Abstract
High density lipoprotein particles (HDL) transport, among other molecules, cholesterol (HDL-C). In epidemiologic studies, plasma HDL-C levels have an inverse relationship to the risk of atherosclerotic cardiovascular disease (CVD). It has been assumed that this reflects the protective functions of HDL, which include their ability to promote cholesterol efflux. Yet, a number of recent pharmacological and genetic studies have failed to demonstrate that increased plasma levels of HDL-C resulted in decreased CVD risk, giving rise to a controversy over whether plasma levels of HDL-C reflect HDL function, or that HDL is even as protective as assumed. On balance, the evidence from pre-clinical and (limited) clinical studies show that HDL can promote the regression of atherosclerosis when the levels of functional particles are increased from endogenous or exogenous sources. The data show that regression results from a combination of reduced plaque lipid and macrophage contents, as well as from a reduction in its inflammatory state. While more research will be needed on basic mechanisms and to establish that these changes translate clinically to reduced CVD events, that HDL can regress plaques suggests that the recent trial failures do not eliminate HDL from consideration as an atheroprotective agent, but emphasizes the important distinction between HDL function and plasma levels of HDL-C.
Keywords: HDL, atherosclerosis, regression, mouse, coronary artery disease
Introduction
High density lipoproteins (HDL) are typically defined as lipoprotein particles with buoyant densities from 1.063–1.21 (g/mL) and having apoAI as the major apolipoprotein species. It has become increasingly appreciated that the HDL population consists of a collection of particles with diverse sizes, structures, and composition, and functional properties that are thought to influence athero-protectiveness. The compositional complexity reflects not only multiple species of proteins and lipids, but also other macromolecules (e.g., micro RNA). Historically, the HDL component of greatest clinical interest has been the cholesterol content (HDL-C), which consists of both the free and the more predominant ester species, because of the many epidemiologic studies demonstrating a strong negative correlation between plasma HDL-C and the risk of cardiovascular disease (e.g., 1–4).
The mechanism for this association has been presumed to be that the plasma level of HDL-C reflects the availability of functional HDL particles with atheroprotective actions, particularly the stimulation of reverse cholesterol transport (RCT) from peripheral cells (including foam cells in coronary plaques) to the liver. Based on relatively small clinical trials or in vitro studies, there is also support for the idea that HDL can protect the endothelium (by activation of the eNOS pathway), inhibit LDL oxidation, and exert anti-inflammatory and anti-thrombotic effects 5–8. The trend of the recently evolving data, however, does not establish tight associations between plasma levels of HDL-C and either these functions or, more significantly from a clinical perspective, cardiovascular disease (CVD) risk. For example, the in vitro ability of plasma samples to promote cholesterol efflux was better than HDL-C as a predictor for angiographically proven coronary artery disease 9, and for genetic polymorphisms that were associated with changes in HDL-C, there were no corresponding variations in CVD risk 10.
Indeed, these types of studies and the failure of a number of drugs that, among other effects, raise plasma HDL-C without reducing CVD risk (e.g., 11, 12) have fueled the skepticism that plasma levels of HDL-C reflect any cardio-protective functions of HDL particles or that, more fundamentally, that HDL has cardio-protective functions. As we 13 and others (including in the present review series) have argued, however, it is important to appreciate the distinction between HDL functions and plasma levels of HDL-C, as well as to consider the evidence from a number of pre-clinical and clinical studies that support atheroprotection by HDL if the number of functional particles is increased. The focus of the present review will be on these studies, though to put them in context, we will also discuss some clinical reports that have contributed to the “HDL controversy”.
Our particular research interest has been in the area of the regression of atherosclerosis by HDL, which will be highlighted. Table 1 summarizes a number of the relevant pre-clinical and clinical studies on this point, but space does not allow for a comprehensive discussion of all of them, and still others, in the sections below, so the interested reader is encouraged to consult the cited references and other sources for more comprehensive information.
Table 1.
Selected HDL and apoA-I regression studies
| Author | Species | Approach/drug | Dosage | Administration | Plaque site |
Main findings in plaques |
|---|---|---|---|---|---|---|
| Badimon et al. 1990 22 |
Rabbit (New Zealandwhite rabbits) |
HDL-VHDL | 50 mg | i.v., weekly over 4 weeks |
Total aorta surface |
Extent of fatty streaks ↓ Aortic lipid accumulation (TC, FC and PL) ↓ |
| Parolini et al. 2008 84 |
Rabbit (New Zealandwhite rabbits) |
apoA-IMilano (ETC-216) |
1) 5 mg/kg 2) 10 mg/kg 3) 20 mg/kg 4) 40 mg/kg 5) 150 mg/kg |
5 i.v. injections, every 4 days |
Carotid arteries (assessed by IVUS and MRI) |
Atheroma volume ↓ with 3 highest dosages Significant regression after 2nd administration of 150 mg/kg |
| Shah et al. 2001 32 |
Mouse (apoE−/−) |
apoA-IMilano | 400 mg/kg | Single i.v. injection |
Aortic root (48 hours post injection) |
Lipid content ↓ Macrophage content ↓ |
| Cho et al. 2009 85 |
Mouse (apoE−/−) |
1) rHDL 2) V156K-rHDL (V156K point mutant of apoA-I) 3) R173C-rHDL (apoA-IMilano) |
120 mg/kg | Single i.v. injection |
Aortic root (24 and 48 hours post injection) |
Lipid content: rHDL↓, V156K ↓↓, R173C ↓↓ Macrophage content: rHDL ↓, V156K ↓↓, R173C ↓↓ |
| Feig et al. 2011 31 |
Mouse (apoE−/−) |
Aortic arch transplant into apoE−/− mice transgenic for human apoA-I |
Aortic arch (7 days post transplant) |
Plaque size ↓ Macrophage content ↓ M1 macrophages ↓ M2 macrophages ↑ CCR7 ↑ in plaque macrophages |
||
| Rayner et al. 2012 52 |
Mouse (LDLr−/−) |
Anti-miR33 | 10 mg/kg | 1st week: 2 s.c. injections, followed by weekly injections |
Aortic root (after 4 weeks of treatment) |
Plaque size ↓ Macrophage content ↓ Lipid content ↓ Collagen content ↑ M1 macrophages ↓ M2 macrophages ↑ |
| Nissen et al. 2003 58 |
Human (ACS) |
apoA-IMilano (ETC 216) |
1) 15 mg/kg 2) 45 mg/kg |
i.v., weekly over 5 weeks |
Coronary artery (assessed by IVUS) |
Combined rHDL groups vs. baseline (Median): - Change in total atheroma volume - 13.3 mm3 (p<0.001) - Change in atheroma volume: −4.2% - Change in percent atheroma volume (PAV): −0.81% (p=0.02) |
| Tardif et al. 2007 (ERASE) 59 |
Human (ACS) |
rHDL (CSL-111) | 1) 40 mg/kg 2) 80 mg/kg (80 mg/kg dosage discontinued due to liver function test abnormalities) |
i.v., weekly over 4 weeks |
Coronary artery (assessed by IVUS) |
rHDL vs. placebo group (Median): - Change in total plaque volume: − 5.3 mm3 vs. −2.3 mm3 (n.s.) - Change in atheroma volume: − 3.4% vs. −1.6% (n.s.) - Significant improvement of plaque characterization index and coronary score |
| Shaw et al. 2008 60 |
Human (PAD) |
rHDL (CSL-111) | 80 mg/kg | i.v. | SFA (after atherectomy 5–7 days post infusion) |
Lipid content ↓ Macrophages cell size ↓ VCAM-1 expression ↓ |
ACS = acute coronary syndrome; Anti-miR = anti-microRNA; ApoA-I = apolipoprotein A-I; apoE = apolipoprotein E; CCR7 = C-C chemokine receptor type 7; ERASE = Effect of rHDL on Atherosclerosis - Safety and Efficacy; FC = free cholesterol; HDL = high-density lipoprotein; i.v. = intravenous; IVUS = intravascular ultrasound; LDLr = low-density lipoprotein receptor; n.s.= non significant; MRI = magnetic resonance imaging; PAD = peripheral artery disease; PAV = percent atheroma volume; PL = phospholipids; rHDL = reconstituted high-density lipoprotein; s.c. = subcutaneous; SFA = superficial femoral artery; TC = total cholesterol; VCAM-1 = vascular cell adhesion molecule 1; VHDL = very-high-density lipoprotein.
