Abstract
The goals of the study were: (1) to explore the communication between human mesenchymal stem cells (MSC) and rat cardiac myocytes resulting in differentiation of the stem cells and, (2) to evaluate the role of mitochondria in it. Light and fluorescence microscopy as well as scanning electron microscopy revealed that after co-cultivation, cells formed intercellular contacts and transient exchange with cytosolic elements could be observed. The transport of cytosolic entity had no specific direction. Noticeably, mitochondria also could be transferred to the recipient cells in a unidirectional fashion (towards cardiomyocytes only). Transmission electron microscopy revealed significant variability in both the diameter of intercellular contacting tubes and their shape. Inside of these nanotubes mitochondria-resembling structures were identified. Moreover, after co-cultivation with cardiomyocytes, expression of human-specific myosin was revealed in MSC. Thus, we speculate that: (1) transport of intracellular elements to MSC possibly can determine the direction of their differentiation and, (2) mitochondria may be involved in the mechanism of the stem cell differentiation. It looks plausible that mitochondrial transfer to recipient cardiomyocytes may be involved in the mechanism of failed myocardium repair after stem cells transplantation.
Keywords: foetal stem cells, cell therapy, mitochondria, tunnelling nanotubes, cardiomyocyte
Introduction
Several cardiovascular diseases (myocardium infarction, ischaemic cardiomyopathy, heart failure) have been shown to involve cell death of cardiac myocytes and failure of myocardium excitation-contraction coupling and subsequently contractility. Global occurrence of these diseases and minimal proliferative capacity of cardiac myocytes make these diseases the great problem of heart medicine. Accordingly, actively developing field of cell therapy used for failed heart is aimed to meet these general demands [1].
Many studies, including clinical ones [2–4], display efficiency of stem and progenitor cells of different types in injured myocardium function refinement. Since pioneer studies revealed cardiomyocyte-directed differentiation of stem cells [5–7], possibilities of using embryonic stem cells [8, 9], mesenchymal [10, 11] and haematopoietic [12] stem cells, endothelial progenitor cells [13], foetal myoblasts [14] and foetal cardiac myocytes [15, 16] and several other cell types for myocardial regeneration have been investigated. However, despite the significant progress in research of opportunities of cell therapy for cardiovascular diseases some questions remain unclear: what types of stem and progenitor cells are most effective for therapy; what are the mechanisms of positive influence of cell transplantation and whether such therapy can have a prolonged effect [17].
Integration of transplanted cells into injured myocardial tissue is of a particular interest. Recently, it was shown that mesenchymal stem cells (MSC) integrate into myocardium [18] and manifest expression of specific markers of heart tissue, moreover MSC show ability to restore electric conductivity of cultivated cardiac myocytes syncytium [19]. Different studies demonstrated ability of cardiac myocytes to fuse with other somatic and progenitor cell types [20, 21], with these newly formed hybrid cells having the predominant phenotype of a cardiomyocyte.
However, there might be another mechanism of host myocardial and donor stem/progenitor cells interaction revealed in several other studies: transdifferentiation of an endothelial progenitor cell into a cardiac myocyte supposed to occur without cell fusion, but due to cytoplasmic information transferred from a cardiac myocyte to progenitor cells [22]. Koyanagi and coworkers [23] have speculated that in this case, the mechanism of the transdifferentiation signal is based on the transfer of cardiomyocyte mitochondria to a progenitor cell. Nevertheless, some researchers challenge this mechanism of transdifferentiation [24] considering it to be an artefact of cultivation or cell staining techniques. However, besides the mitochondria transport from cardio to progenitor cells described by Koyanagi and coworkers, there is an evidence of mitochondria being transferred from stem cells to recipient cells deficient by the oxidative phosphorylation [25] resulting in restoration of aerobic respiration in recipient cells.
In our study, we examined the possibility of transient cell-to-cell exchange of cytoplasm and organelles between bone marrow MSC and cultivated rat embryonic cardiac myocytes during co-culturing.
Materials and methods
Cell culture
Primary culture of cardiac myocytes was isolated from hearts of 15–17 days of gestation rat embryos. Heart tissue was dissociated in 0.1% col-lagenase type II in Hanks solution, followed by centrifugation at 1000 g for 3 min. Cells were re-suspended in Dulbecco's Modified Eagle Medium (DMEM)/F12 (4:1) medium, containing 10% foetal bovine serum and 5% horse serum. This technique allowed to harvest attached cardiac myocytes associates demonstrating spontaneous contractility during whole cultivation period.
MSC were isolated from human foetal long bones (11–12 weeks of gestation), received by routine pregnancy termination carried out in a licensed medical procedure (according to the Ministry of Public Health order N302 of 28.12.1993 and supplement N3 of 05.04.1994). These cells were proved to be MSC since they were positive to the surface epitope CD90 and negative to CD34, CD45 and CD31 [26]. Also, they were shown to differentiate into adipocytes and osteoblasts [26, 27]. Cells were washed out of bone marrow with DMEM, containing 2 mM of ethylenediaminete-traacetic acid (EDTA). Further, cell suspension was layered on ficoll (density 1.077 g/ml) and centrifuged at 2000 g for 30 min. Mononuclear cells were collected, washed out and suspended in DMEM/F12 (1:1) containing 10% foetal bovine serum. After 24 hrs, unattached cells were removed, and adherent cells were cultivated until forming monolayer.
