Abstract
Background: Angiogenesis is an important determinant of the pathophysiology of portal hypertension contributing to the formation of portosystemic collateral vessels and the hyperdynamic splanchnic circulation associated to this syndrome. Somatostatin and its analogues, like octreotide, have been shown to be powerful inhibitors of experimental angiogenesis. Aim: To determine whether octreotide has angioinhibitory effects in portal hypertensive rats. Methods: Partial portal vein-ligated (PPVL) rats were treated with octreotide or vehicle during 4 or 7 days. Splanchnic neovascularization and VEGF expression were determined by histological analysis and western blotting. Expression of the somatostatin receptor subtype 2 (SSTR2), which mediates the anti-angiogenic effects of octreotide, was also analyzed. Formation of portosystemic collaterals (radioactive microspheres) and hemodynamic parameters were also measured. Results: Octreotide treatment during 4 days markedly and significantly decreased splanchnic neovascularization, VEGF expression by 63% and portal pressure by 15%, whereas portosystemic collateralization and splanchnic blood flow were not modified. After 1 week of octreotide injection, portal pressure was reduced by 20%, but inhibition of angiogenesis escaped from octreotide therapy, a phenomenon that could be related to the finding that expression of SSTR2 receptor decreased progressively (up to 78% reduction) during the evolution of portal hypertension. Conclusion: This study provides the first experimental evidence showing that octreotide may be an effective anti-angiogenic therapy early after induction of portal hypertension, but not in advanced stages most likely due to SSTR2 down-regulation during the progression of portal hypertension in rats. These findings shed light on new mechanisms of action of octreotide in portal hypertension.
Keywords: octreotide, somatostatin, portal hypertension, angiogenesis, heme oxygenase
Introduction
Portal hypertension is the most important complication of cirrhosis and is a leading cause of mortality and liver transplantation worldwide [1]. A characteristic feature of the portal hypertensive syndrome is the development of a hyperdynamic splanchnic circulation with an increase in blood flow in splanchnic organs draining into the portal vein and a subsequent increase in portal venous inflow [1]. Such an increased portal venous inflow represents a significant factor maintaining and worsening the portal pressure elevation. Portal hypertension is also characterized by the formation of portosystemic collateral vessels and gastroesophageal varices, which are responsible of most clinical consequences of portal hypertension [1]. We have recently demonstrated that angiogenesis, the development of new blood vessels, is a crucial process both for development and maintenance of the hyperdy-namic splanchnic circulatory syndrome and the portosystemic collateral circulation in portal hypertension [2–4].
Somatostatin is a regulatory peptide characterized by a wide spectrum of biological actions that has been successfully used in the management of bleeding gastroesophageal varices in patients with chronic liver disease [5, 6]. The precise mode of action of somatostatin and its analogues on portal hypertension is, however, still unclear. Recently, several studies have focused on the possible anti-proliferative effect of this peptide. Thus, octreotide, a synthetic octapeptide analogue of naturally occurring somatostatin, with similar pharmacological effects but with a prolonged duration of action, has been shown to be a powerful inhibitor of cell proliferation and neovascularization in several experimental models [7–10], and therefore represents a novel therapeutic avenue to combat several angiogenesis-related diseases [11, 12]. The angioinhibitory properties of octreotide are predominantly mediated by the high affinity somatostatin subtype 2 receptor (SSTR2) [13]. The possible anti-angiogenic effects of octreotide have not been characterized in portal hypertension as yet. Hence, in the present study, we determined the role of octreotide as an inhibitor of angiogenesis in portal hypertensive rats.
Materials and methods
Materials
Octreotide was purchased from Novartis (Basel, Switzerland). Polyclonal antibodies against rat CD31, vascular endothelial growth factor (VEGF), SSTR2 and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were from Santa Cruz Biotechnology (Santa Cruz, California, USA). Monoclonal antibody against rat endothelial nitric oxide synthase (eNOS) was from BD Biosciences (Franklin Lakes, New Jersey, USA). Polyclonal antibody against rat heme oxygenase-1 (HO-1) and peroxidase-conjugated secondary antibodies were from Stressgen (Sidney, Canada). 51Cr-labeled micros-pheres were from Perkin-Elmer (Boston, Massachusetts, USA). Protein assay kit was from Bio-Rad (Hercules, California, USA). Polyvinylidine difluoride (PVDF) membranes were from Millipore (Billerica, Massachusetts, USA). All other reagents and chemicals were from Sigma (St Louis, Missouri, USA).
Animals and treatments
Portal hypertension was induced in male Sprague-Dawley rats (300–350 g body weight) by partial portal vein ligation (PPVL) as previously described [14]. Briefly, under anesthesia (80 mg/kg ketamine plus 12 mg/kg xylacine, intraperitoneally), a calibrated constriction of the portal vein was performed with a single ligature of 3-0 silk tied around the portal vein and a 20-gauge blunt-tipped needle. The needle was then removed, leaving a calibrated constriction of the portal vein. In sham-operated rats, the portal vein was isolated and similarly manipulated but not ligated.
