Abstract
Neurodegenerative diseases are progressive and incurable and are becoming ever more prevalent. To study whether neural stem cell can reactivate or rescue functions of impaired neurons in the human aging and neurodegenerating brain, we co-cultured postmortem slices from Alzheimer patients and control participants with rat embryonic day 14 (E14) neural stem cells. Viability staining based on the exclusion of ethidium bromide by intact plasma membranes showed that there were strikingly more viable cells and fewer dead cells in slices co-cultured with neural stem cells than in untreated slices. The presence of Alzheimer pathology in the brain slices did not influence this effect, although the slices from Alzheimer patients, in general, contained fewer viable cells. Co-culturing with rat E14 fibroblasts did not improve the viability of neurons in the human brain slices. Since the human slices and neural stem cells were separated by a membrane during co-culturing our data show for the first time that neural stem cells release diffusible factors that may improve the survival of aged and degenerating neurons in human brains.
Keywords: rat embryonic neural stem cells, neuronal reactivation, tissue culture, Alzheimer's disease
Introduction
It is generally believed that neural stem cells may constitute a promising source to replace lost neurons in neurodegenerative diseases, such as Parkinson's disease, where only limited brain areas are affected [1]. For other neurodegenerative disorders (e.g. Alzheimer's disease (AD), multiple sclerosis, amyotrophic lateral sclerosis) the prospects are considered to be less positive. Although in AD massive neuronal loss only occurs in very few brain structures, such as the hippocmpal CA1 and CA2 regions, the entorhinal cortex and the locus coeruleus [2–5], large parts of the brain are affected by pathological alterations [6] and decreased neuronal metabolism [7–9]. However, even in the presence of abundant Alzheimer pathology many viable neocortical neurons can be observed [10]. Further, metabolic alterations are already observed in mild cognitive impairment [11], while in this pre-clinical AD stage no substantial neuron loss in a vulnerable brain region such as the hippocampal CA1 area occurs [12]. As a result, it has been argued that AD is predominantly due to reduced neuronal metabolic activity, with aging as a major risk factor, and that reactivation of neurons might be a promising therapeutic strategy for AD [9]. In this respect, it is interesting that a study using an animal model for Parkinson's disease has shown that neural stem cells have the capacity to rescue degenerating neurons rather than to replace lost ones [13]. Similarly, in a model for chronic multiple sclerosis neural stem cells did not only replace lost oligodendrocytes, but also stimulated the survival of persisting oligodendrocytes and reduced reactive astrogliosis, thereby inducing functional recovery [14]. Proliferation of endogenous cells has been described in the sub-granular zone of the adult human brain [15], which reportedly is enhanced in Alzheimer's disease [16, 17]. However, it is unclear whether this increased proliferation in AD may be helpful to devise therapies [16, 17].
To study whether neural stem cells can, in principle, re-activate or rescue human neurons that have experienced prolonged aging or have suffered from a neurodegenerative process, we have co-cultured slices from postmortem human brain with embryonic day 14 (E14) rat neural stem cells.
Materials and methods
Patients
Brains were obtained with informed consent viathe rapid autopsy program from the Netherlands Brain Bank. Permission for the use of tissues and medical information for research purposes and ethical approval for culturing human postmortem human brain tissue was obtained from the Ethical Committee of the Medical Faculty of the Free University (VUMC, Amsterdam, The Netherlands, 971019) where the autopsies were performed. The data presented in this report are based on neocortical tissue from the precentral gyrus (PG) of Alzheimer patients (n = 5) and control individuals without neurological disease (n = 4). The autopsy details of the patients are shown in Table 1.
1.
Clinical data of the patients.
| Patient (NBB- number) | Age | Sex | Brain weight (grams) | pH | Clinical diagnosis | Cause of death |
|---|---|---|---|---|---|---|
| A1 (03-090) | 87 | F | 929 | 6.5 | AD, Vascular dementia Arterial fibrillation Hypertension Diabetes mellitus type II | Infection |
| A2 (05-005) | 94 | F | 1041 | 7.3 | AD, cardiac decompensation Atrial fibrillation | Cardiovascular accident |
| A3 (05-010) | 93 | M | 1210 | 6.5 | AD, Anaemia Prostate cancer | NA |
| A4 (05-011) | 93 | F | 1045 | 6.5 | AD, Chronic malnutrition | Cachexia |
| A5 (05-040) | 69 | M | 1170 | 6.5 | AD, Hypertension | Pneumonia |
| C1 (04-061) | 88 | F | 1168 | 7.0 | Paroxysmal atrium fibrillation | Old age |
| C2 (04-074) | 55 | M>F | 1321 | 6.4 | Trans-sexuality Lung cancer | Lung carcinoma |
| C3 (05-014) | 86 | F | 1127 | 7.1 | Anaemia Heart failure Epilepsy | Heart failure |
| C4 (05-032) | 74 | M | 1522 | 6.4 | Lung carcinoma | Lung carcinoma |
A1–A5: Alzheimer patients; C1–C4: control participants; to facilitate cross-reference with other studies the patient numbers according to the Netherlands
Brain Bank (NBB) are indicated between brackets.
F, female; M, male; M>F, male to female transexual; pH, pH value of cerebrospinal fluid at death; NA, not available.
Culture conditions of postmortem slices, rat E14 neurospheres and rat E14 fibroblasts
As previously reported [10, 18], from each patient pieces of PG were chopped into 200 μm slices, which were allocated in a systematic way to 24-well plates to facilitate later sampling for co-culture experiments. The slices were cultured free-floating in uncoated 24-well plates containing basal medium [19] at 35°C with 5% CO2. Tissue cultures were refreshed three times per week with 100 μl R16.