The percentage of cells carrying contacting nanotubes was evaluated in 20 independent fields of view (at 63 × objective) for each culture.
For determination of a fraction of cells undergoing an exchange of their contents in 10 separate fields of view (at 63 × objective) the number of cells carrying both fluorescent cytosolic probes was evaluated as a percentage of the total number of cells.
Foetal tissue sampling was approved by the Institutional Ethics Committee of the Research Center of Obstetrics, Gynecology and Perinatology in compliance with national guidelines regarding the use of foetal tissue for research. Handling of animals and experimental procedures were conducted in accordance with the international guideless for animal care and use and were approved by the Institutional Ethics
Committee of A.N. Belozersky Institute of Physico-Chemical Biology at Moscow State University.
Co-cultivation of rat cardiomyocytes and MSC
MSC used for co-culture experiments were dissociated in 0.25% Tripsin-EDTA and this suspension was added to cultured cardiomyocytes. Cell suspension contained nearly 105 cells per ml. Mixed culture was incubated in DMEM:F12 (4:1) for different time intervals.
Fluorescent probes staining
Mitochondrial probes, tetramethylrhodamine ethyl ester (TMRE), Mitotracker Green FM and Mitotracker Red (Molecular Probes, USA) were used to visualize mitochondria. Cells were stained with 200 nM Mitotracker Green FM or Mitotracker Red dissolved in DMEM: F12 containing 20 mM HEPES. Excess of the dye was washed out with medium.
Transport of cytoplasmic entity was documented using calcein-AM (Molecular Probes, USA) cell staining. Cells were incubated with 2.5 μM calcein-AM for 60 min at 37°C, and then cells were washed out in the medium.
Cell cultures were studied using laser scanning confocal microscope LSM510 (Carl Zeiss, Germany) with manufacture's software. Fluorescence analysis was performed in glass-bottom dishes with the excitation at 488 nm (for Mitotracker Green and calcein) and 543 nm (for Mitotracker Red) and emission collected at 500–530 nm and >560 nm, respectively.
Flow cytometry analysis
Comparative analysis of calcein content in cells on different stages of co-cultivation was undertaken using flow cytometric analyser Beckman Coulter. Excitation laser 488 nm was used to detect calcein and emission was collected all above 530 nm.
Immunocytochemistry
Cells were washed in phosphate buffered saline (PBS), fixed for 15 min in 4% formaldehyde with PBS at 4°C, and permeabilized in PBS containing 0.1% Triton X-100 for 15 min. followed by blocking in PBS with 1% bovine serum albumin (PBS-BSA) for 60 min. Human cardiac-specific β-myosin H-chain was detected using mouse anti-human myosin monoclonal antibodies (Chemicon, USA) diluted 1:200 in PBS-BSA at 25°C 2 hrs. After three 15-min rinses in PBS-BSA, cells were incubated for 1 hr in fluorescein isothiocyanate (FITC)-conjugated antimouse IgG (Sigma, USA) diluted 1:100. The glasses were washed, placed on microscope slides with a mounting medium and sealed beneath cover slips with nail varnish.
Electron microscopy
For the electron microscopy, cells were fixed in 2.5% glutaraldehyde (Sigma, USA) prepared in the phosphate buffer (pH7.4), post-fixed in 1% OsO4, dehydrated (70% ethanol contained 2% uranyl acetate) and embedded in Epon 812 (Fluka, USA). After Epon polymerization and removal of the coverslips, the samples were cut into ultra thin sections using an LKB Ultratome III. The sections were stained with lead citrate and examined using HU-11B or HU-12 microscopes (Hitachi, Japan).
Results
Formation of cell- to-cell contacts during co-cultivation
In a number of studies, it has been shown that cells are capable of forming ultrathin intracellular structures, stretching towards neighbouring cells. These structures were coined the term, tunnelling nanotubes [28, 29] and described for many cultured cell types of mammals: tumour, stem and normal tissue cells (reviewed in [30]). Nanotubes apparently representing structures of different organization are considered to transfer multi-protein complexes and whole organelles between contacting cells. Recently Koyanagi et al. have demonstrated that mitochondria can be transferred along nanotubes from one cell to another and this process might be linked to endothelial progenitor cells transdifferentiation to cardiomyocytes [23]. We also observed that both MSC and cardiomyocytes make numerous extensive intercellular structures; in some cases, the length of these cytoplasmic extensions is comparable to cell size (Fig. 1). Interestingly, tubes of a small diameter and other cytoplasmic strands can be formed in MSC and cardiomyocytes harvested in monocultures (Fig. 1D). Apparently, for cardiomyocytes this phenomenon may reflect the phenotypic feature to form united syncytium, as it occurs in heart tissue.
1.