In a first experimental protocol, PPVL rats received a 50 μg/kg dose of octreotide (n = 7) or vehicle (500 μl of 0.9% NaCl; n = 7) by subcutaneous injection every 12 hrs for 4 consecutive days, starting immediately after PPVL. In a second protocol, PPVL rats were treated with octreotide (50 μg·kg−1·12 hr−1; n = 8), or vehicle (n = 8) from PPVL to day 7. In all groups, the last dose of octreotide or vehicle was injected 90 min before studies. All procedures were approved by the Ethics Committee of the University of Barcelona.
Hemodynamic studies
Under anesthesia, PE-50 catheters were introduced into a femoral artery and the portal vein and connected to highly sensitive pressure transducers to measure arterial pressure (MAP, mmHg) and portal pressure (PP, mmHg), respectively. Then, a non-constrictive perivascular transit-time ultrasonic flowprobe (Transonic Systems, New York, USA) was placed around the superior mesenteric artery and connected to a flowmeter to measure superior mesenteric artery blood flow (SMABF, ml⋅min−1⋅100 g−1). Superior mesenteric artery resistance (SMAR, mmHg⋅ml−1⋅min−1(100 g−1) was calculated as: (MAP–PP)/SMABF [15].
Determination of the extent of portosystemic collateral vessel formation
The extent of portosystemic collateralization was quantified by injection of 51Cr-labeled microspheres (15 ± 3 μm in diameter; specific activity 52.68 mCi/g) directly into the spleen [2–4]. Rats were then sacrificed and radioactivity in liver and lungs was counted in a gamma-scintillation counter. This allows to quantify the degree of collateral formation in a 0–100% scale by the equation: collateralization (%) = (lungs radioactivi-ty/[lungs radioactivity + liver radioactivity]) x 100.
Western blot analysis
Tissue samples (intestinal mucosa from jejunum) were homogenized in ice-cold lysis buffer containing 50 mM Tris-HCl (pH 7.4), 0.1 mM EGTA, 0.1 mM EDTA, 2 mM leupeptin, 1 mM phenylmethylsulphonyl fluoride, 1% Nonidet P40, 0.1% sodium dodecyl sulfate and 0.1% deoxycholate. The homogenate was centrifuged at 10,000 g for 30 min. The supernatant was processed for determination of total protein concentration by using a colorimetric assay. Proteins were separated by SDS-PAGE electrophoresis and subsequently transferred to PVDF membranes. Non-specific binding sites in the membranes were blocked with 5% non-fat dry milk in incubation buffer before addition of antibodies against rat VEGF, CD31, SSTR2, eNOS or HO-1. Following incubation, membranes were washed and exposed to horseradish peroxidase-conju-gated secondary antibody. After washing, blots were visualized using enhanced chemiluminescence. Quantification of expression normalized to the housekeeping protein GAPDH was measured by computer-assisted densitometry [2–4]. The Western blot analysis was repeated to confirm reproducibility using the samples collected from three individual rats of each experimental group.
Measurement of vascular areas
Tissue samples (mesentery) were fixed in 10% neutral-buffered formalin and embedded in paraffin. Tissues were sectioned (thickness of 2 μm), and slides were stained with hematoxylin and eosin (H&E). Quantitative analysis of angiogenesis was performed with the assistance of ImageJ 1,37v software (NIH, Bethesda, MD) by scanning the entire tissue section and counting the areas occupied by vascular structures. We obtained a value from each tissue section (one section per rat) and then averaged the results from individual sections in each experimental group (four sections per group). Group values reflect the average readings from all sections in the group. The results of neovascularization measurements are expressed as the mean area (±SEM; expressed in square micrometers) occupied by vascular structures within a tissue field measuring 1 mm2.
Statistics
All data were expressed as mean ± SEM. Unpaired Student's t-test and ANOVA were used for statistical analysis and P < 0.05 was accepted as significant.
Results
Effects on splanchnic neovascularization and VEGF expression.
To evaluate the effects of octreotide on splanchnic neovasculariza-tion, we determined the protein expression of CD31, an endothelial cell marker that has been widely used as an index of angiogenesis [2–4], as well as the expression of the angiogenic growth factor VEGF in the intestine from portal hypertensive rats. Western blot analysis showed a significant reduction of the expression of CD31 (38% decrease; P < 0.01) and VEGF (63% decrease; P < 0.05) after a 4-day octreotide treatment compared with vehicle (Fig. 1). However, these anti-angiogenic effects of octreotide were not observed after treatment for 7 days. Thus, CD31 protein expression was not significantly different when comparing octreotide-treated and vehicle-treated portal hypertensive rats (Fig. 1). Similarly, VEGF expression was not significantly modified by the 7-days octreotide treatment in portal hypertensive animals (Fig. 1).
1.
