules membrane have to be demonstrated to be concerned in the granule swelling and may contribute to release of contents in secretory granules, which could provide a new information with AQP functions. In pancreatic zymo-gen granules, functional relationship between AQP1 and K+ channel has been demonstrated [64]. In parotid secretory granules, the presence of AQP5 and AQP6, which are water-selective and Cl−-permeable channels, respectively, has been demonstrated [22, 84]. Therefore, the relationship between water channels and ion channels has to be elucidated. To elucidate the function of AQPs in secretory granules, specific, non-toxic AQP inhibitors and specific AQP antibodies will be useful.
Cell secretion can be greatly accelerated following an appropriate cellular signal, which is called ‘regulatory secretion’. Regulatory secretion is dependent on intracellular Ca2+ or other intracellular signals. AQP4 function has been reported to be regulated by protein kinase C [86]. Studies with such a regulation on AQPs in secretory granules may be important to elucidate the functions.
Acknowledgments
This study was supported in part by a Nihon University Multidisciplinary Research Grant for 2006-2007 and a Grant of Oral Health Science Center HRC7 from Tokyo Dental College.
References
- 1.Smith BL, Agre P. Erythrocyte Mr 28,000 transmembrane protein exists as a multi-subunit oligomer similar to channel proteins. J Biol Chem. 1991;266:6407–15. [PubMed] [Google Scholar]
- 2.Preston GM, Carroll TP, Guggino WB, Agre P. Appearance of water channels in Xenopus oocytes expressing red cell CHIP28 protein. Science. 1992;256:385–7. doi: 10.1126/science.256.5055.385. [DOI] [PubMed] [Google Scholar]
- 3.Preston GM, Agre P. Isolation of the cDNA for erythrocyte integral membrane protein of 28 kilodalton: member of an ancient channel family. Proc Natl Acad Sci USA. 1991;88:11110–4. doi: 10.1073/pnas.88.24.11110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jung JS, Preston GM, Smith BL, Guggino WB, Agre P. Molecular structure of the water channel through aquaporin CHIP. The hourglass model. J Biol Chem. 1994;269:14648–54. [PubMed] [Google Scholar]
- 5.Murata K, Mitsuoka K, Hirai T, Walz T, Agre P, Heymann JB, Engle A, Fujiyoshi Y. Structural determinants of permeation through aquaporin-1. Nature. 2000;407:599–605. doi: 10.1038/35036519. [DOI] [PubMed] [Google Scholar]
- 6.Sui H, Han BG, Lee KJ, Walian P, Jap BK. Structural basis of water-specific transport through the AQP1 water channel. Nature. 2001;414:872–8. doi: 10.1038/414872a. [DOI] [PubMed] [Google Scholar]
- 7.Van Hoek AN, Hom ML, Luthjens LH, De Jong MD, Dempster JA, Van Os CH. Functional unit of 30 kDa for proximal tubule water channels as revealed by radiation inactivation. J Biol Chem. 1991;266:16633–5. [PubMed] [Google Scholar]
- 8.Kong Y, Ma J. Dynamic mechanisms of the membrane water channel aquaporin-1 (AQP1) Proc Natl Acad Sci USA. 2001;98:14345–9. doi: 10.1073/pnas.251507998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tajkhorshid E, Nollert P, Jensen MO, Miercke LJ, O’Connell J, Stroud RM, Schulton K. Control of the selectivity of the aquaporin water channel family by global orientational tuning. Science. 2002;296:525–30. doi: 10.1126/science.1067778. [DOI] [PubMed] [Google Scholar]
- 10.De Groot BL, Frigato T, Helms V, Grubmeller H. The mechanism of proton exclusion in the aquaporin-1 water channel. J Mol Biol. 2003;333:279–93. doi: 10.1016/j.jmb.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 11.Chakrabarti N, Roux B, Pomes R. Structural determinants of proton blockage in aquaporins. J Mol Biol. 2004;343:493–510. doi: 10.1016/j.jmb.2004.08.036. [DOI] [PubMed] [Google Scholar]