We obtained permission from the local Committee on Animal Care and Use for the experiments described in this report. All procedures involving animals were performed in compliance with the guidelines of this committee and were conducted as described by other investigators [20, 21] with minor modifications. Timed pregnant Wistar rats were killed by CO2 inhalation and decapitation (E14, plug day = 0). The foetuses were removed from the uterus, placed in ice-cold phosphate buffered saline (PBS) and kept on ice during subsequent manipulations. After careful removal of blood vessels, the forebrains were incubated in PBS containing 0.25% trypsin for 15 min. at 37°C. During the digestion, the tissue pieces were 2-3 times gently disrupted by pipetting through a flamed-polished pasteur pipette. After a brief centrifugation step, the cells were re-suspended in Dulbecco's modification of Eagle's medium (DMEM)/F12 medium (Life Technologies, Breda, The Netherlands) supplemented with B 27 medium supplement (1:100, Life Technologies), human recombinant basic fibroblast growth factor (b-FGF, 20 ng/ml, Pepro Tech, London, UK), human recombinant epidermal growth factor (EGF, 20 ng/ml, Pepro Tech), heparin (5 μg/ml, Sigma, Zwijndrecht, The Netherlands), gentamicin (50 mg/ml, Life Technologies) and plated into 6-well plate at a density of about 105 cells/ml. After 2-3 days of culture, neurospheres were formed, which were mechanically dissociated and reseeded at regular intervals [20]. Further uncoated coverslips were used during the culture to discourage neurospheres from attachment to the bottom and concomitant differentiation. In this way floating neurosphere cultures consisting of cells with an undifferentiated morphology could be maintained for at least 1 month. Since it has been shown that by 35 days E14 rat neurospheres become senescent [20], for co-culturing purposes no neurospheres older than 30 days were used (i.e. either passage 1 or 2). If the floating neurospheres (Fig. 1A) were allowed to attach to the bottom bipolar shaped nestin positive cells started to migrate out of the spheres (Fig. 1B). If these cells were allowed to stay attached for longer periods, either in mitogen-containing medium, serum-containing medium or serum-free medium, they assumed different morphological shapes. Then various markers for oligodendrocytes (NG2, Fig. 1C), immature neurons (β-tubulin III, doublecortin and gamma aminobutyric acid (GABA), Fig. 1D-F) and astrocytes (glial fibrillary acidic protein [GFAP] and vimentin, Fig. 1G-H) could be detected showing that the E14 neural stem cells were multi-potent.
1.
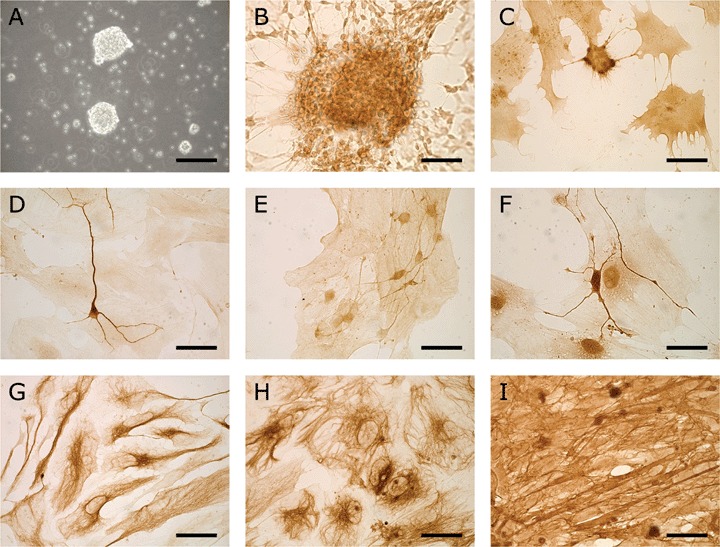
Illustration of the neurospheres used for co-culturing purposes and their multipotency. (A) Phase contrast micrograph of 3-day-old free-floating neurospheres. (B) Cells migrating out of a neurosphere that had been allowed to attach to a substrate 4 days previously (nestin staining). After 11 days in serum containing medium many cells had differentiated towards several neural cell types (C-H). (C) Cells expressing the oligodendrocytic marker NG2. (D) A β-tubulin III expressing immature neuron. (E) Some cells were faintly doublecortin positive. In the developing nervous system doublecortin is a marker for migrating neuroblasts. (F) Also cells expressing the neurotransmitter GABA were observed. Cells with the morphology of astrocytes were positive for GFAP (G) and vimentin (H). (I) Cultured fibroblasts formed a dense layer of cells expressing fibronectin. Scale bars: 60 μm (A); 30 μm (B–I).
For the isolation of fibroblasts the bodies of the foetuses were washed twice in fresh PBS to remove any remaining blood. After the head, heart, liver and intestines were removed, the carcasses were minced and treated with 0.25% trypsin in PBS containing 0.04% EDTA for 20 min. at 37°C and triturated vigorously. After centrifugation the fibroblasts were re-suspended in DMEM/F12 medium supplemented with 10% foetal bovine serum and transferred to a T75 flask. One day before co-culture, the cells were digested and seeded onto uncoated 30 mm coverslips in a 6-well plate (Fig. 1I).
Co-culture conditions
In order to avoid the possibility of cell fusion or trans-differentiation, during co-culture experiments, human postmortem slices were separated by a membrane from either neural stem cells or fibroblasts. Moreover, neural stem cells or fibroblasts growing over the surface of the postmortem slices would prevent accurate counting of viable cells. Therefore, we used Transwell plates (Corning, Acton, MA, USA) containing 24 mm inserts with 8 μm pore size and in a co-culture experiment 2 wells were used: in each well four postmortem slices were placed in the upper compartment. In the lower compartment of one well we put either E14 rat neurospheres (NCC group) or E14 rat fibroblasts (FCC group) while in the other well no cells (untreated group) were added. Autopsy material arrives at unpredictable times whereas neural stem cells need some time to proliferate to form neurospheres and, therefore, the start of co-cultures varied from one experiment to another. If the amount of the tissue permitted, more than one experiment per patient was carried out with different co-culture duration. Thus, for five patients (A1-A4, C3), slices from both the untreated (U) and the co-cultured group were harvested and stained at three different time-points (i.e. 3, 7 and 10 days after co-culturing with neurospheres). The material of patient C1 was sufficient for two experiments (5 and 11 days in co-culture), whereas tissue from patients C2, C4 and A5 each only allowed one experiment (7 days in co-culture). At each time point four untreated slices and four co-cultured slices were analysed.
Pilot experiments have suggested that the human postmortem slices can survive better in R16 medium than in DMEM/F12 with mitogens which are needed for neural stem cell proliferation or in DMEM/F12 with foetal bovine serum (data not shown). Therefore, the slices, neurospheres and fibroblasts were kept in their own medium for different periods before co-culture, but in the co-culture conditions the medium was R16 without addition of any growth factors or mitogens. At the start of co-culture experiments with neurospheres, uncoated sterile coverslips were placed into the Transwell plate and neurospheres harvested in Eppendorf tubes were washed two times with R16. On average 40 neurospheres were transferred into lower compartment of the co-culture well. In the case of co-cultures with fibroblasts, coverslips with attached fibroblasts were washed with R16 and were then placed into Transwell plates with R16 medium. Four postmortem slices were transferred from the 24-well plates into each upper compartment of a Transwell plate in a systematic way such that they constituted comparable groups. The amount of R16 was adjusted to 3 ml in order to cover the slices with medium. During co-culture experiments three times a week 1 ml of R16 was removed and old neurospheres were replaced by new neurospheres, then 1 ml R16 was added. Cells in neurospheres that were replaced after 2-3 days in co-culture conditions were still viable and had retained an undifferentiated morphology, but we have not tested whether they were still multipotent.