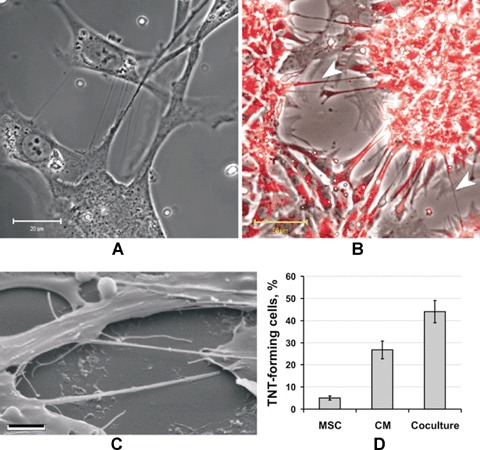
Nanotubes formation under co-cultivation of human mesenchymal stem cells (MSC) and rat cardiac myocytes. (A) Phase contrast. (B) Cells treated with a mitochon-drial probe, TMRE; specific mitochondrial staining is revealed in nanotubes as well (arrowheads). (C) Scanning electron microscopy. Bar, 20, 50 and 3 μm for A, B, C correspondingly. (D) Percentage of cells, forming intercellular contacting elements (tunnelling nanotubes, TNT).
To confirm the possibility of the mitochondrial exchange between cardiomyocytes and MSC, cells were stained with TMRE, specifically accumulating in mitochondria. Confocal microscopy revealed TMRE fluorescence in the intercellular cytoplasmic nanotubes (Fig. 1).
Although both types of cells being in monoculture were able to form connecting tubes, the number of nanotubes significantly increased under co-cultivation of two kinds of cells (Fig. 1D).
Electron microscopic study revealed that morphologically the cells formed numerous contacting tubes (Fig. 2A), which varied in both the diameter and the shape. Some of these tubes had small and almost constant diameter (Fig. 2C) along the major length of the tube with the exception of the initial/terminal part having the shape of the funnel (Fig. 2A and D). Other tubes had a variable diameter over the length (Fig. 2D) and might form branches (Fig. 2B and D). For the simplicity, considering their small size we called them all as tunnelling nanotubes, the term coined by Rustom et al. [29]. All nanotubes were limited by membranes sometimes with non-homogenous content inside. (Fig. 2D).
2.
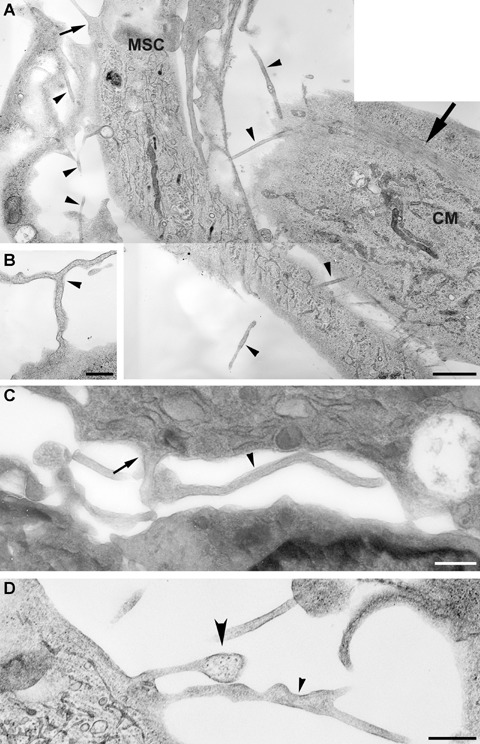
Electron microscopy of intercellular tunnelling nanotubes (shown by black arrowheads). (A) Two communicating cells, MSC and cardiomyocyte (CM); big arrow points to muscle fibre bundles; small arrow points to a funnel-shaped initiation/termination of the nanotube. (B) Small diameter branching nanotubes (arrowhead). (C) Small diameter bending nanotubes (arrowhead) terminating with a funnel (arrow). (D) One nanotube with variable diameter and another with granular content are shown by big and small arrowheads correspondingly. Bar, 1 μm for A; 0.5 μm for B, C, D.
Cytoplasmic transfer from MSC to neonatal cardiomyocytes
We studied an opportunity to transfer cytosolic entity from cell-to-cell contiguous in co-culture through cell contacts (such as gap-junctions and nanotubes). Cells were stained with a fluorescent probe, calcein-AM, to determine the cytoplasm transfer and its direction. Firstly, bone marrow MSC were stained with calcein-AM and transferred to plates with non-stained rat cardiac myocytes. The appearance of low-molecular calcein having green fluorescence (received in stained cells after cleavage of calcein-AM by intracellular esterases) in non-stained cells may report on its possible transfer between two kinds of cells.
It was noticed that approximately 4 hrs after initiation of co-cultivation of calcein-stained MSC and unstained cardiomyocytes, cells began to make contacts and nanotubes can be observed. Still, calcein fluorescence was observed in MSC only, that is transport of cytosolic entity did not occur by this time (data not shown).
When cells were analysed 24 hrs after initiation of co-cultivation, the appearance of multitude connections was obvious but due to cell proliferation and a subsequent increase of cell density in culture, many cells start connecting and possibly form gap junctions. A low level of green fluorescence was observed also in many cardiomyocytes neighbouring to MSC stained with calcein (having high intensity of calcein fluorescence in cytoplasm) (Fig. 3A). Therefore, the dye from MSC cytoplasm was transferred to cytoplasm of cardiomyocytes that proved migration of the MSC cytoplasm content to cytosol of neighbouring cardiomyocytes.
3.