- 12.Verkman AS. More than just water channels: unexpected cellular roles of aquapor-ins. J Cell Sci. 2005;118:3225–32. doi: 10.1242/jcs.02519. [DOI] [PubMed] [Google Scholar]
- 13.Castle NA. Aquaporins as targets for drug discovery. Drug Discov Today. 2005;10:485–93. doi: 10.1016/S1359-6446(05)03390-8. [DOI] [PubMed] [Google Scholar]
- 14.Agre P, King LS, Yasui M, Guggino WB, Ottersen OP, Fujiyoshi Y, Engel A, Nielsen S. Aquaporin water channels – from atomic structure to clinical medicine. J Physiol. 2002;542:3–16. doi: 10.1113/jphysiol.2002.020818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yasui M. Molecular mechanisms and drug development in aquaporin water channel diseases: structure and function of aqua-porins. J Pharmacol Sci. 2004;96:260–3. doi: 10.1254/jphs.fmj04004x4. [DOI] [PubMed] [Google Scholar]
- 16.Anthony TL, Brooks HL, Boassa D, Leonov S, Yanochko GM, Regan JW, Yool AJ. Cloned human aquaporin-1 is a cyclic GMP-gated ion channel. Mol Pharmacol. 2000;57:576–88. doi: 10.1124/mol.57.3.576. [DOI] [PubMed] [Google Scholar]
- 17.Cooper GJ, Boron WF. Effect of PCMBS on CO2 permeability of Xenopus oocytes expressing aquaporin 1 or its C189S mutant. Am J Physiol Cell Physiol. 1998;275:C1481–6. doi: 10.1152/ajpcell.1998.275.6.C1481. [DOI] [PubMed] [Google Scholar]
- 18.Nakhoul NL, Hering-Smith KS, Abdulnour-Nakhoul SM, Hamm LL. Transport of NH3/NH in oocytes expressing aquaporin-1. Am J Physiol Renal Physiol. 2001;281:F255–63. doi: 10.1152/ajprenal.2001.281.2.F255. [DOI] [PubMed] [Google Scholar]
- 19.Liu J, Shen J, Carbrey JM, Mukhopadhyay R, Agre P, Rosen BP. Arsenite transport by mammalian aquagly-seroporins AQP7 and AQP9. Proc Natl Acad Sci USA. 2002;99:6053–8. doi: 10.1073/pnas.092131899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yasui M, Hazama A, Kwon TH, Nielsen S, Guggino WB, Agre P. Rapid gating and anion permeability of an intracellular aqua-porin. Nature. 1999;402:184–7. doi: 10.1038/46045. [DOI] [PubMed] [Google Scholar]
- 21.Cho SJ, Sattar AK, Jeong EH, Satchi M, Cho JA, Dash S, Mayes MS, Stromer MH, Jena BP. Aquaporin 1 regulates GTP-induced rapid gating of water in secretory vesicles. Proc Natl Acad Sci USA. 2002;99:4720–4. doi: 10.1073/pnas.072083499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matsuki M, Hashimoto S, Shimono M, Murakami M, Fujita-Yoshigaki J, Furuyama S, Sugiya H. Involvement of aquaporin-5 water channel in osmoregula-tion in parotid secretory granules. J Membr Biol. 2005;203:119–26. doi: 10.1007/s00232-005-0736-9. [DOI] [PubMed] [Google Scholar]
- 23.Parvin MN, Kurabuchi S, Murdiastuti K, Yao C, KosugiTanaka C, Akamatsu T, Kanamori N, Hosoi K. Subcellular redistribution of AQP5 by vasoactive intestinal polypeptide in the Brunner's gland of the rat duodenum. Am J Physiol Gastrointest Liver Physiol. 2005;288:G1283–91. doi: 10.1152/ajpgi.00030.2004. [DOI] [PubMed] [Google Scholar]
- 24.Smith JK, Siddiqui AA, Motlica LA, Dykes R, Simmons C, Schmidt J, Krishnaswamy GA, Berk SL. Interferon-α upregulates gene expression of aquaporin-5 in human parotid glands. J Interferon Cytokine Res. 1999;19:929–35. doi: 10.1089/107999099313479. [DOI] [PubMed] [Google Scholar]
- 25.Ferri D, Mazzone A, Liquori GE, Gassano G, Svelto M, Calamita G. Ontogeny distribution, and possible functional implications of unusual aquaporin, AQP8, in mouse liver. Hepatology. 2003;38:947–57. doi: 10.1053/jhep.2003.50397. [DOI] [PubMed] [Google Scholar]