HDL and atherosclerosis regression: pre-clinical models
That atheromata can regress at all is a concept that has met resistance over the years. The reason for this may have been that advanced atherosclerotic lesions in humans and in animal models contain calcification and fibrosis, characteristics that seem irreversible 14, 15. Nonetheless, a number of studies beginning over 50 years ago argue that this is not the case. For example, the first interventional study demonstrating substantial shrinkage of atherosclerotic lesions was performed in cholesterol-fed rabbits 16. Animals received intravenous bolus injections of phospholipid (PL). After less than a week and a half of treatment, the remaining plaques were fewer and smaller, with approximately 75% of the arterial cholesterol stores being removed. The basis of this effect was explored in subsequent mechanistic studies (reviewed in 17), which indicated that when intravenously injected at sufficient doses, initially cholesterol-free PL vesicles remained intact in the bloodstream and were capable of extracting cholesterol from lipoproteins, particularly HDL. Thus, these circulating particles act as a sink for cholesterol, which is shuttled to them from tissues by HDL and lipid-poor apoAI. Because the liver serves as the predominant organ for the clearance of PL vesicles, the antiatherogenic effects of these particles likely result from their ability to act as synthetic mediators of RCT from peripheral tissues to the liver.
Using a variety of atherosclerotic animal models, including monkeys, other groups showed similar arterial benefits from a variety of interventions, including, again, the injection of dispersed PLs, as well as from dietary changes, and treatment with hypolipidemic agents (e.g., 16, 18,19, 20,21). Most relevant to the present review, studies in cholesterol-fed rabbits demonstrated shrinkage or delayed progression of atheromata after injections of HDL 22 or apoAI 23, respectively.
The availability of appropriate mouse models would allow more convenient and mechanistic investigations of atherosclerosis regression and the effects of HDL and apoAI. Murine HDL metabolism, however, has three major differences compared to that in humans: HDL, not LDL, is the principal carrier of circulating cholesterol in mouse plasma, mouse HDL is a mono-disperse population (i.e., without HDL2 and HDL3 sub-divisions), and the activity of cholesterol-ester transfer protein (CETP), which in humans serves in the plasma to trade cholesteryl ester carried on HDL for triglycerides on the apoB-containing lipoproteins VLDL and LDL, is absent 7, 8. To make mouse HDL metabolism more human-relevant, some investigators have introduced a human apoAI transgene, which results in more disperse HDL particles from which mouse apoAI appears to be displaced (reviewed in 24). Others have also genetically modified mice with a transgene for human CETP 24.
Another limitation of the mouse for atherosclerosis research is that it is naturally resistant to the disease. To overcome this, mice with deficiency in either apoE (apoE−/−) or the LDL receptor (Ldlr−/−) were created, providing robust models of hypercholesterolemia and atherosclerosis 25. Notably, there are a number of similarities between atherosclerosis pathology in humans and mice, though the latter do not exhibit the plaque rupture. Early studies of atherosclerosis in mice included the demonstration of the delayed progression of plaques upon the increased hepatic expression of apoAI (using a human apoAI transgene; hAI) in apoE−/− mice 26, 27. More recently, mouse models of atherosclerosis have been used for regression studies as well. In the present review, we will emphasize these pre-clinical studies having a focus on apoAI or HDL particles, and not on HDL-C per se.
Hepatic production of human apoAI (hAI) was increased by adenoviral vectors after plaques began to form in a number of studies. In 1999 Daniel Rader and colleagues reported that in Lldr−/− mice this resulted in an ~3X increase in plasma levels of apoAI (vs. control virus injected mice) and 70% and 46% reductions in atherosclerosis lesion area measured by aortic en face analysis or by aortic root plaque cross-sectional area, respectively28. The mice had been fed the atherogenic diet, however, for only 5 weeks before the viral treatments began, meaning that the regression induced by increasing the production of hAI was of early “fatty streak”-like plaques. Using a similar approach, Lawrence Chan and colleagues used an adenoviral vector expressing hAI in Ldlr−/− mice fed an atherogenic diet, but for a considerably longer period than the previous study (36 weeks)29. In contrast to the early lesions in Ldlr−/− mice, hAI expression was no longer sufficient to produce regression of lesion size. One factor underlying the differences between the effects of hAI expression in the two studies is that as plaques advance in mouse models or in humans, the % occupied by macrophages decreases. Thus, if these cells are major beneficiaries of raising the number of functional HDL particles, then the impact on lesion size would be attenuated in advanced plaques. Note that there could still be an improvement in plaque stability if an advanced plaque had its composition altered to become macrophage-poor and collagen-rich, as we have found in apoE−/−mice after raising plasma hAI levels (30, 31 and discussed further below).
In another approach to increasing the number of functional HDL particles, PK Shah and colleagues reported in 2001 the results of studies in which a high dose of recombinant hAI (the milano variant; complexed to PC) was given to apoE−/− mice fed an atherogenic diet for 26 weeks to develop advanced plaques 32. Dramatically, a single intravenous bolus of the hAI preparation resulted in reductions by 48 h in plaque lipid and macrophage contents of up to 50% and 36%, respectively.
In 2001 we reported a new mouse model 33 in which rapid changes in the plasma lipoprotein profile could be made and sustained indefinitely. The initial approach was to transplant either an atherosclerotic thoracic 33 or an aortic arch segment 34 from hypercholesterolemic apoE−/− donor mice to normolipidemic wild-type (WT) recipient mice. As in the Shah study, regression was rapidly apparent (as judged by plaque content of CD68+ monocyte derived cells, which are primarily macrophages), with a 50% reduction by 3 days after transfer 34–37. Notably, the quantitative change in macrophage content was associated with emigration of CD68+ cells from plaques to regional and systemic lymph nodes under regression, but not progression, conditions 35, 37. By analyzing RNA obtained from laser-captured plaque CD68+ cells, we found an increase in the gene expression of the chemokine receptor CCR7, a factor previously shown to be required for the migration of dendritic cells (which like macrophages are primarily monocyte derived38), and only in the regression environment 37. Furthermore, we went on to show a substantial functional contribution of CCR7 for regression in this model 37.