Viability assessment
Viability of cells in the postmortem human brain slices was assessed using the Live/Dead kit (L3224, Molecular Probes, Leiden, The Netherlands) according to the protocol provided by the manufacturer. The procedures for obtaining images and counting cells have been described previously [10]. Briefly, at systematic random sampled locations in the cortical layer of the slices photographs were made using a CLSM (Zeiss 410 Invert confocal laser scanning microscope) with a 25x oil objective. Twenty to 50 images were made in this way from each slice and in each image the cells were counted using in 12 counting quadrants containing inclusion and exclusion lines. Cells with a green fluorescent cytoplasm and a dark nucleus were counted as ‘living’, while cells with only a red nucleus were counted as ‘dead’. Cells that had a red nucleus with green cytoplasm, and were consequently in an intermediate state between living and dead, were denoted as ‘leaky cells' [10].
Immunocytochemistry
Slices were fixed overnight with a 4% paraformaldehyde in PBS at the day of autopsy (day in vitro 0, DIV0) to assess the morphological features of the tissue and the presence of Alzheimer's pathology in all four tissue pieces for each patient. Following three washing steps with Tris-buffered saline (TBS; 0.05 M Tris, 0.15 M NaCl, pH 7.6), slices were incubated in 3% hydrogen peroxide, 10% methanol in TBS for 30 min. to eliminate the endogenous peroxidase activity. Antibody to neuronal nuclear protein (mouse-anti-NeuN, Chemicon, Temecula, CA, 1:200) was used to visualize the cytoarchitecture of neurons in human slices. The antibodies against β-amyloid (mouse-anti-βA4, DAKO, Glostrup Denmark, 1:200) and abnormally phosphorylated tau (mouse-anti-AT-8, Innogenetics, Gent, Belgium, 1:300) were used to evaluate the degree of AD pathology and these antibodies required extra pre-treatment procedures [22, 23]. For staining of β-amyloid, it is necessary to incubate the slices with 100% formic acid for 20 min. to increase the staining intensity. For the AT8 staining, the slices were first incubated with 0.3% hydrogen peroxide in 100% methanol and then with 5% milk powder (in TBS) to block the background before incubation with the primary antibody solution. After overnight incubation, the slices were washed with TBS and incubated with rabbit-anti-mouse antibody conjugated to peroxidase (DAKO, 1:100) for 1 hr at room temperature. Subsequently, the slices were allowed to react with 0.5 mg/ml 3,3’-diaminobenzidine (DAB) in TBS for 5-20 min. until the staining was satisfactory.
The extend of the pathological alterations (β–amyloid and AT8) in the cultured slices was semi-quantitatively evaluated by four independent observers, attributing grades ranging from no (0), mild (1), moderate (2) strong (3) to massive (4), which subsequently were averaged over the observers using medians.
To illustrate the properties of rat E14 neurospheres and fibroblasts, we used several antibodies. As a marker for undifferentiated neural stem cells we used rabbit-anti-nestin (Chemicon, 1:1000). Markers for immature neurons were mouse-anti-β-tubulin III (Sigma, 1:2000), guinea pig-anti-doublecortin (Chemicon, 1:3000) and rabbit-anti-gamma aminobutyric acid (anti-GABA, Sigma, 1:1000). Rabbit-anti-NG2 (Chemicon, 1:1000) was applied to detect precursors for oligodendrocytes. Astrocytes were detected using mouse-anti-vimentin (Chemicon 1:4000) and rabbit-anti-glial fibrillary protein (anti-GFAP, DAKO 1:10000). Secondary antibodies were donkey-anti-rabbit conjugated to peroxidase (Jackson Laboratories, 1:1000), rabbit-anti-mouse conjugated to peroxidase (DAKO, 1:100) and biotinylated-goat-anti-guinea pig (Vector, Burlingame, CA, USA, 1:400). ABC Elite kit (Vector, 1:800) was used in combination with the biotinylated antibody. For neurosphere and fibroblast it was not necessary to pre-treat the preparations with hydrogen peroxide, but the incubation conditions were further the same as used for postmortem slices.
Statistical analysis
The statistical analysis was performed using S-PLUS 6.2 software (Insightful Inc., Seattle, Washington, USA). The Mann-Whitney test and Fisher's exact test were performed to analyse the clinico-pathological data. Linear regression was used to analyse the influence of Alzheimer pathology on the neuronal viability in untreated slices and the improvement due to co-culture with neural stem cells. Further, we had essentially two questions: (1) Is there difference in the viability of cells in untreated slices from control and AD patients? This was tested using the t-test. (2) Does treatment with neural stem cells improve the viability of slices from both control and AD patients? This was tested with the paired t-test. The corresponding questions with respect to leaky, dead and total cells were tested separately. A level-of-significance of 0.05 was considered to be significant.
Results
Clinico-pathological data and morphological features of the human postmortem slices
The clinical data from all patients are summarized in Table 1. Using the Mann-Whitney test we did not find significant differences for age (P= 0.07), brain weight (P= 0.07) and the agonal state at death as indicated by the pH of the colony stimulating factor (CSF) (P= 0.30) between the AD and control group. Also, sex was not different between the groups (Fisher's exact test P= 0.57). Table 2 displays information regarding the postmortem delay, the transport delay, the total delay between death of the patient and placement of slices into culture medium, the grades of local amyloid pathology and hyperphosphorylated tau pathology (AT8) and Braak stage [6]. The postmortem interval and transport delay (including the preparation of the tissue) may have a detrimental effect on the survival of neurons, therefore, we also analysed these factors. It appeared that the postmortem interval (P= 0.06) and transport delay (P= 0.18) did not differ between the control and AD group. However, the total delay (i.e. from death of the subject until placement of slices in culture medium) was higher in the control group (P= 0.01). This indicates that with the possible exception of the total delay, the control and AD groups were matched. The effect of total delay on neuronal viability will be addressed below. Causes of death were very diverse (including cancer, cardiovascular disease, cachexia and infection) and as such not useful in further analysis.
2.