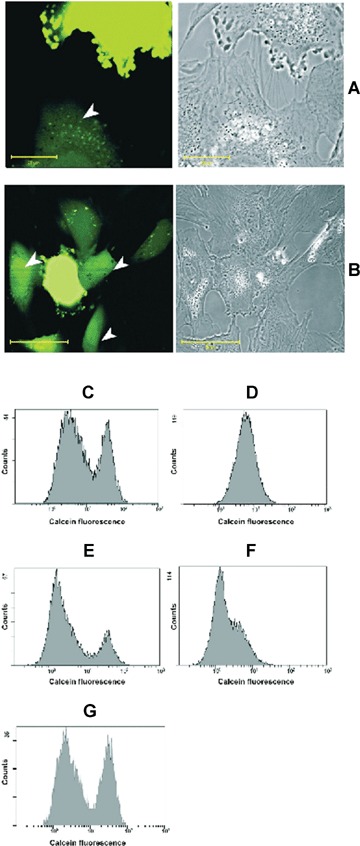
Transport of cytosolic entity from human MSC to rat cardiomyocytes after 24 hrs of co-cultivation. (A–B) MSC are stained with calcein-AM and placed to cardiomyocytes. After 24 hrs, calcein fluorescence is revealed not only in MSC but also in contacting with them cardiomyocytes (pointed with arrowheads). Diffused staining of calcein in cytosol of cardiomyocytes as well as calcein compartmentation within are observed. Calcein transfer may occur as possibly mediated by nanotubes (A) or gap junctions (B) Bar, 20 and 50 μm for A and B correspondingly. (C–G) Flow cytometry of co-cultivated human MSC and rat cardiomyocytes. Cells were stained with calcein-AM and cultivated in two varieties: C and D, MSC stained with calcein-AM while cardiomyocytes are non-stained; E and F, cardiomyocytes are stained with calcein-AM, while MSC are non-stained. Pictures are taken after 3 hrs (C and E) and 24 hrs (D and F) of co-cultivation. (G) Cells were shaken for 24 hrs on horizontal plate with 50 cycles per minute.
Thus, many cells contacted tightly with their cytoplasmic membrane surfaces with possible gap junction formation through which cytoplasmic entity probably was transferred (Fig. 3A), however some cardiomyocytes contacted with MSC far apart by means of nanotubes formation. In this case, cardiomyocytes contacted with calcein-stained MSC exhibited the presence of calcein fluorescence in cytoplasm (Fig. 3B). This observation underlined the possibility of cytoplasm migration not only through structures of gap junction, but also through nanotubes, although effectiveness of this transfer may be quite lower. However, the exact way of cytoplasmic molecules transfer (gap or nanotubes) stays unclear.
The link between cell-to-cell contacts formation and cytoplasmic content exchange between cells was examined using flow cytometry analysis. Two series of experiments with different cell-staining dyes before co-cultivation were undertaken. In one set of experiments, calcein-AM-loaded MSC were passed to dishes with non-stained cardiomyocytes. In another set, non-stained MSC were transferred to dishes with calcein-AM-loaded cardiomyocytes. Cells were analysed by flow cytometry after 3 and 24 hrs of co-cultivation. After 3 hrs of co-culturing in both sets mixed culture apparently contained two sub-populations of cells distinguishing in intensity of calcein fluorescence (Fig. 3C and E). The population with low-fluorescence intensity corresponded to non-stained cells, according to preliminary calibration using control cells. Another population carried high fluorescence corresponding to calcein-loaded cells.
After 24 hrs co-culturing of calcein-loaded MSC and non-stained cardiomyocytes, there was no apparent division of cells into two sub-populations. Suspension of cells demonstrated a broad single peak of the intensity distribution (Fig. 3D). When compared to 3 hrs after co-culturing (Fig. 3C), the quantity of cells with high fluorescence decreased from 40.1% to 17.3%. Herein, statistical analysis of peaks displayed that after 24 hrs of co-culturing the mean of intensity distribution amounted to 7.14 while after 3 hrs of co-culturing it was 2.56 and 42 for non-stained and calcein-loaded cells peaks, respectively. So calcein fluorescence intensity in MSC population dropped while fluorescence in cardiomyocytes increased after 24 hrs of co-cultivation, resulting in the formation of single population with average intensity. It confirms the suggestion that the formation of cell-to-cell contacts in a mixed culture, through which calcein-contained cytoplasmic entity could migrate between co-cultured cells, that is from MSC to cardiomyocytes.
In other experiments, cardiomyocytes were stained with calcein and co-cultured with non-stained MSC. The division of cell suspension into two sub-populations observed after 3 hrs (Fig. 3E) also vanished after 24 hrs of co-cultivation (Fig. 3F), and instead of two intensity peaks only a single peak remained. We revealed that after 24 hrs of co-culturing the mean of intensity distribution amounted to 2.1, whereas after 3 hrs of co-culturing it was 1.52 and 38.8 for non-stained MSC and calcein-loaded cardiomyocytes peaks, respectively. We concluded that migration of calcein-stained cytoplasmic content occurred from cardiomyocytes to MSC as well as in the reverse direction.
The fact that cytosol transfer between cells was blocked under conditions preventing formation of tunnelling nanotubes is in support of their active role in the transfer. For this, after 1 hr of co-cultivation, cells were exposed to 24 hrs of gentle shaking. Under these conditions, nanotubes being highly fragile are disrupted (not shown) and co-cultured cells demonstrate the separation for two subpopulations (Fig. 3G) as in initial period of co-cultivation, that is cytosolic transfer did not occur.