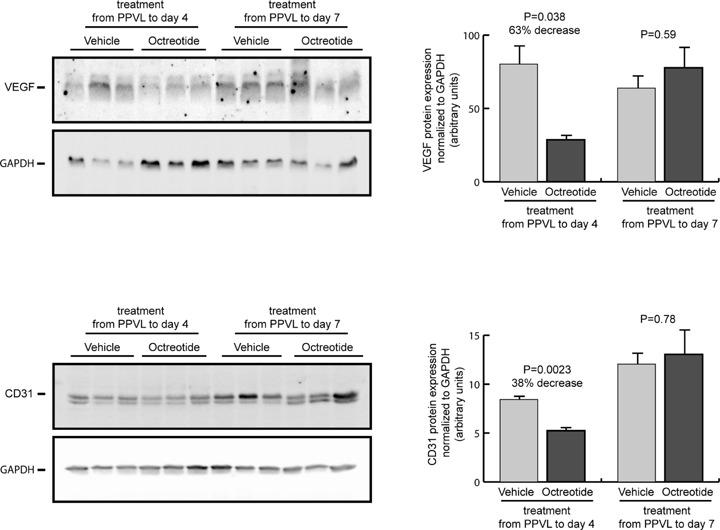
Expression of VEGF and CD31 in the intestine of portal hypertensive rats treated with octreotide or vehicle during 4 or 7 days after induction of portal hypertension by partial portal vein ligation (PPVL). Representative blots for VEGF, CD31 and the housekeeping protein GAPDH are shown at the left and quantification of expression normalized to GAPDH and expressed as arbitrary units is shown at the right.
To further characterize the effects of octreotide on splanchnic neovascularization in portal hypertensive rats, we also performed histological analysis and quantification of vascular areas on rat mesenteric sections. As shown in Figure 2, the mesentery from portal hypertensive rats treated with vehicle was intensely neovascularized both at day 4 and at day 7 after PPVL. This increased vascular area was significantly reduced, by 64%, after treatment with octreotide for a 4-day period, but not when the treatment took place during 7 days. Therefore, these findings further confirmed those observed using the western blot technique.
2.
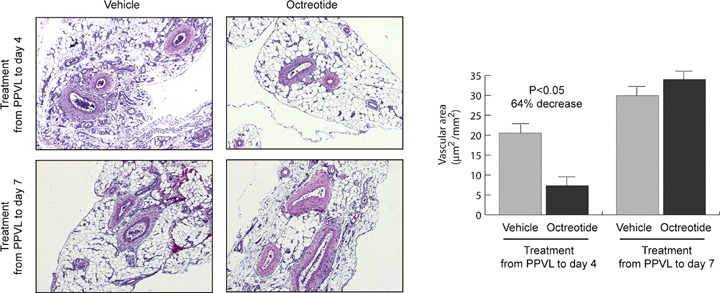
Effect of octreotide administration on the splanchnic neovascularization of portal hypertensive rats. Treatment with octreotide or vehicle was performed from PPVL to day 4th or from PPVL to day 7th. Representative histological images of mesentery sections stained with H&E (original magnification x40) are shown at the left, and quantitative analysis of vascular areas is shown at the right.
Effects on portosystemic collateral circulation
There was a tendency for the extent of collateral vessel formation to be lower (16% decrease) in portal hypertensive rats treated with octreotide from the day of PPVL to day 4th than in vehicle-treated portal hypertensive rats, but this change was not statistically significant (P = 0.18; Fig. 3). This tendency was not observed when treatment with octreotide was started immediately after PPVL and terminated 7 days later (P = 0.57; Fig. 3).
3.
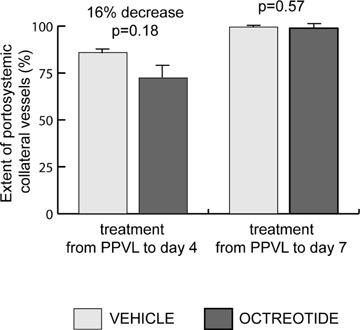
Formation of portosystemic collateral vessels in portal hypertensive rats treated with octreotide or vehicle during 4 or 7 days after induction of portal hypertension by PPVL.
Effects on SSTR2 expression
We also measured the expression of the SSTR2 receptor, which predominantly mediates the anti-angiogenic effects of octreotide [13]. SSTR2 protein was expressed in the intestine from sham-operated rats. Remarkably, after induction of portal hypertension, there was a progressive decline of SSTR2 expression over time, with a 40% decrease at day 4 after PPVL (P = 0.19) and up to a 78% reduction at day 7 after PPVL (P < 0.01), compared with sham-operated rats (Fig. 4). Treatment with octreotide during either 4 or 7 days from PPVL did not significantly modify SSTR2 expression compared with the corresponding vehicle-treated groups (Fig. 4).
4.