Autopsy data and Alzheimer hallmark scores.
| Patient | Postmortem delay (hrs) | Transport delay (hrs) | Total delay (hrs) | Braak stage | Average local neuropathology | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β-amyloid | AT-8 | |||||||||||
| A1 | 4.5 | 2 | 6.5 | V | 3 | 2.75 | ||||||
| A2 | 3.5 | 2.5 | 6 | V | 4 | 4 | ||||||
| A3 | 4 | 1 | 5 | V | 3 | 2.75 | ||||||
| A4 | 2 | 2.5 | 4.5 | IV | 2 | 2.5 | ||||||
| A5 | 4.5 | 1.5 | 6 | VI | 2.75 | 3.25 | ||||||
| C1 | 4 | 2.5 | 6.5 | >0 | 0.25 | 1.25 | ||||||
| C2 | 4.5 | 2.5 | 7 | 0 | 0 | 0 | ||||||
| C3 | 5.5 | 1 | 6.5 | III | 0 | 0 | ||||||
| C4 | 4.5 | 5 | 9.5 | 0 | 0 | 0 | ||||||
A1–A5: Alzheimer patients: C1–C4: control participants.
cf. Braak & Braak (1996); >0 means that some neuritic alterations were noted.
Four observers evaluated the grades of Alzheimer pathology as no (0), mild (1), moderate (2), strong (3) or massive (4) pathology. For each patient the median of these grades is indicated.
Immunocytochemical stainings of slices fixed at day 0 in vitro (DIV 0) provide important information about the cytoarchitectural structure of the brain tissue and the pathological alterations that were present at the start of the experiments. The NeuN staining revealed that there were no overt differences in the cytoarchitecture between patients. Amyloid pathology ranged from non-existent to sparsely distributed small diffuse plaques in control participants (Fig. 2A and C). In accordance with the β-amyloid staining, none or very few AT8-positive dystrophic neurites and tangles were observed in the slices from the control brains (Fig 2B, D). The slices of the Alzheimer patients contained moderate to massive amyloid pathology involving neuritic plaques as illustrated in Figures 2E and G. Considerable amounts of AT8 positive dystrophic neurites and tangles were detected in the slices from the AD patients (Fig. 2F and H). These observations are reflected in the semi-quantitative grades shown in Table 2. There appeared to be a strong correlation between grades for amyloid and hyperphosphorylated tau pathology (Spearman ρ= 0.94, P= 0.009). Further, both the grades for local amyloid load (P= 0.006) and local AT8 staining (P= 0.007) provided a good explanation of the distinction between control participants and AD patients (Mann-Whitney test). Neurofibrillary tangles are more closely associated with the severity of AD than plaques [24], therefore, we chose the AT8 grades for subsequent data analysis with respect to pathological alterations. The sole purpose of this was to minimize repetitious conclusions. However, it should be noted that any conclusion based on AT8 pathology was also reflected by analysis using amyloid pathology scores, although there might be minor differences between P-values. Thus, the AT8 scores of tissue slices from control patients had an overall median value of 0.125, whereas for slices from AD patients the overall median was 2.75 (tendency to strong AD pathology).
2.
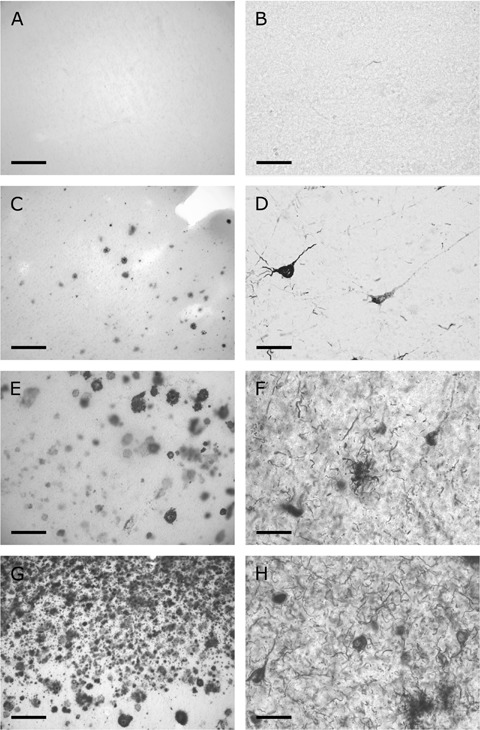
Examples of local amyloid pathology (A, C, E, G) and intracellular deposits of hyper-phosphorylated tau (B, D, F, H) in adjacent 200 μm thick slices fixed at DIV 0. (A, B) Control patient C3 that had no plaques or hyperphosphorylated tau deposits. Only in one control patient (C1) small, mainly diffuse, plaques were observed (C), while isolated tangles and lightly stained neurites were visible (D). The other control patients did not show noticeable pathological alterations as illustrated by patient C3 (A, B). Alzheimer patients (A1) and (A2) showed strong (E) and massive (G) amyloid load, respectively, with both diffuse and neuritic plaques in the three upper cortical layers. (F, H) Clusters of dystrophic neurites in layer III are indicative of the presence of neuritic plaques (arrows) and numerous tangles can be discerned. Further the density of dystrophic neurites and number of tangles are also illustrative for the difference in AT8 scores (F, H). Scale bars: 200 μm (A, C, E, G); 50 μm (B, D, F, H).
Neuronal viability in untreated postmortem human brain slices is negatively correlated with local Alzheimer pathology
As mentioned in the Methods section, observations were made at different time-points in vitro for different patients. In general, the viable, leaky, dead and total cell density in untreated slices did not change significantly over time in vitro, with P-values of 0.92, 0.23, 0.43 and 0.92, respectively. Thus, the numbers of cells per mm3 of tissue in the four categories remained relatively stable within a period of about 1 month after start of the culturing procedure. This is in agreement with previous observations [10]. To eliminate pseudo-replication we used the average cell counts from the experiments at different time-points of each patient for further analysis.
Visual inspection of untreated slices stained with the live/dead kit did not show any obvious differences between tissue from control participants and AD patients. However, cell counts revealed that the number of viable cells in untreated slices of AD patients was reduced by 55% as compared with untreated slices from control participants (Table 3). As is shown in Table 4 this reduction was significant (P= 0.02), whereas the differences in leaky, dead and total cell number were not significant. It appeared from linear regression that the presence of local AD pathology was a major factor determining the neuronal viability in untreated neocortex tissue slices. The presence of pathological alterations explained about 60% (R2= 0.58, P= 0.02 (AT8); R2= 0.61, P= 0.01 (amyloid)) of the variability of viable cell counts. Braak scores give a general estimate of the neuritic pathology in the brain and appeared to have a predictive value about the intensity of local AT8 alterations (R2= 0.74, P= 0.003). However, Braak scores were less strongly correlated with viability of neurons (R2= 0.44, P= 0.05) than the local hyperphosphorylated tau scores. As stated above, AD and control patients were matched with respect to age, sex, brain weight, pH of the CSF, postmortem interval and transport delay, therefore, we did not test their effect on viability. Total delay between death of the patient and start of the cultures appeared to have no significant effect on the viable cell density (P= 0.16). Hence, we concluded that differences in neuronal survival between the control and AD group were not associated with the longer total processing delay of tissue from control participants. Linear regression showed that local AT8 pathology had no influence on the density of leaky cells (P= 0.96), dead cells (P= 0.61) or the total cell density (P= 0.94) in untreated slices.