- 26.Jeremic A, Cho WJ, Jena BP. Involvement of water channels in synaptic vesicle swelling. Exp Biol Med. 2005;230:674–80. doi: 10.1177/153537020523000910. [DOI] [PubMed] [Google Scholar]
- 27.Yasui M, Kwon TH, Knepper MA, Nielsen S, Agre P. Aquaporin-6: An intracellular vesicle water channel protein in renal epithelia. Proc Natl Acad Sci USA. 1999;96:5808–13. doi: 10.1073/pnas.96.10.5808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arvan P, Castle D. Sorting and storage during secretory granule biogenesis: looking backward and looking forward. Biochem J. 1998;332:593–610. doi: 10.1042/bj3320593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tooze SA, Martens GJ, Huttner WB. Secretory granule biogenesis: rafting to the SNARE. Trends Cell Biol. 2001;11:116–22. doi: 10.1016/s0962-8924(00)01907-3. [DOI] [PubMed] [Google Scholar]
- 30.Dannies PS. Mechanism for storage of prolactin and growth hormone in secretory granules. Mol Genet Metabolism. 2002;76:6–13. doi: 10.1016/s1096-7192(02)00011-2. [DOI] [PubMed] [Google Scholar]
- 31.Palade GE, Bruns RR. Structural modulation of plasmalemmal vesicles. J Cell Biol. 1968;37:633–649. doi: 10.1083/jcb.37.3.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jena BP. Discovery of the porosome: revealing the molecular mechanism of secretion and membrane fusion in cells. J Cell Mol Med. 2004;8:1–21. doi: 10.1111/j.1582-4934.2004.tb00255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jena BP. Molecular machinery and mechanism of cell secretion. Exp Biol Med. 2005;230:307–19. doi: 10.1177/153537020523000504. [DOI] [PubMed] [Google Scholar]
- 34.Anderson LL. Discovery of the ‘poro-some’; the universal seceretory machinery in cells. J Cell Mol Med. 2006;10:126–31. doi: 10.1111/j.1582-4934.2006.tb00294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jeftinija S. The story of cell sectetion: events leading to the discovery of the ‘porosome’– the universal seceretory machinery in cells. J Cell Mol Med. 2006;10:273–79. doi: 10.1111/j.1582-4934.2006.tb00398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leabu M. Membrane fusion in cells: molecular machinery and mechanisms. J Cell Mol Med. 2006;10:423–27. doi: 10.1111/j.1582-4934.2006.tb00409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jena BP, Schneider SW, Geibel JP, Webster P, Oberleithner H, Sritharan KC. Gi regulation of secretory vesicle swelling examined by atomic force microscopy. Proc Natl Acad Sci USA. 1997;94:13317–22. doi: 10.1073/pnas.94.24.13317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thévenod F. Ion channels in secretory granules of the pancreas and their role in exocytosis and release of secretory proteins. Am J Physiol Cell Physiol. 2002;283:C651–72. doi: 10.1152/ajpcell.00600.2001. [DOI] [PubMed] [Google Scholar]
- 39.Kelly ML, Cho WJ, Jeremic A, Abu-Hamdah R, Jena BP. Vesicle swelling regulates content expulsion during secretion. Cell Biol Int. 2004;28:709–16. doi: 10.1016/j.cellbi.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 40.Raina S, Preston GM, Guggino WB, Agre P. Molecular cloning and characterization of an aquaporin cDNA from salivary, lacrimal, and respiratory tissues. J Biol Chem. 1995;270:1908–12. doi: 10.1074/jbc.270.4.1908. [DOI] [PubMed] [Google Scholar]
- 41.Matsuzaki T, Suzuki T, Koyama H, Tanaka S, Takata K. Aquaporin-5 (AQP5), a water channel protein, in the rat salivary and lacrimal glands: immunolocalization and effect of secretory stimulation. Cell Tissue Res. 1999;295:513–21. doi: 10.1007/s004410051257. [DOI] [PubMed] [Google Scholar]