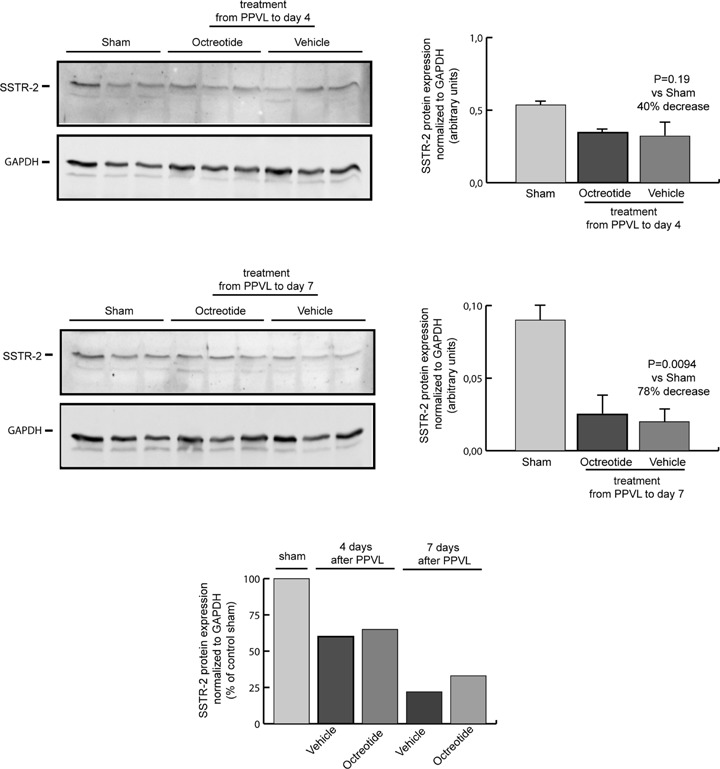
Expression of the somatostatin subtype 2 receptor (SSTR2) in the intestine of sham-operated control rats and portal hypertensive rats treated with octreotide or vehicle during 4 or 7 days after induction of portal hypertension by PPVL. Representative blots for SSTR2 and the housekeeping protein GAPDH are shown at the left and quantification of expression normalized to GAPDH and expressed as arbitrary units is shown at the right. Quantification of SSTR2 expression normalized to GAPDH and expressed as percentage of sham-operated control rats is also shown at the bottom, illustrating the progressive down-regulation of SSTR2 expression during the evolution of the portal hypertensive syndrome in rats.
Hemodynamic effects
Octreotide treatment during 4 days, starting immediately after PPVL, caused no significant changes in MAP, SMABF and SMAR (Fig. 5), but octreotide significantly decreased PP by 15% (P < 0.05; Fig. 4) and heart rate by 11% (326.86 ± 8.49 versus291.0 ± 10.99 beats/min; P < 0.05), compared with vehicle-treated PPVL rats. Octreotide administration during 7 days, commencing the day of PPVL, did not cause any significant change in MAP, SMABF and SMAR (Fig. 5). PP decreased significantly, by 20%, in PPVL rats treated with octreotide for 7 days, compared with vehicle-treated PPVL rats (P < 0.05; Fig. 5), and heart rate decreased by 16% (345.3 ± 22.16 beats/min in the vehicle group and 291.7 ± 14.54 beats/min in the octreotide group; P < 0.05).
5.
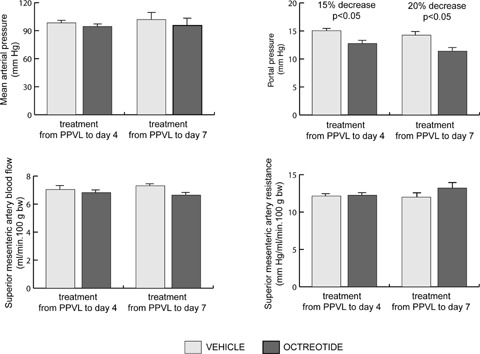
Mean arterial pressure, portal pressure, superior mesenteric artery blood flow and resistance in portal hypertensive rats treated with octreotide or vehicle during 4 or 7 days after induction of portal hypertension by PPVL.
Effects on eNOS and HO-1 expression
To assess whether the lack of an effect on SMABF after octreotide treatment, despite a decrease in splanchnic neovascularization, was due to a compensatory increase in vasodilatation, we determined the effects of octreotide on the expression of the vasodila-tory enzymatic systems eNOS and HO-1. Protein expression of eNOS was not significantly modified either by the 4-days or 7-days octreotide treatment in the intestine from portal hypertensive rats, compared with vehicle (Fig. 6). Interestingly, the intestinal expression of HO-1 increased markedly and significantly after octreotide treatment in portal hypertensive rats, both in the 4-days treatment group (148% increase; P < 0.05) and in the 7-days treatment group (113% increase; P < 0.01) compared with vehicle-treated portal hypertensive rats (Fig. 6).
6.
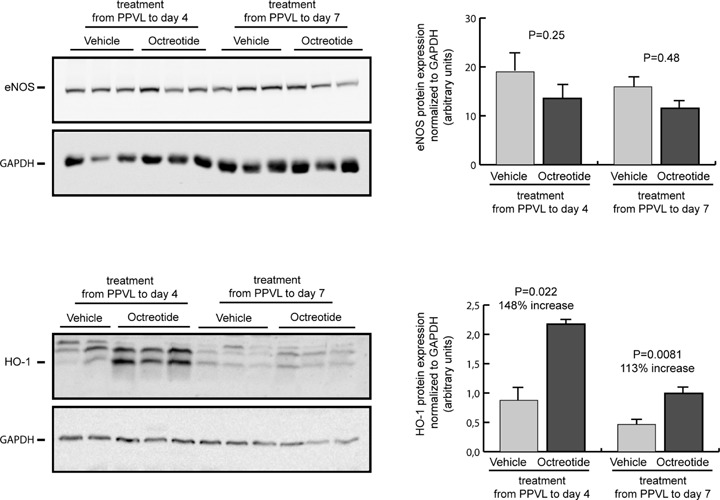
Expression of eNOS and HO-1 in the intestine of portal hypertensive rats treated with octreotide or vehicle during 4 or 7 days after induction of portal hypertension by PPVL. Representative blots for eNOS, HO-1 and the housekeeping protein GAPDH are shown at the left and quantification of expression normalized to GAPDH and expressed as arbitrary units is shown at the right.