3.
Comparison of the averaged cell numbers in untreated slices and in slices co-cultured with neural stem cells.
| Group (±S.E.M.) | Treatment | Viable cells (±S.E.M.) | Leaky cells (±S.E.M.) | Dead cells (±S.E.M.) | Total cells | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C | U | 3.41 | 0.66 | 5.34 | 0.39 | 17.16 | 2.21 | 25.91 | 1.39 | ||||||||||||||||||||
| C | NCC | 5.93 | 0.38 | 3.74 | 0.81 | 6.03 | 1.86 | 15.71 | 2.47 | ||||||||||||||||||||
| AD | U | 1.60 | 0.39 | 7.67 | 2.70 | 21.87 | 3.40 | 31.14 | 4.81 | ||||||||||||||||||||
| AD | NCC | 4.24 | 0.83 | 7.33 | 1.89 | 13.20 | 3.97 | 24.77 | 5.07 | ||||||||||||||||||||
C, control group; AD, Alzheimer patients; U, untreated slices; NCC, slices that have been co-cultured with neural stem cells. The cell numbers are per mm3 (X1000).
4.
Summary of statistical analysis of the cell density data using t-tests.
| df | Viable cells t-value p | Leaky cells t-value p | Dead cells t-value p | total cells t-value p | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Untreated tissue: | |||||||||||||||||||||||||||||
| AD versus C | 6 | 3.10 | 0.02 | −0.13 | 0.90 | −0.22 | 0.84 | 0.05 | 0.96 | ||||||||||||||||||||
| AD and C combined: | |||||||||||||||||||||||||||||
| U versus NCC | 7 | 5.65 | <0.001 | −0.098 | 0.36 | −5.57 | <0.001 | −3.59 | 0.009 | ||||||||||||||||||||
df, degrees of freedom; t-value is the Student-t statistic; p, the corresponding level of significance
Neuronal viability in human postmortem slices was improved by co-culturing with rat E14 neural stem cells
Co-culture of postmortem human brain slices with rat E14 neurospheres resulted in an amazingly strong improvement of neuronal viability (Fig. 3A and B; Table 3). It may be noted that the vast majority of the viable cells in both untreated and co-cultured slices had a pyramidal shape indicating that they were neurons (Fig. 3). Viability of small cells was more difficult to establish, because of the criterium of the presence of a dark nucleus. Leaky pyramidal neurons could be identified, but a non-pyramidal shaped leaky cell or a dead cell (single red nucleus) could pertain to any cell type including pyramidal neurons, interneurons, glia and vascular cells. For this reason the number of viable cells may have been underestimated. Taking controls and Alzheimer patients together, the effect of treatment with neural stem cells consisted of an average increase of 2600 viable cells per mm3 (P < 0.001; Table 4). The number of leaky cells roughly remained the same (P= 0.36), while the mean number of dead cells and the mean total number of cells decreased with about 9500 (P < 0.001) and 7800 (P= 0.009) per mm3, respectively (cfTable 4). Part of the 7800 missing cells may have been degraded during the co-culture period, but the difficulty to detect small cells if they do not have a red nucleus may also play a role. Further studies are required to address this point.
3.
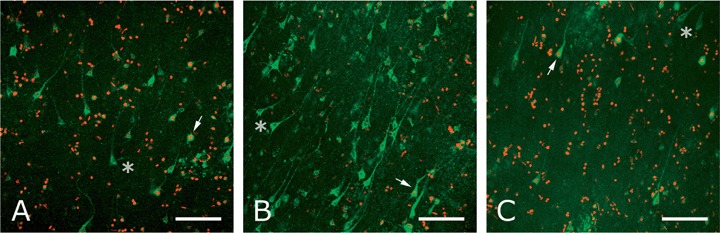
Viability staining of slices from patient (A5) at DIV 7. (A) Untreated slices contained few viable, several leaky and a large number of dead cells. (B) In slices co-cultured with E14 rat neural stem cells the number of viable cells was significantly increased, the number of leaky cells was roughly the same and there was a marked decrease of dead cells. (C) After co-culturing with E14 rat fibroblasts the viability staining was comparable with that of untreated slices. Viable cells have a green cytoplasm with an unstained nucleus (asterisk); leaky cells have a green cytoplasm with a red nucleus (arrow); dead cells only have a red nucleus. Scale bar: 100 μm.
The effects of neural stem cells on the viability in tissue slices from the different patients are shown in Figure 4A and B for the control and AD group separately. The enhancement of viability was similar in slices from the different control participants (Fig. 4A). The vertical bars in Figure 4A and B indicate that the average improvement of viability in slices from AD patients was only slightly less than the average improvement of viability in tissue from control participants. The high variability of the viability in co-cultured slices from AD patients, however, suggests that tissue from some AD patients responded less well to treatment with neural stem cells (Fig. 4B). By contrast, tissue from other AD patients may be stimulated to attain the same viability level as tissue from control participants can reach (Fig. 4A and B). This may be indicative of a heterogeneity in responsiveness of the AD tissue. However, the improvement of neuronal viability was not related to local AT8 pathology (linear regression incorporating both C and AD patients: R2= 0.002, P= 0.90). Likewise, the presence of neurofibrillary pathology also had no effect on the differences of leaky (R2= 0.23, P= 0.18) and dead cells (R2= 0.08, P= 0.49) and on the total cell density (R2= 0.18, P= 0.30) due to treatment with neural stem cells.
4.