- 42.Matsuzaki T, Tajika Y, Suzuki T, Aoki T, Hagiwara H, Takata K. Immunolicalization of water channel, aquaporin-5 (AQP5) in the digestive system. Arch Histol Cytol. 2003;66:307–15. doi: 10.1679/aohc.66.307. [DOI] [PubMed] [Google Scholar]
- 43.Kirkegaard P, Lundberg JM, Poulsen SS, Fahrenkrug J, Hokfelt T, Christiansen L. Vasoactive intestinal polypeptidergic nerves and Brunner's gland secretion in the rat. Gastroenterol. 1981;81:872–8. [PubMed] [Google Scholar]
- 44.Ishikawa Y, Eguchi T, Skowronski MT, Ishida H. Acetylcholine acts on M3 muscarinic receptors and induces the translocation of aquaporin 5 water channel via cytosolic Ca2+ elevation in rat parotid glands. Biochem Biophys Res Commun. 1998;245:835–40. doi: 10.1006/bbrc.1998.8395. [DOI] [PubMed] [Google Scholar]
- 45.Gresz V, Kwon TH, Gong H, Agre P, Steward MC, King LS, Nielsen S. Immunolocalization of AQP5 in rat parotid and submandibular salivary glands after stimulation or inhibition of secretion in vivo. Am J Physiol Gastrointest Liver Physiol. 2004;287:G151–61. doi: 10.1152/ajpgi.00480.2003. [DOI] [PubMed] [Google Scholar]
- 46.Matsuzaki T, Ablimit A, Suzuki T, Aoki T, Hagiwara H, Takata K. Changes of aquaporin 5-distribution during release and reaccumulation of secretory granules in isoproterenol-treated mouse parotid gland. J Electron Microscopy. 2006;55:183–9. doi: 10.1093/jmicro/dfl023. [DOI] [PubMed] [Google Scholar]
- 47.Gasser KW, DiDomenico J, Hopfer U. Secretagogues activate chloride transport pathways in pancreatic zymogen granules. Am J Physiol Gastrointest Liver Physiol. 1988;254:G93–9. doi: 10.1152/ajpgi.1988.254.1.G93. [DOI] [PubMed] [Google Scholar]
- 48.Gasser KW, Goldsmith A, Hopfer U. Regulation of chloride transport in parotid secretory granules by membrane fluidity. Biochemistry. 1990;29:7282–8. doi: 10.1021/bi00483a018. [DOI] [PubMed] [Google Scholar]
- 49.Nemoto T, Kimura R, Ito K, Tachikawa A, Miyashita Y, Lino M, Kasai H. Sequential-replenishment mechanism of exocytosis in pancreatic acini. Nat Cell Biol. 2001;3:73–6. doi: 10.1038/35060042. [DOI] [PubMed] [Google Scholar]
- 50.Zimmerberg J, Curran M, Cohen FS, Brodwick M. Simultaneous electrical and optical measurements show that membrane fusion proceeds secretory granule swelling during exocytosis of beige mouse mast cells. Proc Natl Acad Sci USA. 1987;84:1585–9. doi: 10.1073/pnas.84.6.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Breckenridge LJ, Aimers W. Final steps in exocytosis observed in a cell with giant secretory granules. Proc Natl Acad Sci USA. 1987;84:1945–9. doi: 10.1073/pnas.84.7.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pazoles CJ, Pollard HB. Evidence for stimulation of anion transport in ATP-evoked transmitter release from isolated secretory vesicles. J Biol Chem. 1978;253:3962–9. [PubMed] [Google Scholar]
- 53.Pollard HB, Pazoles CJ, Creutz CE, Zinder O. The chromaffin granule and possible mechanisms of exocytosis. Int Rev Cytol. 1979;58:159–97. doi: 10.1016/s0074-7696(08)61475-8. [DOI] [PubMed] [Google Scholar]
- 54.Stanley EF, Ehrenstein G. A model for exocytosis based on the opening of calcium-activated potassium channels in vesicles. Life Sci. 1985;37:1985–95. doi: 10.1016/0024-3205(85)90029-3. [DOI] [PubMed] [Google Scholar]
- 55.Finkelstein A, Zimmerberg J, Cohen FS. Osmotic swelling of vesicles: its role in the fusion of vesicles with planner phospho-lipids bilayer membranes and its possible role in exocytosis. Annu Rev Physiol. 1986;48:163–74. doi: 10.1146/annurev.ph.48.030186.001115. [DOI] [PubMed] [Google Scholar]