By staining by of the cell culture before co-cultivation with cytosolic probes showing different fluorescence (calcein AM having green fluorescence and Red-Orange calcein AM having red fluorescence) we determined a percentage of cells carrying both probes, that is, of those who could exchange with their cytosol. It occurred that the number of cells where the exchange with cytosol took place corresponded to 38% of the total number of cells in the population.
Transfer of mitochondria from MSC to cardiomyocytes
Since Koyanagi and co-authors in the recent study [23] revealed mitochondria transfer between adult human endothelial progenitor cells and neonatal rat cardiomyocytes, we investigated such transfer between other cell types. We used two types of mitochondrial dyes: Mitotracker Green FM (green fluorescence) and Mitotracker Red (red fluorescence) to examine mitochondria transfer between MSC and cardiomyocytes. MSC were loaded with Mitotracker Green FM before co-cultivation and placed into plates with Mitotracker Red-loaded cardiomyocytes. After 3 hrs of co-culturing, mitochondria transfer was not detected, that correlated with our data that cytoplasm transport did not take place. Then cells were analysed after 24 hrs of co-cultivation. We observed three types of mitochondrial staining in cultural cells. The majority of cells (type 1) contained mitochondria with the green stain solely or with the red stain solely (Type 1 and 2 correspondingly, Fig. 4A). However, some cells contained inclusions of green-fluorescing organelles among red-fluorescing mitochondria (Type 3) (Fig. 4B). Lower percentage of green-fluorescing mitochondria among the majority of red-fluorescing ones apparently demonstrates the exclusive transportation of green-fluorescing mitochondria, which originate from MSC. Interestingly, in this co-culture we never observed MSC containing mitochondria stained with Mitotracker Red, so there was no transfer of mitochondria from cardiomyocytes to MSC. At the same time, after 24 hrs of co-culturing green and red fluorescence did not co-localize, underlining that inside the cell there is no fusion of mitochondria taken from different sources.
4.
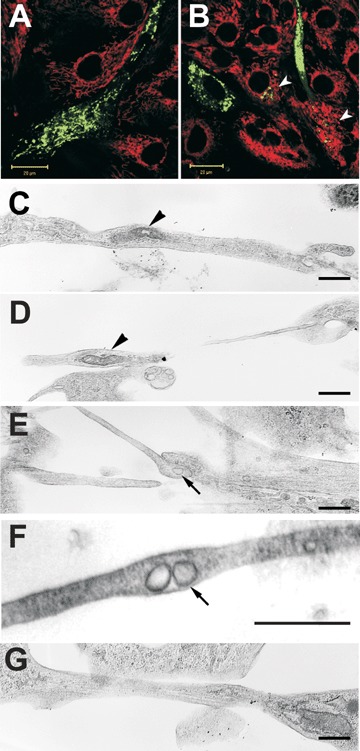
Mitochondria transfer from MSC to cardiomyocytes. MSC are stained with Mitotracker Green FM (green fluorescence), while cardiomyocytes with Mitotracker Red (red fluorescence). In majority of cells, mono-coloured fluorescing mitochondria are dominating (A) but in some cardiomyocytes (arrowheads), green-fluorescing mitochondria derived from MSC are present. (B) Electron microscopic micrographs (C–F) demonstrate the presence of mitochondria-like structures displaying double membrane vesicles with apparent cristae (black arowheads) and vesicles possibly limited by a single membrane (arrows). (G) Some filaments apparently representing cytoskeletal elements can be seen within nanotubes. Bars, 20 μm for A–B and 0.5 μm for C–G.
Electron microscopic study revealed that cytoplasmic extensions often contained vesicular structures limited with a double membrane thus resembling mitochondria in the appearance (Fig. 4C and D). Sometimes in these vesicles the cristae structures could be recognized. The diameter of these mitochondria-like structures was of the order of 0.1–0.2 microns. More often in nanotubes of smaller diameter (" 0.1 micron), we were able to detect small-size vesicles where double membranes were not so apparent (Fig. 4F). In some of nanotubes we identified the cytoskeletal elements (Fig. 4G).
Myosin expression in MSC
We used specific anti-human cardiac-specific β-myosin heavy-chain antibodies to detect expression of β-myosin H-chain in human MSC. Antibodies did not cross-react with rat myosin in cardiomyocytes, so potentially we were able to observe the existence of myosin only in hMSC. Expression of the protein was not detected in MSC monoculture (Fig. 5A), but it appeared after co-culturing with cardio cells. After 24 hrs of culturing with cardiomyocytes, MSC exhibited myosin-positive staining (Fig. 5B), and after 3 days, myosin-positive compartments were clearly observed in the majority of stem cells (Fig. 5C).
5.