Discussion
The present study demonstrates that, early after the induction of portal hypertension, the somatostatin analogue octreotide significantly decreases the extent of splanchnic neovascularization as well as the expression of the growth factor VEGF in splanchnic organs from PPVL rats compared with vehicle-treated portal hypertensive animals. To our knowledge, our study uncovers a novel role of the somatostatin/octreotide signaling in mediating anti-angiogenic effects during portal hypertension.
Interestingly, this angioinhibitory activity was displayed only when octreotide was administered from the day of PPVL to day 4th. However, after 1 week of daily octreotide injection, inhibition of angiogenesis escaped from octreotide therapy. Thus, levels of intestinal CD31 and VEGF proteins in portal hypertensive rats treated with octreotide from the day of PPVL to day 7th were found to be comparable to those in vehicle-treated portal hypertensive animals. Furthermore, quantitative analysis of neovascu-larization demonstrated that the mesentery from PPVL rats was intensely neovascularized and this increased vascular area was markedly decreased after treatment with octreotide for 4 days, but not for 7 days.
The molecular basis of the escape phenomenon experienced by these rats are yet to be understood. It should be noted that the anti-angiogenic activity of octreotide is predominantly mediated by the receptor subtype SSTR2 [13]. Our study demonstrates for the first time the presence of SSTR2 in the intestinal mucosa of portal hypertensive rats and a progressive down-regulation of this receptor during the evolution of portal hypertension. Thus, SSTR2 expression decreased by 40% at day 4 and by 78% at day 7 after PPVL, compared with sham-operated rats. This robust decline in SSTR2 expression may be a major reason explaining why octreotide treatment for 7 days had no marked anti-angiogenic effect on portal hypertensive rats. It is worthwhile mentioning that SSTR2 expression in portal hypertensive rats was not affected by octreotide treatment, suggesting that octreotide administration by itself did not contribute significantly to further down-regulate the expression of the SSTR2 receptor. These results also illustrate an important point; that the prevalence of SSTR2 expression on splanchnic blood vessels is a key factor governing the efficacy of somatostatin analogues as inhibitors of angiogenesis in rats with portal hypertension.
The mechanism by which SSTR2 expression diminishes during the evolution of portal hypertension is at present unknown. Interestingly, several lines of evidence suggest that SSTR2 is uniquely down-regulated in endothelial cells during angiogenesis [10–12]. Thus, it has been previously demonstrated that SSTR2 is strongly expressed in normal pancreas, whereas expression of this receptor is frequently lost in pancreatic tumors [16, 17]. In addition, normal human retina displays strong SSTR2 expression, whereas in eyes with late stages of age-related maculopathy, characterized by choroidal neovascularization, SSTR2 is expressed at low levels or not expressed at all [18]. In our present study, SSTR2 was progressively down-regulated in the intestinal mucosa upon the onset of angiogenic signaling in the splanchnic vascula-ture of portal hypertensive rats, suggesting that angiogenesis could trigger SSTR2 down-regulation in this experimental model. By contrast, other studies have found that a large variety of tumors are rich in SSTR2 [5]. The reason for this discrepancy is unclear but may be due in part to tissue-specific differences in the expression of somatostatin receptors.
In the present study, we also determined the effects of octreotide on the formation of portosystemic collateral vessels, which is, in part, a VEGF-dependent angiogenic process in portal hypertension. Octreotide decreased the extent of collateral formation by 16% after 4 days of treatment compared with vehicle-treated portal hypertensive rats, but the difference did not reach statistical significance (P = 0.18). After 7 days of treatment, there was no change in collateral formation. The dissociation observed between a marked and significant anti-angiogenic response to octreotide in splanchnic organs and a modest non-significant effect on portosystemic collateral vessel formation may reflect that SSTR2 is expressed in collateral vessels but with a relatively lower abundance than in the splanchnic blood vessels. Moreover, the lack of effect after 7 days of octreotide treatment suggests reduction in SSTR2 expression in collateral vessels during the progression of the portal hypertensive syndrome, similar to the observed in the splanchnic vasculature.
Another novel finding of the present study is that octreotide treatment resulted in a marked and significant up-regulation of HO-1 expression in the intestinal mucosa of portal hypertensive rats. This HO-1 induction was observed both after 4 days of octreotide treatment (148% increase) and after treatment for 7 days (113% increase). Therefore, there was not an escape phenomenon to the octreotide-induced HO-1 up-regulation, suggesting that this effect of octreotide was not mediated through binding to the SSTR2 receptor.