- 56.Aimers W. Exocytosis. Annu Rev Physiol. 1990;52:607–24. doi: 10.1146/annurev.ph.52.030190.003135. [DOI] [PubMed] [Google Scholar]
- 57.Verkman AS, Mitra AK. Structure and function of aquaporin water channels. Am J Physiol Renal Physiol. 2000;278:F13–28. doi: 10.1152/ajprenal.2000.278.1.F13. [DOI] [PubMed] [Google Scholar]
- 58.Nadin CY, Rogers J, Tomlinson S, Edwardson JM. A specific interaction in vitro between pancreatic zymogen granules and plasma membranes: stimulation by G-protein activators but not by Ca2+ J Cell Biol. 1989;109:2801–8. doi: 10.1083/jcb.109.6.2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sattar AA, Boinpally R, Stromer MH, Jena BP. Gαi3 in pancreatic zymogen granules participates in vesicular fusion. J Biochem. 2002;131:815–20. doi: 10.1093/oxfordjournals.jbchem.a003170. [DOI] [PubMed] [Google Scholar]
- 60.Schneider SW. Kiss and run mechanism in exocytosis. J Membr Biol. 2001;181:67–76. [PubMed] [Google Scholar]
- 61.De Lisle RC, Hopfer C. Electrolyte permeabilities of pancreatic zymogen granules: implications for pancreatic secretion. Am J Physiol Gastrointest Liver Physiol. 1986;250:G489–96. doi: 10.1152/ajpgi.1986.250.4.G489. [DOI] [PubMed] [Google Scholar]
- 62.Watoson EL, DiJulio D, Kauffman D, Iversen J, Robinovitch MR, Izutsu KT. Evidence for G proteins in rat parotid plasma membranes and secretory granules. Biochem J. 1992;285:441–9. doi: 10.1042/bj2850441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Watson EL, Izutsu KT, Jacobson KL, Dijulio DH. The heterotrimeric GTP-bind-ing protein, Gs, modulates the Cl− conductance of rat parotid acinar secretory granules. Biochem Biophys Res Commun. 1997;238:638–42. doi: 10.1006/bbrc.1997.7354. [DOI] [PubMed] [Google Scholar]
- 64.Abu-Hamdah R, Cho W-J, Sho S-J, Jeremic A, Kelly M, Hie AE, Jena BP. Regulation of the water channel aqua-porin-1: isolation and reconstitution of the regulatory complex. Cell Biol Int. 2004;28:7–17. doi: 10.1016/j.cellbi.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 65.Anthony TL, Brooks HL, Boassa D, Leonov S, Yanochko GM, Regan JW, Yool AJ. Cloned human aquaporin-1 is a cyclic GMP-gated ion channel. Mol Pharmacol. 2000;57:576–88. doi: 10.1124/mol.57.3.576. [DOI] [PubMed] [Google Scholar]
- 66.Brooks HL, Regan JW, Yool AJ. Inhibition of aquaproin-1 water permeability by tetraethylammonium: involvement of the loop E pore region. Mol Pharmacol. 2000;57:1021–6. [PubMed] [Google Scholar]
- 67.Saparov SM, Kozono D, Rothe U, Agre P, Pohl P. Water and ion permeation of aquaporin-1 in planar lipid bilayers. Major differences in structural determinants and stoichiometry. J Biol Chem. 2001;276:31515–20. doi: 10.1074/jbc.M104267200. [DOI] [PubMed] [Google Scholar]
- 68.Yool AJ, Weinstein AM. New roles for old holes: ion channel function in aquaproin-1. News Physiol Sci. 2002;17:68–72. doi: 10.1152/nips.01372.2001. [DOI] [PubMed] [Google Scholar]
- 69.Agre P, Lee MD, Devidas S, Guggino WB. Aquaporins and ion conductance. Science. 1997;275:1490. author reply 1492. [PubMed] [Google Scholar]
- 70.Gasser KW, Hopfer U. Chloride transport across the membrane of parotid secretory granules. Am J Physiol Cell Physiol. 1990;259:C413–20. doi: 10.1152/ajpcell.1990.259.3.C413. [DOI] [PubMed] [Google Scholar]
- 71.Jacquot J, Puchelle E, Hinnraky J, Fuchey C, Bettinger C, Spilmont C, Bonnet N, Dieterle A, Dreyer D, Pavirani A, Dalemans W. Localization of the cystic fibrosis transmembrane conductance regylator in airway secretory glands. Eur Respir J. 1993;6:169–76. [PubMed] [Google Scholar]