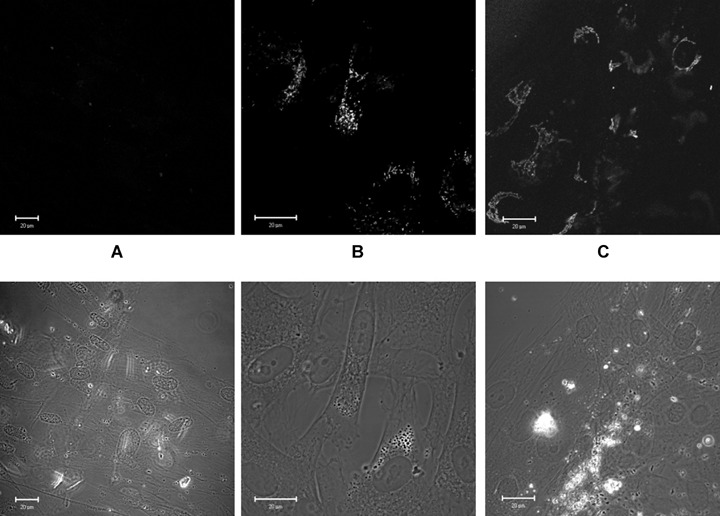
Immune-cytochemistry of cardiac-specific human β-myosin H-chain. (A) Control human MSC. (B) Human MSC, co-cultivated with rat cardiomyocytes for 1 day. (C) Human MSC co-cultivated with rat cardiomyocytes for 3 days.
Discussion
Our findings confirmed that cells could form ultra thin structures connecting neighbouring cells and described as nanotubes [30, 31]. Nanotubes have been demonstrated mostly in cell systems consisting of different cell types but occasionally were reported for cells in monocultures. This interaction was shown for macrophages and erythrocytes [32], endothelium progenitor cells and cardiomyocytes [23], immune cells [33], prostate cancer cells [34] and others. In the present study, both MSC and cardiomyocytes exhibited limited nanotubes formation before co-culture starting. After co-cultivation, the number of contacting nanotubes significantly rose. It has been proposed that tunnelling nanotubes represent a novel and general biological principle of cell-to-cell communication and it becomes increasingly apparent that they fulfil important functions in physiological processes of multi-cellular organisms.
Furthermore, we also inquired the possibility of mitochondria transport through nanotubes, recently discovered [23, 30]. Despite ultra small diameter of tubes, we observed mitochondria-specific staining inside of them. Although we failed to clarify what kind of cells they belonged to and could not monitor realtime movements, we revealed the result of such transport—mitochondria transfer from one type of cells to another. The electron microscopic data gave additional support for the possibility of mitochondrial transfer along nanotubes. Inside of these we found different types of vesicles some of which displayed classical mitochondrial ultra structure. It is possible to speculate that cytoskeleton filaments found within nanotubes may be an important element of directed vesicle-transporting machinery.
Another important finding was the phenomenon of cytoplasm exchange between contacted cells. Although calcein transfer is frequently used for detection of gap junctions, we believe that in our model, the dye could be transferred along nanotubes as well. This speculation is confirmed by confocal images where calcein transport was observed between cells making only nanotubes contacts. Nevertheless, tight cell contacts with possible formation of gap junctions between such different cell types as MSC and cardiomyocytes also took place, providing cytoplasm transfer as effective as mediated by nanotubes (Fig. 3B).
Cytoplasm entity transfer did not show a precise direction since the difference between patterns from Figure 3D and F is not essential when being normalized to C and E correspondingly. Thus, we can conclude that this transport had no specific carriers and occurred by diffusion of molecules through nanotubes and gap junctions. However, mitochondria transfer, also taking place in co-culture, was more specific. We observed that the organelles migrated only from MSC to cardiomyocytes, resembling data of Spees and coworkers [25], but there was no evidence for cardiomyocyte-to-MSC mitochondria transfer at least within first 24 hrs of co-cultivation. Probably, direction of mitochondria transfer could strongly depend on the type of cross-talking cells.
Another significant result of co-culturing was the appearance of myosin H-chain in MSC. We proposed that penetration of cardiomyocyte's cytoplasm to MSC caused such differentiation by donation of some signalling molecules to ignite MSC differentiation on the cardiac muscle pathway.
Thus, we discovered two consequences of MSC-cardiomyocyte cross-talk and cellular content exchange, both of them extremely important for clinical cell therapy of myocardium failures. On the one hand, cardiomyocytes-to-MSC cross-talk caused turning on differentiation of stem cells towards contractile cells, and this process took place already after 24 hrs of co-culture. It may mean that potentially transplanted MSC could replace damaged myocardial cells.
On the other hand, there is a distinct way of cell therapy effect on heart disorders. MSC can donate functional mitochondria to cardiomyocytes and restore their energetic state under the conditions when their own mitochondria are damaged or dysfunctional. If so, this may be an essential repair mechanism of mitochondria in heart cells damaged both under infarct and ischaemia and due to genetic defects, that is in cardiomiopathies.
Acknowledgments
This work was supported by Russian Foundation of Basic research (06-04-48614, 08-04-01667, 08-04-01692).