In the WT recipients, relative to the donor mice, non-HDL-C levels decreased and HDL-C levels were restored from ~33% of normal to WT levels. Importantly, the differences in HDL-C levels between WT and apoE−/− mice reflect their respective plasma levels of apoAI, and hence, the number of HDL and lipid-poor apoAI particles. To selectively test the effects of the changes in these levels on plaque regression, we now used as recipients hAI transgenic/apoE−/− (hAI/EKO) mice 30, 39, which, as noted above, have increased hepatic production of apoAI and suppressed atherosclerosis progression, despite persistent non-HDL hypercholesterolemia 26, 27.
Remarkably, despite the persistent non-HDL hypercholesterolemia in hAI/apoEKO recipients, plaque CD68+ cell content decreased by >50% by one week after transplantation, whereas there was little change in apoAI−/− recipient mice despite hypolipidemia, indicating a need for functional HDL particles. As in the WT recipient study, the decreased content of plaque CD68+ cells in the hAI/apoEKO recipients was associated with their emigration and induction of their CCR7 31. One molecular mechanism for the induction of CCR7 in plaque macrophages by increasing functional HDL is that following cholesterol depletion of the plaque 31, there is stimulation of CCR7 transcription in the macrophages through the sterol response element (SRE) in the murine and human gene promoters 40. Because HDL can also decrease the circulating pool of monocytes 41 as well as the expression of monocyte adhesion factors expressed by endothelial cells in hypercholesterolemic mice (e.g., 42), it is likely that ongoing monocyte recruitment is also decreased, which would be expected to contribute to plaque regression 43.
An increasingly recognized goal of atherosclerosis treatment is the resolution of the plaque inflammatory state (e.g., see 44), which can be accomplished not only by a decrease in the content of activated macrophages, but also by an enrichment in macrophages with anti-inflammatory properties. Based on primarily studies in vitro, activated and anti-inflammatory/tissue-repairing macrophages have been characterized by their differential expression levels of a number of molecules, and have been broadly classified as M1 and M2, respectively (e.g., 45). Using such markers, macrophages with characteristics of the M1 or M2 state have been found in both human and murine atherosclerotic plaques (e.g., 46, 47).
As noted earlier, HDL is thought to contribute to athero-protection as an anti-inflammatory, through, for example, anti-oxidant properties of its enzymatic and non-enzymatic components, the ability to remove normal and toxic lipid species from cells, and the dampening of TLR signaling by regulating plasma membrane cholesterol content 7, 48, 49. To probe whether the anti-inflammatory effects HDL included changes in the balance between M1 and M2 macrophages, markers of these states were assessed in plaques in donor (i.e., apoE−/− mice after 16 weeks of an atherogenic diet) and in recipient (hAI/EKO) mice. The increases in plasma levels of apoAI and HDL particles were associated with decreased expression of inflammatory factors and enrichment of M2 markers 31, 50. Furthermore, by using an inhibitor of miR-33, which increases lipid-poor apoAI production by the liver and enhances ABCA1/G1-mediated cholesterol efflux from macrophages 51, in collaboration with Kathryn Moore we found similar results as in the transplant model; i.e., decreased macrophage content in plaques in Ldlr−/− mice, as well as reduced and increased M1 and M2 marker expression, respectively 52.
Recently, the balance between the two macrophage states has been therapeutically manipulated and effects on atherosclerosis progression measured. Ldlr−/− mice were treated with M2 polarizing factors, which, consistent with the favorable association of the M2 state in regression, attenuated atherosclerosis progression53, 54. How HDL contributes to a reduction in M1 or an enhancement in M2 polarization may depend on a number of its properties. As noted above, there is growing evidence that it or apoAI may limit TLR responsiveness to inflammatory stimuli (by regulating plasma membrane cholesterol content and micro-environments), and more recent evidence from our group that it can promote the phosphorylation of STAT6, an integral signaling component of the M2 polarization pathway31, 48, 49, 55–57.
In summary, the pre-clinical data convincingly demonstrate the ability of functional HDL and lipid-poor apoAI particles to promote the regression of atherosclerosis by effects on the number and the inflammatory state of plaque macrophages. A diagrammatic summary of the current pre-clinical understanding of how HDL accomplishes this is given in Figure 1. The availability of an expanding variety of mouse models of regression will allow deeper studies of the associated mechanisms, and will likely inform the human biology.
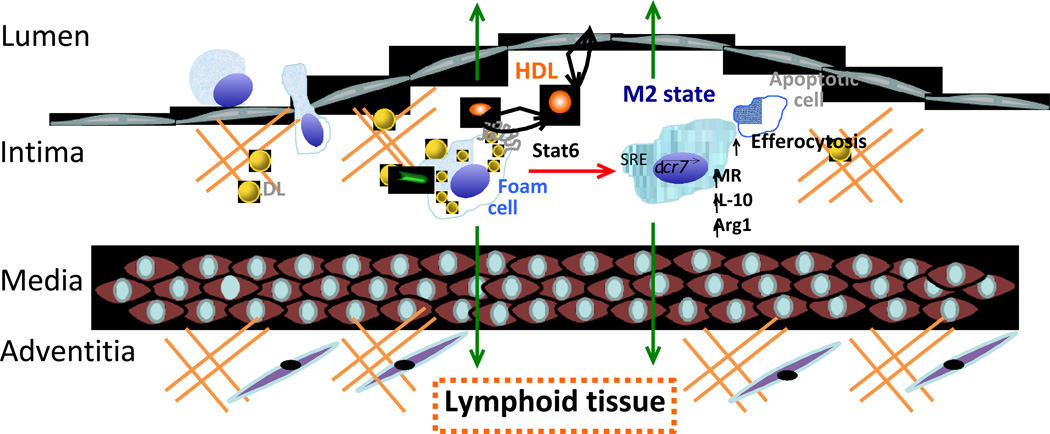
Figure 1. The promotion of atherosclerosis regression by HDL in an aortic transplantation mouse model.
Monocytes are recruited into plaques and become macrophages. These macrophages become activated, cholesterol-laden foam cells, as a result of ingesting normal and modified apoB-containing lipoproteins, and are retained in the plaque. Based on in vitro and pre-clinical studies, recently recognized ways in which HDL can contribute to plaque regression include 1) reduced monocyte recruitment because of reduced leukocytosis or endothelial cell adhesion molecule expression, 2) the stimulation of CCR7 expression by the promotion of cholesterol efflux from foam cells, which results in emigration of macrophages to lymphoid tissue and to the systemic circulation, and, 3) the stimulation of the STAT6 pathway to polarize macrophages to the M2 state, as indicated by the increase in the markers mannose receptor (MR), IL-10, and arginase I (Arg I). As tissue repair cells, M2 macrophages also exhibit enhanced efferocytosis (disposal) of apoptotic cells. See text for details and for additional mechanisms.