- 72.Baconnais S, Delavoie F, Zahm JM, Milliot M, Terryn C, Castillon N, Banchet B, Michel J, Danos O, Merten M, Chinet T, Zierold K, Bonnet N, Puchelle E, Balossier G. Abnormal ion content, hydra-tion and granule expansion of the secretory granules from cystic fibrosis airway glandular cells. Exp Cell Res. 2005;309:296–304. doi: 10.1016/j.yexcr.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 73.Shachar-Hill B, Hill AE. Paracellular fluid transport by epithelia. Int Rev Cytol. 2002;215:319–50. doi: 10.1016/s0074-7696(02)15014-5. [DOI] [PubMed] [Google Scholar]
- 74.Hill AE, Shachar-Hill B, Shachar-Hill Y. What are aquaporins for? J Membr Biol. 2004;197:1–32. doi: 10.1007/s00232-003-0639-6. [DOI] [PubMed] [Google Scholar]
- 75.Raina S, Preston GM, Guggino WB, Agre P. Molecular cloning and characterization of an aquaporin cDNA from salivary, lacrimal, and respiratory tissues. J Biol Chem. 1995;270:1908–12. doi: 10.1074/jbc.270.4.1908. [DOI] [PubMed] [Google Scholar]
- 76.Preston GM, Jung JS, Guggino WB, Agre P. The mercury-sensitive residue at cysteine 189 in the CHIP28 water channel. J Biol Chem. 1993;268:17–20. [PubMed] [Google Scholar]
- 77.Sugiya H, Matsuki M. AQPs and control of vesicle volume in secretory cells. J Membr Biol. 2006;210:155–9. doi: 10.1007/s00232-005-0853-5. [DOI] [PubMed] [Google Scholar]
- 78.Frigeri A, Gropper MA, Umenishi F, Kawashima M, Brown D, Verkman AS. Localization of MIWC and GLIP water channel homologs in neuromuscular, epithelial and glandular tissues. J Cell Sci. 1995;108:2993–3002. doi: 10.1242/jcs.108.9.2993. [DOI] [PubMed] [Google Scholar]
- 79.Yasui M, Hazama A, Kwon TH, Nielsen S, Guggino WB, Agre P. Rapid gating and anion permeability of an intracellular aqua-porin. Nature. 1999;402:184–7. doi: 10.1038/46045. [DOI] [PubMed] [Google Scholar]
- 80.Holm LM, Klaerke DA, Zeuthen T. Aquaporin 6 is permeable to glycerol and urea. Pflügers Arch. 2004;448:181–6. doi: 10.1007/s00424-004-1245-x. [DOI] [PubMed] [Google Scholar]
- 81.Ma T, Frigeri A, Skach W, Verkman AS. Cloning of a novel rat kidney cDNA homologous to CHIP28 and WCH-CD water channels. Biochem Biophys Res Commun. 1993;197:654–9. doi: 10.1006/bbrc.1993.2529. [DOI] [PubMed] [Google Scholar]
- 82.Ma T, Yang B, Kuo WL, Verkman AS. cDNA cloning and gene structure of a novel water channel expressed exclusively in human kidney: evidence for a gene cluster of aquaporins at chromosome locus 12q13. Genomics. 1996;35:543–50. doi: 10.1006/geno.1996.0396. [DOI] [PubMed] [Google Scholar]
- 83.Hazama A, Kozono D, Guggino WB, Agre P, Yasui M. Ion permeation of AQP6 water channel protein. Single channel recordings after Hg2+ activation. J Biol Chem. 2002;277:29224–30. doi: 10.1074/jbc.M204258200. [DOI] [PubMed] [Google Scholar]
- 84.Matsuki-Fukushima M, Hashimoto S, Shimono M, Satoh K, Fujita-Yoshigaki J, Sugiya H. Presence and localization of aquaporin-6 in rat parotid acinar cells. Cell Tissue Res. 2008;332:73–80. doi: 10.1007/s00441-007-0558-4. [DOI] [PubMed] [Google Scholar]
- 85.Ikeda M, Beitz E, Kozono D, Guggino WB, Agre P, Yasui M. Characterization of aqua-porin-6 as a nitrate channel in mammalian cells. Requirement of pore-lining residue threonine 63. J Biol Chem. 2002;277:39873–9. doi: 10.1074/jbc.M207008200. [DOI] [PubMed] [Google Scholar]
- 86.Zelenina M, Zelenin S, Bondar AA, Brismar H, Aperia A. Water permeability of aqua-porin-4 is decreased by protein kinase C and dopamine. Am J Physiol Renal Physiol. 2002;283:F309–18. doi: 10.1152/ajprenal.00260.2001. [DOI] [PubMed] [Google Scholar]