This splanchnic HO-1 overexpression after octreotide treatment may have relevant implications, mainly taking into account that it occurs on top of the previously demonstrated HO-1 upreg-ulation in splanchnic organs of portal hypertensive rats [19–21]. HO-1 activity plays an important role in cellular protection against oxidative injury [13]. This is related to the fact that this enzymatic system breaks down heme, thereby mitigating the hazardous cellular effects of this pro-oxidant. In addition, HO activity produces biliverdin, which is converted to bilirubin by the action of biliverdin reductase. Both biliverdin and bilirubin are potent antioxidants. Moreover, the enzymatic HO activity generates carbon monoxide (CO), which in addition of being a potent vasodilator also has anti-inflammatory properties [21]. Recently, we have demonstrated that HO-1 up-regulation constitutes an important antioxidant mechanism in portal hypertensive rats [22, 23]. Therefore, by up-regulating HO-1, octreotide may have a beneficial effect reducing oxidative stress and inflammatory responses in splanchnic organs from portal hypertensive rats. Interestingly, recent studies have demonstrated that octreotide protects against intestinal and colonic damage associated with enteritis or colitis, respectively, through anti-inflammatory and antioxidant effects mediated by octreotide-induced HO-1 overexpression [24, 25]. Altogether, these results suggest that the already demonstrated anti-inflammatory activity of somatostatin and its analogues could be at least partly mediated through HO-1 upregulation [26].
HO-1 produces the powerful vasodilator CO, which contributes significantly to the development and maintenance of splanchnic hyperdynamic circulation in chronic portal hypertension [19–21]. An increased CO-mediated splanchnic vasodilatation in response to octreotide could explain why, in the present study, splanchnic blood flow was not significantly reduced despite a marked decrease in splanchnic neovascularization in portal hypertensive rats receiving octreotide from PPVL to day 4. In other words, octreotide treatment could decrease the number of splanchnic blood vessels but simultaneously increase the vasodilatation of these vessels the net effect being a lack of change in splanchnic blood flow. The presence of an adaptive response between vasodilators aimed at maintaining vasodilatation in portal hypertension has also been previously reported [15, 27]. Our results suggest that such compensatory mechanism does not involve increased NO production since eNOS protein expression was not significantly modified after octreotide in portal hypertensive rats.
Emerging evidence indicates the participation of HO-1 in angio-genesis [28, 29], and we have also recently demonstrated that HO-1 activity up-regulates VEGF expression in portal hypertensive rats [22]. Based on these findings, it could be possible that the effect of octreotide decreasing VEGF expression and angiogenesis observed in our present study was partly counteracted by a simultaneous increase in VEGF expression stimulated by the octreotide-dependent HO-1 induction. Such dual effect of octreotide inhibiting and stimulating VEGF expression may have important therapeutical implications. Thus, the pronounced ability of octreotide to decrease VEGF expression seems to be primarily an SSTR2-dependent effect, which may not be observed when SSTR2 expression is down-regulated, like in advanced portal hypertension. These results suggest that an anti-angiogenic therapy with octreotide will be effective only if administered in the earliest stages of portal hypertension, when predominates the angioin-hibitory activity of octreotide mediated through SSTR2-dependent VEGF inhibition. However, in advanced stages of portal hypertension, the ability of octreotide to inhibit angiogenesis mostly disappears as a result of the marked decline in SSTR2 expression, prevailing the HO-1-dependent pro-angiogenic effect of octreotide.
Regarding the hemodynamic effects of the somatostatin analogue in portal hypertensive rats, we found a significant decrease in PP both after 4 days (15% decrease) and 7 days (20% decrease) octreotide treatment, without any significant effect on SMABF or SMAR. Heart rate was also significantly reduced after octreotide treatment during 4 as well as 7 days. Our present results are in line with previous reports, although others have shown a lack of effect of octreotide in chronic portal hypertension [6, 27]. In our current study, measurement of hemodynamic parameters was carried out 90 min after the last octreotide injection. In preliminary studies, we measured hemodynamics 4 hrs after the final dose of octreotide and we did not find any significant effect (data not shown). Such evidence suggests that the hemody-namic effects of octreotide are short lasting. These issues also stress that carefully designed experimental studies are essential to investigate the effects of octreotide in portal hypertension, emphasizing determination of tissular SSTR status as well as optimization of doses and regimens of administration [30].
In conclusion, the present study demonstrates that the somato-statin analogue octreotide inhibits splanchnic neovascularization and VEGF expression in portal hypertensive rats by a mechanism primarily SSTR2-dependent. Our results also indicate that the expression of SSTR2 is down-regulated during the progression of the portal hypertensive syndrome in rats. This progressive decline in SSTR2 expression determines that octreotide may be an effective anti-angiogenic therapy only in early stages of splanchnic neo-vascularization in portal hypertension, but not in advanced portal hypertension, when SSTR2 is down-regulated. In addition, this is the first study showing that octreotide up-regulates HO-1 expression in rats with portal hypertension. Overall, our findings shed light on new mechanisms of action of somatostatin and its agonists in portal hypertension, and may have implications for many other diseases whose pathogenicity is mediated by angiogenesis.