HDL and Atherosclerosis Regression: Clinical Studies
In addition to pre-clinical studies, there are a limited number of clinical studies in which plasma levels of HDL particles have been manipulated and the effects on plaques assessed (a partial summary is in Table 1). For example58, patients at high risk for cardiovascular disease were infused with either an artificial form of HDL (apoAI milano/phospholipid complexes) or saline (placebo) once a week for 5 weeks. By intravascular ultrasound (IVUS), there was a significant reduction in atheroma volume (−4.2%) in the combined (high and low dose) treatment group, though no enhancement by the higher dose was observed. In the second infusion study (ERASE), high-risk patients received 4 weekly infusions with reconstituted HDL (rHDL; containing wild type apoAI) or saline (placebo) 59. Similar to the previous study, there was a significant decrease in atheroma volume (−3.4%) (as assessed by IVUS) after treatment with rHDL compared to baseline, but not compared to placebo (a comparison for which the study was not powered). However, the rHDL group had statistically significant improvements in a plaque characterization index and in a coronary stenosis score on quantitative coronary angiography compared to the placebo group. In the third infusion trial 60, a single dose of reconstituted human HDL was infused into patients undergoing femoral atherectomies, with the procedure performed 5–7 days later. Compared to the control group (receiving saline solution), in the excised plaque samples in the HDL infusion group, macrophage activation state (e.g., diminished VCAM-1 expression) as well as cell size (due to diminished lipid content) were reduced, consistent with the results from the pre-clinical studies reviewed above.
HDL-C and Atherosclerosis Regression: The Clinical Controversy
In contrast to the small number of clinical studies on increasing the number of HDL particles, there is a larger literature on the effects on plaques and CVD risk of pharmacologic manipulations that raise HDL-C. There have been 2 major such strategies, niacin, and more recently, CETP inhibition. The presumption has been that the increases in HDL-C would reflect the actions of an increased supply of functional HDL and lipid-poor apoAI particles, which would be expected to benefit plaque size, composition, and CVD risk. We will consider each agent in in turn.
Niacin modestly raises plasma levels of HDL-C and lowers those of LDL-C, triglycerides and Lp(a) through mechanisms that remain largely undefined (see 61for a recent review). Its effect on CVD risk has been studied for decades, but we will confine our survey to those most relevant to atherosclerosis regression. Niacin’s pleiotropic effects on plasma lipids/lipoproteins and its frequent use with other lipid-lowering agents, however, makes it difficult to attribute its effects in most clinical studies solely to changes in HDL metabolism.
The major clinical studies on niacin that have included effects on plaques are (oldest first): the Familial Atherosclerosis Treatment Study (FATS), the Cholesterol-Lowering Atherosclerosis Study (CLAS), the HDL-Atherosclerosis Treatment Study (HATS), and the Arterial Biology for the Investigation of Treatment Effects of Reducing Cholesterol (ARBITER) series of studies. The patient population sizes in all were small (120–162 patients). In FATS 62, patients with documented coronary artery disease (CAD) were randomly assigned to treatment groups: Niacin and the bile acid resin colestipol; the statin lovastatin alone; colestipol alone; or placebo. After 2.5 y, HDL-C in the niacin-colestipol group increased by 43% and was associated with angiographic atherosclerotic regression in 39%. There was also an associated significant outcome benefit with a 73% reduction in clinical events (death, myocardial infarction or revascularization). In CLAS 63, placebo or niacin and colestipol was given to patients with known CAD. Repeat angiography 4 y later showed significantly more patients with non-progression (52% vs. 15%) and regression (18% vs. 6%) in the treatment vs. the placebo group. In HATS 64, niacin-simvastatin alone or together with anti-oxidant vitamin therapy or placebo were given to patients with coronary artery disease. At 3 y follow-up, niacin-simvastatin was associated with significant regression of coronary stenosis and a combined 90% reduction in major clinical events (including death from coronary causes, nonfatal myocardial infarction, stroke or revascularization for worsening angina). Finally, in ARBITER 2 65, once daily extended-release niacin with and without statin therapy was given to patients with coronary artery disease. At 1 y, mean carotid intimal-medial thickness, a controversial surrogate marker of coronary plaque burden, increased significantly in the statin alone group, but was unchanged in the niacin-statin group. Because of these successes, the lack of efficacy of niacin to reduce CV events in the much larger AIM-HIGH (3,414 subjects; 11) and HPS2-Thrive (25,673 patients; 66) was an unexpected surprise.
There have been a number of speculations for these disappointing results, but perhaps the most commonly voiced explanation has been that in both studies, the concurrent use of statins to lower LDL-C aggressively (to the 60 mg/dL range) made showing an additional benefit of raising HDL-C a challenge. Furthermore, the increase in HDL-C was small in both studies (6–7 mg/dL). Another interpretation is that improvements in plaque size or characteristics may not translate into reduced event rates, though the few small-scale studies that have included imaging do not supply sufficient data to definitively assess this possibility.
Alternatively, increases in plasma HDL-C may not necessarily affect plaque biology if HDL’s functions, such as its effectiveness to mediate RCT, are not consistently reflected by plasma levels of HDL-C. Indeed, a number of pre-clinical studies have shown disconnections between plasma HDL-C and the level of RCT and atheroprotection (e.g., in SR-BI transgenic mice 67). This alternative has been also borne out in one relatively small clinical study in which the in vitro efficacy of patients’ apoB-lipoprotein-depleted serum (i.e., enriched in HDL) was correlated with CVD risk, but not plasma levels of HDL-C 9. The mechanistic bases for the “disconnect” between plasma levels of HDL-C and HDL function are discussed below in the section “Dysfunctional apoAI and HDL.”
Turning to CETP, its activity to transfer cholesterol form HDL to apoB-lipoproteins in plasma means that the route back to the liver of cholesterol effluxed to HDL has two pathways-one direct with HDL docking with hepatic SR-BI and unloading its cholesteryl ester, and the other indirect, via apoB-lipoprotein uptake by the hepatic LDL receptor. If CETP is blocked, not only is the direct pathway favored, there is also accumulation of HDL particles in the plasma compartment because they tend to be larger, thereby delaying their clearance, which is inverse to HDL size. This is in contrast to the infusion studies in mice, rabbits, and humans, as well as in the hAI transgenic mice, in which increased HDL-C is a result of supplying more HDL particles by exogenous or endogenous means. Still, as proposed by Alan Tall and others (e.g.,68), CETP inhibition may still lead to the entry of increased numbers of HDL particles into the artery wall where they can act as acceptors of macrophage cholesterol.
In the Japanese population loss-of-function mutations of CETP have been associated in some families with very high levels of HDL-C (>60 mg/dL) and reduced rates of cardiovascular disease 69. In other families, however, particularly those with lower levels of HDL-C, the risk was elevated. The potential requirement for very high levels of HDL-C to be achieved (presumably reflecting a sufficiently expanded plasma pool of efflux-competent HDL particles) may explain the recent failure of the CETP inhibitor (CETPi) dalcetrapib to reduce cardiovascular events in the dal-OUTCOMES trial 12.
Another CETPi, torcetrapib, which potently raises plasma levels of HDL-C and apoAI, and significantly lowers that of LDL-C, was also judged a failure in the ILLUMINATE trial 70. It has been presumed that this involved off-target actions of the compound, as there were elevations in blood pressure and serum potassium, presumably from aldosterone stimulation. CETPi partisans, nevertheless, were encouraged by the posthoc analysis that showed that treated subjects achieving the greatest increases of plasma levels of HDL-C or apoAI had both evidence of atherosclerosis regression on IVUS and also a lower rate of major cardiovascular events 71, 72.