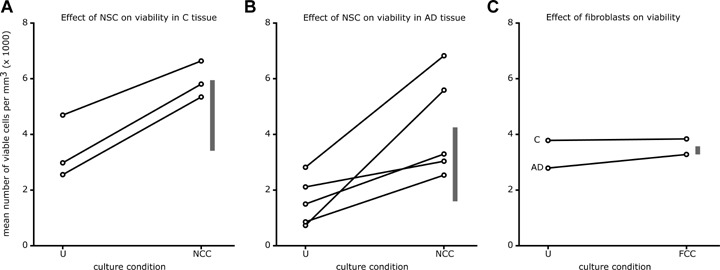
Quantitative evaluation of the viability of postmortem human neurons after co-culture with rat E14 neural stem cells (NSC) or fibroblasts. (A) All three control participants showed an enhanced viability after co-culturing with E14 rat neural stem cells. Each circle denotes the mean value of counts in 4–12 slices and the lines connect measurements within one patient. (B) The effect of co-culturing with neural stem cells was much more variable in tissue from Alzheimer's disease (AD) patients. In slices from some patients the viability reached values also obtained in co-cultured slices from control participants, whereas in others the improvement was much less impressive. Again, each circle is based on measurements of 4–12 slices. (C) The improvement of neuronal viability in slices from one AD and one C participant induced by E14 fibroblasts is slight. The vertical bars in each panel indicate the differences between the mean values of untreated and co-cultured slices. Thus, the lower end of the bar denotes the average viability of untreated slices, the top denotes the average viability of co-cultured slices and the length indicates the improvement due to co-culturing. Note that the average improvement of viability due to factors released by neural stem cells was similar for C participants (2500 cells per mm3) and AD patients (2600 cells per mm3), whereas the mean enhancement of viability induced by E14 fibroblasts was 10-fold less (270 cells per mm3). The culture conditions are denoted by U (untreated slices), NCC (slices co-cultured with E14 neural stem cells) or FCC (slices co-cultured with E14 fibroblasts).
E14 fibroblasts have no effects on neuron viability in human postmortem slices
To study whether the effects of co-culture with stem cells could be evoked by any other cell type, we decided to co-culture slices from one control patient (C4) and one Alzheimer patient (A5) with rat E14 fibroblasts obtained from the bodies of the same embryo's that were used to isolate the neural stem cells. Visual inspection suggested that there was no difference in the number of viable cells between the fibroblast co-cultured and the untreated group (Fig. 3A and C). Comparing panel A with panel C in Figure 4 shows that the mean number of viable cells in the slices from the control participants was not affected by the presence of fibroblasts (mean improvement: 270 viable cells per mm3; S.E.M. value: 400). Fibroblasts also appeared to have no effects on leaky (mean difference: (1130 ± 3790 per mm3), dead (mean difference: (1220 ± 1580 per mm3) and total (mean difference: (2070 ± 5560 per mm3) cells.
Discussion
In previous studies concerning cultured human postmortem brain tissue slices we have shown that viable cells both in cortical and sub-cortical areas can survive for relatively long periods in vitro[10, 25]. Some of these neurons also survive in the presence of massive AD pathology, even if they contain deposits of hyperphosphorylated tau themselves [10, 20]. Here, we present evidence that the density of viable cells in cultured neocortex slices from patients with AD is lower than in control participants. This reduction in viability was strongly correlated with the local presence of amyloid plaques and neurofibrillary tangles. However, our observations do not distinguish between the view that Alzheimer pathology causes neuronal cell death [26] and the view that pathological alterations may be the consequence of neuronal malfunctioning [27]. Braak scores [6] which provide a global evaluation of the extent to which the brain is affected by the Alzheimer disease process correlated less well with the viability of neurons than the local neuritic pathology did. Qualitative assessment of the NeuN staining indicated that the reduced viability in AD patients was not reflected in gross cytoarchitectural changes or altered neuronal morphology between control participants and Alzheimer patients. With respect to this feature it may be noted that we have previously found that histological observations do not provide a faithful impression of cellular viability [10]. Lucassen et al. [28] studied DNA damage, as indicated by in situ end-labelling, in AD patients and found that AD neurons are more vulnerable to the postmortem delay than neurons in control participants, but they could not relate this to the presence of AD pathology. It is noteworthy that the present data do not support a negative effect on the survival of neurons due to the time interval between the death of the patient and the moment that the tissue was placed in culture medium. In the present study we found that the average number of viable cells in untreated slices from control patients was 3400 per mm3 (13% of the total number). In a previous study [10] the number of viable cells slices from three control participants was in the order of 10,000 per mm3 (25–30% of the total cell number). It is not known whether the group reported in Verwer et al.[10] consisted of exceptionally healthy patients. However, with respect to the variables listed in Table 1 the patient data of the previous study were comparable with those recorded in the present report.
Co-culture with rat E14 neural stem cells enhanced the density of viable cells in both AD patients and control participants to the same average extent. This improvement occurred within 3 days after start of the treatment, but stayed at roughly the same level after longer treatment. Considering that ante- and postmortem processes and transfer of tissue to in vitro conditions are likely to be harmful for cells it may not be unexpected that also the viability in untreated slices from control participants was impaired and could, subsequently, be improved by factors released by E14 rat neurospheres. Cells in postmortem slices from two AD patients reacted much more positively to the stimulation by co-culture with neural stem cells than the other three AD patients. They attained the same viability level as the co-cultured slices from control participants. This may be indicative that the AD group is not homogeneous with respect to the responsiveness of their neurons to survival enhancing factors, but there were no indications that this might be related to the presence of pathological alterations. This supports the view that the pathological alterations per se need not be harmful [9, 10, 12, 29, 30] and that even in late stages of the disease neuronal reactivation might be a possible target for therapeutic intervention. To better investigate such properties future studies should include more patients with mild to moderate levels of AD pathology and patients with other neurodegenerative disorders. E14 rat fibroblasts failed to enhance the viability of neurons in aged human postmortem brain slices which suggests that the promotion of neuronal survival by rat neural stem cells is specific.
The postmortem brain tissue was cultured separated by a membrane (8 μm pore size) from the neural stem cells, thus preventing direct interaction. This suggests that neural stem cells release diffusible factors of unknown nature and size that can enhance the survival of human neurons that have experienced aging or a neurodegenerative disease. Such soluble factors have also been reported to enhance the survival of neural stem cells themselves [31]. The nature of these factors is at present unknown, but they may well be neurotrophin-like [32, 33]. Neurotrophic factors have been applied in clinical trials, either by direct infusion [34] or via the transplantation of genetically engineered autologous fibroblasts [35] and both, glial cell line derived neurotrophic factor and nerve growth factor, respectively, induced outgrowth of nerve fibres. In the present study we did concentrate on neuronal viability rather than sprouting. The assessment of viability is based on the exclusion of ethidium bromide, and therefore, it could be that the released factors do not act via signalling pathways but insert directly into the membranes of the human neurons. This could constitute an alternative way to improve their membrane integrity and help the neurons to better maintain their ion homeostasis. Pilot experiments have failed so far to identify putative factors (Lei Wu, unpublished data). Recent reports illustrate that the modes of action of neural stem cells to rescue impaired neuronal cells, indeed, may be very diverse, ranging from intervening in ganglioside metabolism, reduction of inflammation [36], and suppression of apoptosis [33], to support of mitochondrial function, dendritic growth and synap-togenesis [37].