Acknowledgments
This study was supported in part by a grant from the Ministerio de Educacion y Ciencia (SAF2005-05825). MM is a recipient of a Fellowship from the Ministerio de Educacion y Ciencia. The authors thank Dr Juan Carlos Garcia-Pagan and Dr Raul Mendez for helpful discussions.
References
- 1.Bosch J, Pizcueta P, Feu F, Fernandez M, Garcia-Pagan JC. Pathophysiology of portal hypertension. Gastroenterol Clin North Am. 1992;21:1–13. [PubMed] [Google Scholar]
- 2.Fernandez M, Vizzutti F, Garcia-Pagan JC, Rodes J, Bosch J. Anti-VEGF receptor-2 monoclonal antibody prevents portal-systemic collateral vessel formation in portal hypertensive mice. Gastroenterology. 2004;126:886–94. doi: 10.1053/j.gastro.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 3.Fernandez M, Mejias M, Angermayr B, Garcia-Pagan JC, Rodes J, Bosch J. Inhibition of VEGF receptor-2 decreases the development of hyperdynamic splanchnic circulation and portal-systemic collateral vessels in portal hypertensive rats. J Hepatol. 2005;43:98–103. doi: 10.1016/j.jhep.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 4.Fernandez M, Mejias M, Garcia-Pras E, Mendez R, Garcia-Pagan JC, Bosch J. Reversal of portal hypertension and hyper-dynamic splanchnic circulation by combined vascular endothelial growth factor and platelet-derived growth factor blockade in rats. Hepatology. 2007;46:1208–17. doi: 10.1002/hep.21785. [DOI] [PubMed] [Google Scholar]
- 5.Patel YC. Somatostatin and its receptor family. Front Neuroendocrinol. 1999;20:157–98. doi: 10.1006/frne.1999.0183. [DOI] [PubMed] [Google Scholar]
- 6.Abraldes JG, Bosch J. Somatostatin and analogues in portal hypertension. Hepatology. 2002;35:1305–12. doi: 10.1053/jhep.2002.33469. [DOI] [PubMed] [Google Scholar]
- 7.Woltering EA, Barrie R, O'Dorisio TM, Arce D, Ure T, Cramer A, Holmes D, Robertson J, Fassler J. Somatostatin ana-logues inhibit angiogenesis in the chick chorioallantoic membrane. J Surg Res. 1991;50:245–51. doi: 10.1016/0022-4804(91)90186-p. [DOI] [PubMed] [Google Scholar]
- 8.Danesi R, Agen C, Benelli U, Paolo AD, Nardini D, Bocci G, Basolo F, Campagni A, Tacca MD. Inhibition of experimental angiogenesis by the somatostatin analogue octreotide acetate (SMS 201–995) Clin Cancer Res. 1997;3:265–72. [PubMed] [Google Scholar]
- 9.Dasgupta P. Somatostatin analogues: Multiples roles in cellular proliferation, neoplasia, and angiogenesis. Pharmacol Ther. 2004;102:61–85. doi: 10.1016/j.pharmthera.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 10.Woltering EA, Watson JC, Alperin-Lea RC, Sharma C, Keenan E, Kurozawa D, Barrie R. Somatostatin analogs: angiogen-esis inhibitors with novel mechanisms of action. Invest New Drugs. 1997;15:77–86. doi: 10.1023/a:1005774713202. [DOI] [PubMed] [Google Scholar]
- 11.Lamberts SW, De Herder WW, Hofland LJ. Somatostatin analogs in the diagnosis and treatment of cancer. Trends Endocrinol Metab. 2002;13:451–7. doi: 10.1016/s1043-2760(02)00667-7. [DOI] [PubMed] [Google Scholar]
- 12.Woltering EA. Development of targeted somatostatin-based antiangiogenic therapy: a review and future perspectives. Cancer Biother Radiopharm. 2003;18:601–9. doi: 10.1089/108497803322287691. [DOI] [PubMed] [Google Scholar]
- 13.Raynor K, Murphy WA, Coy DH, Taylor JE, Moreau JP, Yasuda K, Bell GI, Reisine T. Cloned somatostatin receptors: identification of subtype-selective peptides and demonstration of high affinity binding of linear peptides. Mol Pharmacol. 1993;43:838–44. [PubMed] [Google Scholar]
- 14.Fernandez M, Garcia-Pagan JC, Casadevall M, Bernadich C, Piera C, Whittle BJ, Pique JM, Bosch J, Rodes J. Evidence against a role for inducible nitric oxide synthase in the hyperdynamic circulation of portal hypertensive rats. Gastroenterology. 1995;108:1487–95. doi: 10.1016/0016-5085(95)90698-3. [DOI] [PubMed] [Google Scholar]
- 15.Fernandez M, Garcia-Pagan JC, Casadevall M, Mourelle MI, Pique JM, Bosch J, Rodes J. Acute and chronic cyclooxygenase blockade in portal-hypertensive rats: influence on nitric oxide biosynthesis. Gastroenterology. 1996;110:1529–35. doi: 10.1053/gast.1996.v110.pm8613060. [DOI] [PubMed] [Google Scholar]