There are 2 other CETPi’s in clinical trials, anacetrapib and evacetrapib. Like torcetrapib, both significantly raise plasma levels of HDL-C and lower LDL-C. Unlike torcetrapib, neither has exhibited adverse effects on blood pressure or serum potassium 73, 74. Completion of the phase III studies (anacetrapib: REVEAL; evacetrapib: ACCELERATE) is expected to provide definitive answers to the question of whether CETP inhibition is an effective strategy to reduce cardiovascular events. Whether the increase in the type of HDL particles produced by CETP inhibition will promote the regression of atherosclerosis will not be addressed, however, because there are no imaging studies included in these trials. Even if there were, the effects of the significant reduction in LDL-C, which itself can lead to regression 75, will be hard to separate out from those of the increase in HDL-C. In fact, it is generally challenging to establish the clinical relationships among HDL function, HDL-C levels, plaque size/composition, and cardiovascular risk, given that all of these are not simultaneously assessed in large-scale intervention or observational studies, and that detecting CVD risk change is not in the time-frame of short-term intervention studies, such as those with apoAI or HDL infusions.
Dysfunctional apoAI and HDL: mechanistic considerations
Although apoAI is the major protein constituent of HDL particles, they are not functionally equivalent, as exemplified by the preferences of the cholesterol efflux factors for one or the other (ABCA1: lipid-poor apoAI; SR-BI and ABCG1: HDL; 76). Lipid-poor apoAI (pre-beta HDL on gels; e.g., 77) constitutes ~5% of plasma apoAI , and may be derived either from its primary secretion by liver or intestine, or released from chylomicrons, VLDL, or HDL upon lipoprotein remodeling. The majority of cholesterol released from macrophages in mouse models is apoAI-dependent (e.g., 78), indicating that lipid-poor apoAI may have an exceptionally important role in RCT. In contrast, lipid-poor apoAI does not possess several of the endothelial cell protective and anti-inflammatory activities of HDL that are mediated by binding to SR-BI (e.g., 79–81).
Multiple studies have shown that apoAI recovered from the human artery wall exhibits extensive post translational modifications through oxidative processes, particularly those mediated by myeloperoxidase (MPO) and nitric oxide derived oxidants (reviewed recently in 82). Further, ex vivo modification of apoAI to a comparable extent by the MPO pathway markedly inhibits cholesterol efflux and LCAT activity of the lipoprotein. MPO oxidation of HDL not only causes it to lose its endothelial cell protective effects, it also gains a proinflammatory activity, inducing endothelial cell adhesion molecule expression 81.
In a recent study 83, we examined both the function and distribution of apoAI recovered from normal and atherosclerotic human arterial tissues. Remarkably, both the function and distribution (HDL particle association) of apoAI recovered from human arterial tissues were markedly different from those observed in plasma. Specifically, in contrast to what is observed in plasma, the overwhelming majority of apoAI (>95%) within both normal and atherosclerotic human arterial tissue was found to be predominantly lipid-poor and not to reside on an HDL particle. Further, the majority of apoAI within arterial tissues was found to be extensively oxidized and cross-linked. In addition, apoAI recovered from human aorta was found to be “dysfunctional”, with 80–90% reductions in cholesterol efflux activity and ability to activate LCAT when incorporated into reconstituted HDL particles. Finally, examination of the relatively lipid-poor fraction of apoAI in the circulation was found to be substantially more oxidatively cross-linked than the apoAI recovered in circulating HDL. These results collectively suggest that in addition to the plasma level of HDL-C not necessarily being functionally relevant, even studies that focus on biological activities of apoAI recovered from plasma or serum HDL may not reflect the biology of apoAI within the artery wall.
Conclusions
The cardioprotective effects of HDL were initially suggested by the strong inverse relationship between plasma HDL-C levels and CVD risk in observational studies. It was assumed that the levels reflected the efficacy of HDL particles to efflux cholesterol from macrophage foam cells in atherosclerotic plaques, as well as other athero-protective functions. More recently, a number of pharmacological and genetic studies have raised the questions of whether HDL-C is a reliable biomarker of HDL functionality, and in a further erosion of the “HDL hypothesis”, whether HDL function itself is important, especially once plaques advance significantly or LDL-C is sufficiently lowered. This controversy has obscured the pre-clinical and human studies to date that have generally shown that when the levels of functional HDL particles are increased, either by stimulating endogenous production of (lipid-poor) apoAI or by providing HDL or apoAI exogenously, regressive changes in plaques result that would be expected to translate to the reduction in CVD risk.
Going forward, this clinical translation remains to be rigorously established by incorporating outcome and imaging data within large-scale, sufficiently powered, studies. Also remaining is the unraveling at progressively deeper levels the mechanistic bases for the beneficial effects of apoAI and HDL on plaque size, composition, and inflammatory state, and how their modifications can impair these effects. Despite the incompleteness of our current clinical and pre-clinical knowledge, if further investigations continue to support the power of HDL to favorably modify plaque biology, rather than abandon the “HDL hypothesis” entirely as a therapeutic strategy, a more prudent approach would be to shift the target of simply raising HDL-C to that of increasing the supply of functional HDL particles or the intrinsic functions through other means.
Supplementary Material
Acknowledgments
We thank Kathryn Moore for helpful discussions.
Sources of Funding
This authors’ HDL-related research was supported by NIH grants HL-084312, HL-098055 (EAF, JDS, SLH), NIH fellowship AG-029748 (JEF), and fellowship from the German Research Foundation (DFG: HE 6092/1-1)(BH).
Non-standard Abbreviations and Acronyms
- ACS
acute coronary syndrome
- Anti-miR
anti-microRNA
- apoAI
apolipoprotein AI
- apoB
apolipoprotein B
- apoE
apolipoprotein E
- apoE−/−
apolipoprotein E deficient
- arg I
arginase I
- CAD
coronary artery disease
- CCR7
C-C chemokine receptor type 7
- CETPi
cholesteryl ester transfer protein inhibitor
- CVD
cardiovascular disease
- FC
free cholesterol
- g/mL
grams per milliliter
- hAI
human apolipoprotein AI
- hAI/EKO
human apolipoprotein AI transgenic-apolipoprotein E knock out
- HDL
high density lipoproteins
- HDL-C
high density lipoprotein cholesterol
- i.v.
intravenous
- IVUS
intravascular ultrasound
- LDL
low density lipoprotein
- LDL-C
low density lipoprotein cholesterol
- Ldlr
low-density lipoprotein receptor
- Ldlr−/−
low density lipoprotein receptor deficient
- Lp(a)
lipoprotein (a)
- MPO
myeloperoxidase
- MR
mannose receptor
- MRI
magnetic resonance imaging
- PAD
peripheral artery disease
- PAV
percent atheroma volume
- PL
phospholipids
- RCT
reverse cholesterol transport
- rHDL
reconstituted high-density lipoprotein
- s.c.
subcutaneous
- SFA
superficial femoral artery
- SRE
sterol response element
- TC
total cholesterol
- TLR
toll-like receptor
- VCAM-1
vascular cell adhesion molecule 1
- VHDL
very-high-density lipoprotein
- WT
wild type.