Future therapeutic implementation would require that human stem cells or human neural precursor cells have under similar conditions the same effect. Several transplantation studies using human embryonic stem cell lines in animal models for human neurological diseases suggest that they too are capable of secreting factors that can rescue degenerating cells (e.g.[13, 14, 33, 36]). Elucidation of the nature of the factors released by neural stem cells would be very important for clinical applications as they might eliminate the necessity for cell transplantation. Although it might be true that different factors need to be isolated for different neurodegener-ative disorders this would still be particularly useful in the treatment of disorders with widespread degeneration, such as AD.
Thus, our data show for the first time that although aged human neurons suffer from impaired viability in the presence of amyloid plaques and tangles, they can still be stimulated by factors released by neural stem cells to survive better.
Acknowledgments
We are grateful to the autopsy team of the Netherlands Brain bank and Dr. R.E. Baker. We thank Ilse van Soelen and Karlijn van Soest for technical assistance. This project was supported by the China Project of the Royal Netherlands Academy of Arts and Sciences (KNAW, 05CD9027), a Delta grant from the Amsterdam Medical Center (AMC), the Innovation project of the Chinese Academy of Science (KSCX2-SW-217), the National key project for basic research of the Ministry of Science and Technology of China (2006CB500705), the Internationale Stichting Alzheimer Onderzoek (ISAO), the Jan Dekkerstichting and dr. Lutgardine Bouwmanstichting.
References
- 1.Lindvall O, Kokaia Z, Martinez-Serrano A. Stem cell therapy for human neurodegenerative disorders – how to make it work. Nature Med. 2004;10:S42–50. doi: 10.1038/nm1064. [DOI] [PubMed] [Google Scholar]
- 2.West MJ, Coleman PD, Flood DG, Troncoso JC. Differences in the pattern of hippocampal neuronal loss in normal ageing and Alzheimer's disease. Lancet. 1994;344:769–72. doi: 10.1016/s0140-6736(94)92338-8. [DOI] [PubMed] [Google Scholar]
- 3.Hoogendijk WJ, Pool CW, Troost D, Van Zwieten E, Swaab DF. Image analyser-assisted morphometry of the locus coeruleus in Alzheimer's disease, Parkinson's disease and amyotrophic lateral sclerosis. Brain. 1995;118:131–43. doi: 10.1093/brain/118.1.131. [DOI] [PubMed] [Google Scholar]
- 4.Gomez-Isla T, Price JL, McKeel DW, Jr, Morris JC, Growdon JH, Hyman BT. Profound loss of layer II entorhinal cortex neurons occurs in very mild Alzheimer's disease. J Neurosci. 1996;16:4491–500. doi: 10.1523/JNEUROSCI.16-14-04491.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zarow C, Vinters HV, Ellis WG, Weiner MW, Mungas D, White L, Chui HC. Correlates of hippocampal neuron number in Alzheimer's disease and ischemic vascular dementia. Ann Neurol. 2005;57:896–903. doi: 10.1002/ana.20503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Braak H, Braak E. Evolution of the neuropathology of Alzheimer's disease. Acta Neurol Scand Suppl. 1996;165:3–12. doi: 10.1111/j.1600-0404.1996.tb05866.x. [DOI] [PubMed] [Google Scholar]
- 7.Haxby JV, Grady CL, Koss E, Horwitz B, Schapiro M, Friedland RP, Rapoport SI. Heterogeneous anterior-posterior metabolic patterns in dementia of the Alzheimer type. Neurology. 1988;38:1853–63. doi: 10.1212/wnl.38.12.1853. [DOI] [PubMed] [Google Scholar]
- 8.Mielke R, Schröder R, Fink GR, Kessler J, Herholz K, Heiss WD. Regional cerebral glucose metabolism and postmortem pathology in Alzheimer's disease. Acta Neuropathol. 1996;91:174–9. doi: 10.1007/s004010050410. [DOI] [PubMed] [Google Scholar]
- 9.Swaab DF, Dubelaar EJG, Hofman MA, Scherder EJA, Van Someren EJW, Verwer RWH. Brain aging and Alzheimer's disease; use it or lose it. Prog Brain Res. 2002;138:343–73. doi: 10.1016/S0079-6123(02)38086-5. [DOI] [PubMed] [Google Scholar]
- 10.Verwer RWH, Hermens WTMC, Dijkhuizen PA, Ter Brake O, Baker RE, Salehi A, Verhaagen J, Swaab DF. Cells in human postmortem brain tissue slices remain alive for several weeks in culture. FASEB J. 2002a;16:54–60. doi: 10.1096/fj.01-0504com. [DOI] [PubMed] [Google Scholar]
- 11.Falini A, Bozzali M, Magnani G, Pero G, Gambini A, Benedetti B, Mossini R, Franceschi M, Comi G, Scotti G, Filippi M. A whole brain MR spectroscopy study from patients with Alzheimer's disease and mild cognitive impairment. Neuroimage. 2005;26:1159–63. doi: 10.1016/j.neuroimage.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 12.West MJ, Kawas CH, Stewart WF, Rudow GL, Troncoso JC. Hippocampal neurons in pre-clinical Alzheimer's disease. Neurobiol Aging. 2004;25:1205–12. doi: 10.1016/j.neurobiolaging.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 13.Ourednik J, Ourednik V, Lynch WP, Schachner M, Snyder EY. Neural stem cells display an inherent mechanism for rescueing dysfunctional neurons. Nature Biotechnol. 2002;20:1103–10. doi: 10.1038/nbt750. [DOI] [PubMed] [Google Scholar]
- 14.Pluchino S, Quattrini A, Brambilla E, Gritti A, Salani G, Dina G, Galli R, Del Caro U, Amadio S, Bergami A, Furlan R, Comi G, Vescovi AL, Martino G. Injection of adult neurospheres induces recovery in a chronic model of multiple sclerosis. Nature. 2003;422:688–94. doi: 10.1038/nature01552. [DOI] [PubMed] [Google Scholar]