- 16.Buscail L, Saint-Laurent N, Chastre E, Vaillant JC, Gespach C, Capella G, Kalthoff H, Lluis F, Vaysse N, Susini C. Loss of sst2 somatostatin receptor gene expression in human pancreatic and col-orectal cancer. Cancer Res. 1996;56:1823–7. [PubMed] [Google Scholar]
- 17.Reubi J, Horisberger U, Essed CE, Jeekel J, Klijn JG, Lamberts SW. Absence of somatostatin receptors in human exocrine pancreatic adenocarcinomas. Gastro-enterology. 1988;95:760–3. doi: 10.1016/s0016-5085(88)80025-8. [DOI] [PubMed] [Google Scholar]
- 18.Lambooij AC, Kuijpers RWAM, Van Lichtenauer-Kaligis EGR, Kliffen M, Baarsma GS, Van Hagen PM, Mooy CM. Somatostatin receptor 2A expression in choroidal neovascularization secondary to age-related macular degeneration. Invest Ophthalmol Vis Sci. 2000;41:2329–35. [PubMed] [Google Scholar]
- 19.Fernandez M, Bonkovsky HL. Increased heme oxygenase-1 gene expression in liver cells and splanchnic organs from portal hypertensive rats. Hepatology. 1999;29:1672–9. doi: 10.1002/hep.510290621. [DOI] [PubMed] [Google Scholar]
- 20.Fernandez M, Lambrecht RW, Bonkovsky HL. Increased heme oxygenase activity in splanchnic organs from portal hypertensive rats: role in modulating mesenteric vascular reactivity. J Hepatol. 2001;34:812–7. doi: 10.1016/s0168-8278(01)00010-1. [DOI] [PubMed] [Google Scholar]
- 21.Lambrecht RW, Fernandez M, Shan Y, Bonkovsky HL. Heme oxygenase in portal hypertension and liver disease. In: Gines P, Arroyo V, Rodes J, Schrier RW, editors. Ascites and Renal Dysfunction in Liver Disease. MA: Blackwell Publishing Malden; 2005. pp. 125–36. [Google Scholar]
- 22.Angermayr B, Mejias M, Gracia-Sancho J, Garcia-Pagan JC, Bosch J, Fernandez M. Heme oxygenase attenuates oxidative stress and inflammation, and increases VEGF expression in portal hypertensive rats. J Hepatol. 2006;44:1033–9. doi: 10.1016/j.jhep.2005.09.021. [DOI] [PubMed] [Google Scholar]
- 23.Angermayr B, Fernandez M, Mejias M, Gracia-Sancho J, Garcia-Pagan JC, Bosch J. NAD(P)H oxidase modulates angiogenesis and the development of por-tosystemic collaterals and splanchnic hyperaemia in portal hypertensive rats. Gut. 2007;56:560–4. doi: 10.1136/gut.2005.088013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abbasoglu SD, Erbil Y, Eren T, Giris M, Barbaros U, Yucel R, Olgac V, Uysal M, Toker G. The effects of heme oxygenase-1 induction by octreotide on radiation enteritis. Peptides. 2006;27:1570–6. doi: 10.1016/j.peptides.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 25.Erbil Y, Giris M, Abbasoglu SD, Barbaros U, Yanik BT, Necefli A, Olgac V, Toker GA. Effect of heme oxygenase-1 induction by octreotide on TNBS-induced colitis. J Gastroenterol Hepatol. 2007;22:1852–8. doi: 10.1111/j.1440-1746.2007.04838.x. [DOI] [PubMed] [Google Scholar]
- 26.Paran D, Paran H. Somatostatin analogs in rheumatoid arthritis and other inflammatory and immune-mediated condi-tions. Curr Opin Invest Drugs. 2003;4:578–82. [PubMed] [Google Scholar]
- 27.Yang YY, Lin HC, Huang YT, Lee TY, Lee WC, Hou MC, Lee FY, Chang FY, Lee SD. Adaptive vasodilatory response after octreotide treatment. Am J Physiol Gastrointest Liver Physiol. 2001;281:G117–23. doi: 10.1152/ajpgi.2001.281.1.G117. [DOI] [PubMed] [Google Scholar]
- 28.Elbirt KK, Bonkovsky HL. Heme oxyge-nase: recent advances in understanding its regulation and role. Proc Assoc Am Physicians. 1999;111:438–47. [PubMed] [Google Scholar]
- 29.Fernandez M, Bonkovsky HL. Vascular endothelial growth factor increases heme oxygenase-1 protein expression in the chick embryo chorioallantoic membrane. Br J Pharmacol. 2003;139:634–40. doi: 10.1038/sj.bjp.0705272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cirera I, Feu F, Luca A, Garcia-Pagan JC, Fernandez M, Escorsell A, Bosch J, Rodes J. Effects of bolus injections and continuous infusions of somatostatin and placebo in patients with cirrhosis: a double-blind hemodynamic investigation. Hepatology. 1995;22:106–11. [PubMed] [Google Scholar]