References
- 1.Anversa P, Sussman MA, Bolli R. Molecular genetic advances in cardiovascular medicine: focus on the myocyte. Circulation. 2004;109:2832–8. doi: 10.1161/01.CIR.0000132469.85026.46. [DOI] [PubMed] [Google Scholar]
- 2.Tse HF, Yiu KH, Lau CP. Bone marrow stem cell therapy for myocardial angiogen-esis. Curr Vasc Pharmacol. 2007;5:103–12. doi: 10.2174/157016107780368299. [DOI] [PubMed] [Google Scholar]
- 3.Britten MB, Abolmaali ND, Assmus B, Lehmann R, Honold J, Schmitt J, Vogl TJ, Martin H, Schachinger V, Dimmeler S, Zeiher AM. Infarct remodeling after intracoronary progenitor cell treatment in patients with acute myocardial infarction (TOPCARE-AMI): mechanistic insights from serial contrast-enhanced magnetic resonance imaging. Circulation. 2003;108:2212–8. doi: 10.1161/01.CIR.0000095788.78169.AF. [DOI] [PubMed] [Google Scholar]
- 4.Wollert KC, Drexler H. Clinical applications of stem cells for the heart. Circ Res. 2005;96:151–63. doi: 10.1161/01.RES.0000155333.69009.63. [DOI] [PubMed] [Google Scholar]
- 5.Doetschman TC, Eistetter H, Katz M, Schmidt W, Kemler R. The in vitro development of blastocyst-derived embryonic stem cell lines: formation of visceral yolk sac, blood islands and myocardium. J Embryol Exp Morphol. 1985;87:27–45. [PubMed] [Google Scholar]
- 6.Maltsev VA, Wobus AM, Rohwedel J, Bader M, Hescheler J. Cardiomyocytes differentiated in vitro from embryonic stem cells developmentally express cardiac-specific genes and ionic currents. Circ Res. 1994;75:233–44. doi: 10.1161/01.res.75.2.233. [DOI] [PubMed] [Google Scholar]
- 7.Wobus AM, Wallukat G, Hescheler J. Pluripotent mouse embryonic stem cells are able to differentiate into cardiomyocytes expressing chronotropic responses to adrenergic and cholinergic agents and Ca2+ channel blockers. Differentiation. 1991;48:173–82. doi: 10.1111/j.1432-0436.1991.tb00255.x. [DOI] [PubMed] [Google Scholar]
- 8.Dai W, Kloner RA. Myocardial regeneration by embryonic stem cell transplantation: present and future trends. Expert Rev Cardiovasc Ther. 2006;4:375–83. doi: 10.1586/14779072.4.3.375. [DOI] [PubMed] [Google Scholar]
- 9.Passier R, Oostwaard DW, Snapper J, Kloots J, Hassink RJ, Kuijk E, Roelen B, De La Riviere AB, Mummery C. Increased cardiomyocyte differentiation from human embryonic stem cells in serum-free cultures. Stem Cells. 2005;23:772–80. doi: 10.1634/stemcells.2004-0184. [DOI] [PubMed] [Google Scholar]
- 10.Li X, Yu X, Lin Q, Deng C, Shan Z, Yang M, Lin S. Bone marrow mesenchymal stem cells differentiate into functional cardiac phenotypes by cardiac microenviron-ment. J Mol Cell Cardiol. 2007;42:295–303. doi: 10.1016/j.yjmcc.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 11.Behfar A, Terzic A. Optimizing adult mesenchymal stem cells for heart repair. J Mol Cell Cardiol. 2007;42:283–4. doi: 10.1016/j.yjmcc.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 12.Nygren JM, Jovinge S, Breitbach M, Sawen P, Roll W, Hescheler J, Taneera J, Fleischmann BK, Jacobsen SE. Bone marrow-derived hematopoietic cells generate cardiomyocytes at a low frequency through cell fusion, but not transdifferenti-ation. Nat Med. 2004;10:494–501. doi: 10.1038/nm1040. [DOI] [PubMed] [Google Scholar]
- 13.Numaguchi Y, Sone T, Okumura K, Ishii M, Morita Y, Kubota R, Yokouchi K, Imai H, Harada M, Osanai H, Kondo T, Murohara T. The impact of the capability of circulating progenitor cell to differentiate on myocardial salvage in patients with primary acute myocardial infarction. Circulation. 2006;114:I114–9. doi: 10.1161/CIRCULATIONAHA.105.000588. [DOI] [PubMed] [Google Scholar]
- 14.Fuchs JR, Nasseri BA, Vacanti JP, Fauza DO. Postnatal myocardial augmentation with skeletal myoblast-based fetal tissue engineering. Surgery. 2006;140:100–7. doi: 10.1016/j.surg.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 15.Etzion S, Holbova R, Miller L, Leor J. Protocols for myocardial infarction repair using fetal cardiac myocytes. Methods Mol Med. 2005;112:205–21. doi: 10.1385/1-59259-879-x:205. [DOI] [PubMed] [Google Scholar]
- 16.Etzion S, Battler A, Barbash IM, Cagnano E, Zarin P, Granot Y, Kedes LH, Kloner RA, Leor J. Influence of embryonic cardiomyocyte transplantation on the progression of heart failure in a rat model of extensive myocardial infarction. J Mol Cell Cardiol. 2001;33:1321–30. doi: 10.1006/jmcc.2000.1391. [DOI] [PubMed] [Google Scholar]
- 17.Dai W, Hale SL, Martin BJ, Kuang JQ, Dow JS, Wold LE, Kloner RA. Allogeneic mesenchymal stem cell transplantation in postinfarcted rat myocardium: short- and long-term effects. Circulation. 2005;112:214–23. doi: 10.1161/CIRCULATIONAHA.104.527937. [DOI] [PubMed] [Google Scholar]