- 15.Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn A, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1–5. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- 16.Jin K, Peel AL, Mao XO, Xie L, Cottrell BA, Henshall DC, Greenberg DA. Increased hippocampal neurogenesis in Alzheimer's disease. Proc Natl Acad Sci USA. 2004;101:343–47. doi: 10.1073/pnas.2634794100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boekhoorn K, Joels M, Lucassen PJ. Increased proliferation reflects glial and vascular-associated changes, but not neuro-genesis in the presenile Alzheimer hippocampus. Neurobiol Dis. 2006;24:1–14. doi: 10.1016/j.nbd.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 18.Verwer RWH, Baker RE, Boiten EFM, Dubelaar EJG, Van Ginkel CJM, Sluiter AA, Swaab DF. Post-mortem brain tissue cultures from elderly control subjects and patients with a neurodegenerative disease. Exp Gerontol. 2003;38:167–72. doi: 10.1016/s0531-5565(02)00154-7. [DOI] [PubMed] [Google Scholar]
- 19.Romijn HJ, De Jong BM, Ruijter JM. A procedure for culturing rat neocortex explants in a serum-free nutrient medium. J Neurosci Meth. 1988;23:75–83. doi: 10.1016/0165-0270(88)90025-8. [DOI] [PubMed] [Google Scholar]
- 20.Ostenfeld T, Jloy E, Tai Y-T, Peters A, Caldwell M, Jauniaux E, Svendsen CN. Regional specification of rodent and human neurospheres. Dev Brain Res. 2002;134:43–55. doi: 10.1016/s0165-3806(01)00291-7. [DOI] [PubMed] [Google Scholar]
- 21.Johe KK, Hazel TG, Muller T, Dugich-Djordjevic MM, McKay RDG. Single factors direct the differentiation of stem cells from the fetal and adult central nervous system. Genes Dev. 1996;10:3129–40. doi: 10.1101/gad.10.24.3129. [DOI] [PubMed] [Google Scholar]
- 22.Augustinack JC, Sanders JL, Tsai LH, Hyman BT. Colocalization and fluorescence resonance energy transfer between cdk5 and AT8 suggests a close association in pre-neurofibrillary tangles and neurofibrillary tangles. J Neuropathol Exp Neurol. 2002;61:557–64. doi: 10.1093/jnen/61.6.557. [DOI] [PubMed] [Google Scholar]
- 23.Cummings BJ, Mason AJ, Kim RC, Sheu PC, Anderson AJ. Optimization of techniques for the maximal detection and quantification of Alzheimer's-related neuropathology with digital imaging. Neurobiol Aging. 2002;23:161–70. doi: 10.1016/s0197-4580(01)00316-5. [DOI] [PubMed] [Google Scholar]
- 24.Giannakopoulos P, Herrmann FR, Bussière T, Bouras C, Kövari E, Perl DP, Morrison JH, Gold G, Hof PR. Tangle and neuron numbers, but not amyloid load, predict cognitive status in Alzheimer's disease. Neurology. 2003;60:1495–1500. doi: 10.1212/01.wnl.0000063311.58879.01. [DOI] [PubMed] [Google Scholar]
- 25.Verwer RWH, Dubelaar EJG, Hermens WTJMC, Swaab DF. Tissue cultures from adult human postmortem subcortical brain areas. J Cell Mol Med. 2002b;6:429–32. doi: 10.1111/j.1582-4934.2002.tb00522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353–6. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 27.Lee H-G, Perry G, Moreira PI, Garrett MR, Liu Q, Zhu X, Takeda A, Nunomura A, Smith MA. Tau phosphorylation in Alzheimer's disease: pathogen or protection? Trends Mol Med. 2005;11:164–9. doi: 10.1016/j.molmed.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 28.Lucassen PJ, Chung WCJ, Kamphorst WK, Swaab DF. DNA damage distribution in the human brain as shown by in situ end labeling; area-specific differences in aging and Alzheimer Disease in the absence of apoptotic morphology. J Neuropath Exp Neurol. 1997;56:887–900. doi: 10.1097/00005072-199708000-00007. [DOI] [PubMed] [Google Scholar]
- 29.Salehi A, Pool CW, Mulder M, Ravid R, Gonatas NK, Swaab DF. Activity of hippocampal CA1 neurons in Alzheimer's disease is not affected by the presence of adjacent neuritic plaques. J Alzheimer's Dis. 1998;1:107–18. doi: 10.3233/jad-1998-1204. [DOI] [PubMed] [Google Scholar]
- 30.Hof PR, Bussière T, Gold G, Köväri E, Giannakopoulos P, Bouras C, Perl DP, Morrison JH. Stereologic evidence for persistence of viable neurons in layer II of the entorhinal cortex and the CA1 field in Alzheimer's disease. J Neuropathol Exp Neurol. 2003;62:55–67. doi: 10.1093/jnen/62.1.55. [DOI] [PubMed] [Google Scholar]
- 31.Chang MY, Park CH, Lee SH. Embryonic cortical stem cells secrete diffusible factors to enhance their survival. Neuroreport. 2003;14:1191–205. doi: 10.1097/00001756-200307010-00001. [DOI] [PubMed] [Google Scholar]
- 32.Llado J, Haenggeli C, Maragakis NJ, Snyder EY, Rothstein JD. Neural stem cells protect against glutamate- induced excitotoxicity and promote survival of injured motor neurons through the secretion of neurotrophic factors. Mol Cell Neurosci. 2004;27:322–31. doi: 10.1016/j.mcn.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 33.Yasuhara T, Matsukawa N, Hara K, Yu G, Maki M, Kim SU, Borlonga CV. Transplantation of human neural stem cells exerts neuroprotection in a rat model of Parkinson's disease. J Neurosci. 2006;26:12497–511. doi: 10.1523/JNEUROSCI.3719-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Love S, Plaha P, Patel NK, Hotton GR, Brooks DJ, Gill SS. Glial cell line-derived neurotrophic factor induces neuronal sprouting in human brain. Nature Med. 2005;11:703–4. doi: 10.1038/nm0705-703. [DOI] [PubMed] [Google Scholar]
- 35.Tuszynski MH, Thal L, Pay M, Salmon DP, HS U, Bakay R, Patel P, Blesch A, Vahlsing HL, Ho G, Tong G, Potkin S, Fallon J, Hansen L, Muffson EJ, Kordower JH, Gall C, Conner J. A phase 1 clinical trial of nerve growth factor gene therapy for Alzheimer disease. Nature Med. 2005;11:551–5. doi: 10.1038/nm1239. [DOI] [PubMed] [Google Scholar]
- 36.Lee JP, Jeyakumar M, Gonzalez R, Takahashi H, Lee PJ, Baek RC, Clark D, Rose H, Fu G, Clarke J, McKercher S, Meerloo J, Muller FJ, Park KI, Butters TD, Dwek RA, Schwartz P, Tong G, Wenger D, Lipton SA, Seyfried TN, Platt FM, Snyder EY. Stem cells act through mulptiple mechamisms to benefit mice with neurodegenerative metabolic disease. Nature Med. 2007;13:439–47. doi: 10.1038/nm1548. [DOI] [PubMed] [Google Scholar]
- 37.Li J, Imitola J, Snyder EY, Sidman RL. Neural stem cells rescue nervous Purkinje neurons by restoring molecular homeostasis of tissue plasminogen activator and downstream targets. J Neurosci. 2006;26:7839–48. doi: 10.1523/JNEUROSCI.1624-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]