Footnotes
Disclosures
Drs. Hazen and Smith report being listed as coinventor on pending and issued patents held by the Cleveland Clinic relating to cardiovascular diagnostics. Dr. Hazen reports having been paid as a consultant for the following companies: AstraZeneca Pharmaceuticals LP, Cleveland Heart Laboratory, Esperion, Lilly, Liposcience Inc, Merck & Co, Inc, Pfizer Inc, and Takeda. Dr. Hazen reports receiving research funds from Abbott, Cleveland Heart Laboratory, and Liposcience Inc. Dr. Smith reports having the right to receive royalty payments for inventions or discoveries related to cardiovascular diagnostics from Cleveland Heart Laboratory and being paid as a consultant for Esperion. Dr. Hazen reports having the right to receive royalty payments for inventions or discoveries related to cardiovascular diagnostics and the companies shown below: Cleveland Heart Laboratory, Frantz Biomarkers, LLC, Liposcience Inc, and Siemens. Dr. Fisher reports that he is a member of the Merck Atherosclerosis Global Advisory Board. The other authors report no disclosures.
References
- 1.Castelli WP, Garrison RJ, Wilson PW, Abbott RD, Kalousdian S, Kannel WB. Incidence of coronary heart disease and lipoprotein cholesterol levels. The framingham study. Jama. 1986;256:2835–2838. [PubMed] [Google Scholar]
- 2.Franceschini G. Epidemiologic evidence for high-density lipoprotein cholesterol as a risk factor for coronary artery disease. Am J Cardiol. 2001;88:9N–13N. doi: 10.1016/s0002-9149(01)02146-4. [DOI] [PubMed] [Google Scholar]
- 3.Gordon DJ, Rifkind BM. High-density lipoprotein--the clinical implications of recent studies. N Engl J Med. 1989;321:1311–1316. doi: 10.1056/NEJM198911093211907. [DOI] [PubMed] [Google Scholar]
- 4.Miller NE, Miller GJ. Letter: High-density lipoprotein and atherosclerosis. Lancet. 1975;1:1033. doi: 10.1016/s0140-6736(75)91977-7. [DOI] [PubMed] [Google Scholar]
- 5.Banka CL. High density lipoprotein and lipoprotein oxidation. Curr Opin Lipidol. 1996;7:139–142. doi: 10.1097/00041433-199606000-00005. [DOI] [PubMed] [Google Scholar]
- 6.Fielding CJ, Fielding PE. Molecular physiology of reverse cholesterol transport. J Lipid Res. 1995;36:211–228. [PubMed] [Google Scholar]
- 7.Feig JE, Shamir R, Fisher EA. Atheroprotective effects of hdl: Beyond reverse cholesterol transport. Curr Drug Targets. 2008;9:196–203. doi: 10.2174/138945008783755557. [DOI] [PubMed] [Google Scholar]
- 8.Stein Y, Stein O. Does therapeutic intervention achieve slowing of progression or bona fide regression of atherosclerotic lesions? Arterioscler Thromb Vasc Biol. 2001;21:183–188. doi: 10.1161/01.atv.21.2.183. [DOI] [PubMed] [Google Scholar]
- 9.Khera AV, Cuchel M, de la Llera-Moya M, Rodrigues A, Burke MF, Jafri K, French BC, Phillips JA, Mucksavage ML, Wilensky RL, Mohler ER, Rothblat GH, Rader DJ. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med. 2011;364:127–135. doi: 10.1056/NEJMoa1001689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Voight BF, Peloso GM, Orho-Melander M, Frikke-Schmidt R, Barbalic M, Jensen MK, Hindy G, Holm H, Ding EL, Johnson T, Schunkert H, Samani NJ, Clarke R, Hopewell JC, Thompson JF, Li M, Thorleifsson G, Newton-Cheh C, Musunuru K, Pirruccello JP, Saleheen D, Chen L, Stewart A, Schillert A, Thorsteinsdottir U, Thorgeirsson G, Anand S, Engert JC, Morgan T, Spertus J, Stoll M, Berger K, Martinelli N, Girelli D, McKeown PP, Patterson CC, Epstein SE, Devaney J, Burnett MS, Mooser V, Ripatti S, Surakka I, Nieminen MS, Sinisalo J, Lokki ML, Perola M, Havulinna A, de Faire U, Gigante B, Ingelsson E, Zeller T, Wild P, de Bakker PI, Klungel OH, Maitland-van der Zee AH, Peters BJ, de Boer A, Grobbee DE, Kamphuisen PW, Deneer VH, Elbers CC, Onland-Moret NC, Hofker MH, Wijmenga C, Verschuren WM, Boer JM, van der Schouw YT, Rasheed A, Frossard P, Demissie S, Willer C, Do R, Ordovas JM, Abecasis GR, Boehnke M, Mohlke KL, Daly MJ, Guiducci C, Burtt NP, Surti A, Gonzalez E, Purcell S, Gabriel S, Marrugat J, Peden J, Erdmann J, Diemert P, Willenborg C, Konig IR, Fischer M, Hengstenberg C, Ziegler A, Buysschaert I, Lambrechts D, Van de Werf F, Fox KA, El Mokhtari NE, Rubin D, Schrezenmeir J, Schreiber S, Schafer A, Danesh J, Blankenberg S, Roberts R, McPherson R, Watkins H, Hall AS, Overvad K, Rimm E, Boerwinkle E, Tybjaerg-Hansen A, Cupples LA, Reilly MP, Melander O, Mannucci PM, Ardissino D, Siscovick D, Elosua R, Stefansson K, O'Donnell CJ, Salomaa V, Rader DJ, Peltonen L, Schwartz SM, Altshuler D, Kathiresan S. Plasma hdl cholesterol and risk of myocardial infarction: A mendelian randomisation study. Lancet. 2012;380:572–580. doi: 10.1016/S0140-6736(12)60312-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boden WE, Probstfield JL, Anderson T, Chaitman BR, Desvignes-Nickens P, Koprowicz K, McBride R, Teo K, Weintraub W. Niacin in patients with low hdl cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365:2255–2267. doi: 10.1056/NEJMoa1107579. [DOI] [PubMed] [Google Scholar]
- 12.Schwartz GG, Olsson AG, Abt M, Ballantyne CM, Barter PJ, Brumm J, Chaitman BR, Holme IM, Kallend D, Leiter LA, Leitersdorf E, McMurray JJ, Mundl H, Nicholls SJ, Shah PK, Tardif JC, Wright RS. Effects of dalcetrapib in patients with a recent acute coronary syndrome. N Engl J Med. 2012;367:2089–2099. doi: 10.1056/NEJMoa1206797. [DOI] [PubMed] [Google Scholar]
- 13.Hewing B, Moore KJ, Fisher EA. Hdl and cardiovascular risk: Time to call the plumber? Circ Res. 2012;111:1117–1120. doi: 10.1161/CIRCRESAHA.112.280958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Williams KJ, Feig JE, Fisher EA. Cellular and molecular mechanisms for rapid regression of atherosclerosis: From bench top to potentially achievable clinical goal. Curr Opin Lipidol. 2007;18:443–450. doi: 10.1097/MOL.0b013e32823bcb15. [DOI] [PubMed] [Google Scholar]
- 15.Williams KJ, Feig JE, Fisher EA. Rapid regression of atherosclerosis: Insights from the clinical and experimental literature. Nat Clin Pract Cardiovasc Med. 2008;5:91–102. doi: 10.1038/ncpcardio1086. [DOI] [PubMed] [Google Scholar]
- 16.Friedman M, Byers SO, Rosenman RH. Resolution of aortic atherosclerotic infiltration in the rabbit by phosphatide infusion. Proc Soc Exp Biol Med. 1957;95:586–588. doi: 10.3181/00379727-95-23300. [DOI] [PubMed] [Google Scholar]