- 18.Tang YL. Autologous mesenchymal stem cells for post-ischemic myocardial repair. Methods Mol Med. 2005;112:183–92. doi: 10.1385/1-59259-879-x:183. [DOI] [PubMed] [Google Scholar]
- 19.Beeres SL, Atsma DE, Van Der Laarse A, Pijnappels DA, Van Tuyn J, Fibbe WE, De Vries AA, Ypey DL, Van Der Wall EE, Schalij MJ. Human adult bone marrow mesenchymal stem cells repair experimental conduction block in rat cardiomyocyte cultures. J Am Coll Cardiol. 2005;46:1943–52. doi: 10.1016/j.jacc.2005.07.055. [DOI] [PubMed] [Google Scholar]
- 20.Ishikawa F, Shimazu H, Shultz LD, Fukata M, Nakamura R, Lyons B, Shimoda K, Shimoda S, Kanemaru T, Nakamura K, Ito H, Kaji Y, Perry AC, Harada M. Purified human hematopoietic stem cells contribute to the generation of cardiomyocytes through cell fusion. FASEB J. 2006;20:950–2. doi: 10.1096/fj.05-4863fje. [DOI] [PubMed] [Google Scholar]
- 21.Matsuura K, Wada H, Nagai T, Iijima Y, Minamino T, Sano M, Akazawa H, Molkentin JD, Kasanuki H, Komuro I. Cardiomyocytes fuse with surrounding noncardiomyocytes and reenter the cell cycle. J Cell Biol. 2004;167:351–63. doi: 10.1083/jcb.200312111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Badorff C, Brandes RP, Popp R, Rupp S, Urbich C, Aicher A, Fleming I, Busse R, Zeiher AM, Dimmeler S. Transdifferentia-tion of blood-derived human adult endothe-lial progenitor cells into functionally active cardiomyocytes. Circulation. 2003;107:1024–32. doi: 10.1161/01.cir.0000051460.85800.bb. [DOI] [PubMed] [Google Scholar]
- 23.Koyanagi M, Brandes RP, Haendeler J, Zeiher AM, Dimmeler S. Cell-to-cell connection of endothelial progenitor cells with cardiac myocytes by nanotubes: a novel mechanism for cell fate changes? Circ Res. 2005;96:1039–41. doi: 10.1161/01.RES.0000168650.23479.0c. [DOI] [PubMed] [Google Scholar]
- 24.Gruh I, Beilner J, Blomer U, Schmiedl A, Schmidt-Richter I, Kruse ML, Haverich A, Martin U. No evidence of transdifferentia-tion of human endothelial progenitor cells into cardiomyocytes after coculture with neonatal rat cardiomyocytes. Circulation. 2006;113:1326–34. doi: 10.1161/CIRCULATIONAHA.105.559005. [DOI] [PubMed] [Google Scholar]
- 25.Spees JL, Olson SD, Whitney MJ, Prockop DJ. Mitochondrial transfer between cells can rescue aerobic respiration. Proc Natl Acad Sci USA. 2006;103:1283–8. doi: 10.1073/pnas.0510511103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Musina RA, Bekchanova ES, Belyavskii AV, Sukhikh GT. Differentiation potential of mesenchymal stem cells of different origin. Bull Exp Biol Med. 2006;141:147–51. doi: 10.1007/s10517-006-0115-2. [DOI] [PubMed] [Google Scholar]
- 27.Musina RA, Bekchanova ES, Sukhikh GT. Comparison of mesenchymal stem cells obtained from different human tissues. Bull Exp Biol Med. 2005;139:504–9. doi: 10.1007/s10517-005-0331-1. [DOI] [PubMed] [Google Scholar]
- 28.Hodneland E, Lundervold A, Gurke S, Tai XC, Rustom A, Gerdes HH. Automated detection of tunneling nanotubes in 3D images. Cytometry. 2006;69:961–72. doi: 10.1002/cyto.a.20302. [DOI] [PubMed] [Google Scholar]
- 29.Rustom A, Saffrich R, Markovic I, Walther P, Gerdes HH. Nanotubular highways for intercellular organelle transport. Science. 2004;303:1007–10. doi: 10.1126/science.1093133. [DOI] [PubMed] [Google Scholar]
- 30.Gerdes HH, Bukoreshtliev NV, Barroso JF. Tunneling nanotubes: A new route for the exchange of components between animal cells. FEBS Lett. 2007;581:2194–201. doi: 10.1016/j.febslet.2007.03.071. [DOI] [PubMed] [Google Scholar]
- 31.Onfelt B, Purbhoo MA, Nedvetzki S, Sowinski S, Davis DM. Long-distance calls between cells connected by tunneling nanotubules. Sci STKE. 2005:pe55. doi: 10.1126/stke.3132005pe55. [DOI] [PubMed] [Google Scholar]
- 32.Galkina SI, Molotkovsky JG, Ullrich V, Sud’ina GF. Scanning electron microscopy study of neutrophil membrane tubulovesicu-lar extensions (cytonemes) and their role in anchoring, aggregation and phagocytosis. The effect of nitric oxide. Exp Cell Res. 2005;304:620–9. doi: 10.1016/j.yexcr.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 33.Watkins SC, Salter RD. Functional connectivity between immune cells mediated by tunneling nanotubules. Immunity. 2005;23:309–18. doi: 10.1016/j.immuni.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 34.Vidulescu C, Clejan S, O’connor KC. Vesicle traffic through intercellular bridges in DU 145 human prostate cancer cells. J Cell Mol Med. 2004;8:388–96. doi: 10.1111/j.1582-4934.2004.tb00328.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