- 17.Rodrigueza WV, Mazany KD, Essenburg AD, Pape ME, Rea TJ, Bisgaier CL, Williams KJ. Large versus small unilamellar vesicles mediate reverse cholesterol transport in vivo into two distinct hepatic metabolic pools. Implications for the treatment of atherosclerosis. Arterioscler Thromb Vasc Biol. 1997;17:2132–2139. doi: 10.1161/01.atv.17.10.2132. [DOI] [PubMed] [Google Scholar]
- 18.Williams KJ, Werth VP, Wolff JA. Intravenously administered lecithin liposomes: A synthetic antiatherogenic lipid particle. Perspect Biol Med. 1984;27:417–431. doi: 10.1353/pbm.1984.0031. [DOI] [PubMed] [Google Scholar]
- 19.Armstrong ML, Warner ED, Connor WE. Regression of coronary atheromatosis in rhesus monkeys. Circ Res. 1970;27:59–67. doi: 10.1161/01.res.27.1.59. [DOI] [PubMed] [Google Scholar]
- 20.Armstrong ML. Evidence of regression of atherosclerosis in primates and man. Postgrad Med J. 1976;52:456–461. doi: 10.1136/pgmj.52.609.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wissler RW, Vesselinovitch D. Studies of regression of advanced atherosclerosis in experimental animals and man. Ann N Y Acad Sci. 1976;275:363–378. doi: 10.1111/j.1749-6632.1976.tb43368.x. [DOI] [PubMed] [Google Scholar]
- 22.Badimon JJ, Badimon L, Fuster V. Regression of atherosclerotic lesions by high density lipoprotein plasma fraction in the cholesterol-fed rabbit. J Clin Invest. 1990;85:1234–1241. doi: 10.1172/JCI114558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miyazaki A, Sakuma S, Morikawa W, Takiue T, Miake F, Terano T, Sakai M, Hakamata H, Sakamoto Y, Natio M, et al. Intravenous injection of rabbit apolipoprotein a-i inhibits the progression of atherosclerosis in cholesterol-fed rabbits. Arterioscler Thromb Vasc Biol. 1995;15:1882–1888. doi: 10.1161/01.atv.15.11.1882. [DOI] [PubMed] [Google Scholar]
- 24.Breslow JL. Transgenic mouse models of lipoprotein metabolism and atherosclerosis. Proc Natl Acad Sci U S A. 1993;90:8314–8318. doi: 10.1073/pnas.90.18.8314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Breslow JL. Mouse models of atherosclerosis. Science. 1996;272:685–688. doi: 10.1126/science.272.5262.685. [DOI] [PubMed] [Google Scholar]
- 26.Plump AS, Scott CJ, Breslow JL. Human apolipoprotein a-i gene expression increases high density lipoprotein and suppresses atherosclerosis in the apolipoprotein e-deficient mouse. Proc Natl Acad Sci U S A. 1994;91:9607–9611. doi: 10.1073/pnas.91.20.9607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paszty C, Maeda N, Verstuyft J, Rubin EM. Apolipoprotein ai transgene corrects apolipoprotein e deficiency-induced atherosclerosis in mice. J Clin Invest. 1994;94:899–903. doi: 10.1172/JCI117412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tangirala RK, Tsukamoto K, Chun SH, Usher D, Pure E, Rader DJ. Regression of atherosclerosis induced by liver-directed gene transfer of apolipoprotein a-i in mice. Circulation. 1999;100:1816–1822. doi: 10.1161/01.cir.100.17.1816. [DOI] [PubMed] [Google Scholar]
- 29.Li R, Chao H, Ko KW, Cormier S, Dieker C, Nour EA, Wang S, Chan L, Oka K. Gene therapy targeting ldl cholesterol but not hdl cholesterol induces regression of advanced atherosclerosis in a mouse model of familial hypercholesterolemia. Journal of genetic syndrome & gene therapy. 2011;2:106. doi: 10.4172/2157-7412.1000106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rong JX, Li J, Reis ED, Choudhury RP, Dansky HM, Elmalem VI, Fallon JT, Breslow JL, Fisher EA. Elevating high-density lipoprotein cholesterol in apolipoprotein e-deficient mice remodels advanced atherosclerotic lesions by decreasing macrophage and increasing smooth muscle cell content. Circulation. 2001;104:2447–2452. doi: 10.1161/hc4501.098952. [DOI] [PubMed] [Google Scholar]
- 31.Feig JE, Rong JX, Shamir R, Sanson M, Vengrenyuk Y, Liu J, Rayner K, Moore K, Garabedian M, Fisher EA. Hdl promotes rapid atherosclerosis regression in mice and alters inflammatory properties of plaque monocyte-derived cells. Proc Natl Acad Sci U S A. 2011;108:7166–7171. doi: 10.1073/pnas.1016086108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shah PK, Yano J, Reyes O, Chyu KY, Kaul S, Bisgaier CL, Drake S, Cercek B. High-dose recombinant apolipoprotein a-i(milano) mobilizes tissue cholesterol and rapidly reduces plaque lipid and macrophage content in apolipoprotein e-deficient mice. Potential implications for acute plaque stabilization. Circulation. 2001;103:3047–3050. doi: 10.1161/hc2501.092494. [DOI] [PubMed] [Google Scholar]
- 33.Reis ED, Li J, Fayad ZA, Rong JX, Hansoty D, Aguinaldo JG, Fallon JT, Fisher EA. Dramatic remodeling of advanced atherosclerotic plaques of the apolipoprotein e-deficient mouse in a novel transplantation model. J Vasc Surg. 2001;34:541–547. doi: 10.1067/mva.2001.115963. [DOI] [PubMed] [Google Scholar]
- 34.Chereshnev I, Trogan E, Omerhodzic S, Itskovich V, Aguinaldo JG, Fayad ZA, Fisher EA, Reis ED. Mouse model of heterotopic aortic arch transplantation. J Surg Res. 2003;111:171–176. doi: 10.1016/s0022-4804(03)00039-8. [DOI] [PubMed] [Google Scholar]
- 35.Llodra J, Angeli V, Liu J, Trogan E, Fisher EA, Randolph GJ. Emigration of monocyte-derived cells from atherosclerotic lesions characterizes regressive, but not progressive, plaques. Proc Natl Acad Sci U S A. 2004;101:11779–11784. doi: 10.1073/pnas.0403259101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Trogan E, Fayad ZA, Itskovich VV, Aguinaldo JG, Mani V, Fallon JT, Chereshnev I, Fisher EA. Serial studies of mouse atherosclerosis by in vivo magnetic resonance imaging detect lesion regression after correction of dyslipidemia. Arterioscler Thromb Vasc Biol. 2004;24:1714–1719. doi: 10.1161/01.ATV.0000139313.69015.1c. [DOI] [PubMed] [Google Scholar]
- 37.Trogan E, Feig JE, Dogan S, Rothblat GH, Angeli V, Tacke F, Randolph GJ, Fisher EA. Gene expression changes in foam cells and the role of chemokine receptor ccr7 during atherosclerosis regression in apoe-deficient mice. Proc Natl Acad Sci U S A. 2006;103:3781–3786. doi: 10.1073/pnas.0511043103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Forster R, Schubel A, Breitfeld D, Kremmer E, Renner-Muller I, Wolf E, Lipp M. Ccr7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell. 1999;99:23–33. doi: 10.1016/s0092-8674(00)80059-8. [DOI] [PubMed] [Google Scholar]
- 39.Feig JE, Shamir R, Joaquin V, Grauer L, Fisher EA. Apoai is required for the regression of atherosclerosis and is a potent regulator of plaque monocyte-derived emigration and inflammatory state. Arterioscler Thromb Vasc Biol. 2009;29:e13. [Google Scholar]
- 40.Feig JE, Shang Y, Rotllan N, Vengrenyuk Y, Wu C, Shamir R, Torra IP, Fernandez-Hernando C, Fisher EA, Garabedian MJ. Statins promote the regression of atherosclerosis via activation of the ccr7-dependent emigration pathway in macrophages. PLoS One. 2011;6:e28534. doi: 10.1371/journal.pone.0028534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yvan-Charvet L, Pagler T, Gautier EL, Avagyan S, Siry RL, Han S, Welch CL, Wang N, Randolph GJ, Snoeck HW, Tall AR. Atp-binding cassette transporters and hdl suppress hematopoietic stem cell proliferation. Science. 2010;328:1689–1693. doi: 10.1126/science.1189731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dansky HM, Charlton SA, Barlow CB, Tamminen M, Smith JD, Frank JS, Breslow JL. Apo a-i inhibits foam cell formation in apo e-deficient mice after monocyte adherence to endothelium. J Clin Invest. 1999;104:31–39. doi: 10.1172/JCI6577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Potteaux S, Gautier EL, Hutchison SB, van Rooijen N, Rader DJ, Thomas MJ, Sorci-Thomas MG, Randolph GJ. Suppressed monocyte recruitment drives macrophage removal from atherosclerotic plaques of apoe−/− mice during disease regression. J Clin Invest. 2011;121:2025–2036. doi: 10.1172/JCI43802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tabas I. Macrophage death and defective inflammation resolution in atherosclerosis. Nature reviews. Immunology. 2010;10:36–46. doi: 10.1038/nri2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gordon S, Mantovani A. Diversity and plasticity of mononuclear phagocytes. European journal of immunology. 2011;41:2470–2472. doi: 10.1002/eji.201141988. [DOI] [PubMed] [Google Scholar]
- 46.Bouhlel MA, Derudas B, Rigamonti E, Dievart R, Brozek J, Haulon S, Zawadzki C, Jude B, Torpier G, Marx N, Staels B, Chinetti-Gbaguidi G. Ppargamma activation primes human monocytes into alternative m2 macrophages with anti-inflammatory properties. Cell Metab. 2007;6:137–143. doi: 10.1016/j.cmet.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 47.Khallou-Laschet J, Varthaman A, Fornasa G, Compain C, Gaston AT, Clement M, Dussiot M, Levillain O, Graff-Dubois S, Nicoletti A, Caligiuri G. Macrophage plasticity in experimental atherosclerosis. PLoS One. 2010;5:e8852. doi: 10.1371/journal.pone.0008852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yvan-Charvet L, Welch C, Pagler TA, Ranalletta M, Lamkanfi M, Han S, Ishibashi M, Li R, Wang N, Tall AR. Increased inflammatory gene expression in abc transporter-deficient macrophages: Free cholesterol accumulation, increased signaling via toll-like receptors, and neutrophil infiltration of atherosclerotic lesions. Circulation. 2008;118:1837–1847. doi: 10.1161/CIRCULATIONAHA.108.793869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhu X, Lee JY, Timmins JM, Brown JM, Boudyguina E, Mulya A, Gebre AK, Willingham MC, Hiltbold EM, Mishra N, Maeda N, Parks JS. Increased cellular free cholesterol in macrophage-specific abca1 knock-out mice enhances pro-inflammatory response of macrophages. J Biol Chem. 2008;283:22930–22941. doi: 10.1074/jbc.M801408200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Feig JE, Parathath S, Rong JX, Mick SL, Vengrenyuk Y, Grauer L, Young SG, Fisher EA. Reversal of hyperlipidemia with a genetic switch favorably affects the content and inflammatory state of macrophages in atherosclerotic plaques. Circulation. 2011;123:989–998. doi: 10.1161/CIRCULATIONAHA.110.984146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rayner KJ, Suarez Y, Davalos A, Parathath S, Fitzgerald ML, Tamehiro N, Fisher EA, Moore KJ, Fernandez-Hernando C. Mir-33 contributes to the regulation of cholesterol homeostasis. Science. 2010;328:1570–1573. doi: 10.1126/science.1189862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rayner KJ, Sheedy FJ, Esau CC, Hussain FN, Temel RE, Parathath S, van Gils JM, Rayner AJ, Chang AN, Suarez Y, Fernandez-Hernando C, Fisher EA, Moore KJ. Antagonism of mir-33 in mice promotes reverse cholesterol transport and regression of atherosclerosis. J Clin Invest. 2011;121:2921–2931. doi: 10.1172/JCI57275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cardilo-Reis L, Gruber S, Schreier SM, Drechsler M, Papac-Milicevic N, Weber C, Wagner O, Stangl H, Soehnlein O, Binder CJ. Interleukin-13 protects from atherosclerosis and modulates plaque composition by skewing the macrophage phenotype. EMBO molecular medicine. 2012;4:1072–1086. doi: 10.1002/emmm.201201374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wolfs IM, Stoger JL, Goossens P, Pottgens C, Gijbels MJ, Wijnands E, van der Vorst EP, van Gorp P, Beckers L, Engel D, Biessen EA, Kraal G, van Die I, Donners MM, de Winther MP. Reprogramming macrophages to an anti-inflammatory phenotype by helminth antigens reduces murine atherosclerosis. Faseb J. 2013 doi: 10.1096/fj.13-235911. [DOI] [PubMed] [Google Scholar]
- 55.Smythies LE, White CR, Maheshwari A, Palgunachari MN, Anantharamaiah GM, Chaddha M, Kurundkar AR, Datta G. Apolipoprotein a-i mimetic 4f alters the function of human monocyte-derived macrophages. American journal of physiology. Cell physiology. 2010;298:C1538–C1548. doi: 10.1152/ajpcell.00467.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Odegaard JI, Chawla A. Alternative macrophage activation and metabolism. Annual review of pathology. 2011;6:275–297. doi: 10.1146/annurev-pathol-011110-130138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sanson M, Distel E, Fisher EA. Hdl induces the expression of the m2 macrophage markers arginase 1 and fizz-1 in a stat6-dependent process. PLoS One. 2013;8:e74676. doi: 10.1371/journal.pone.0074676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nissen SE, Tsunoda T, Tuzcu EM, Schoenhagen P, Cooper CJ, Yasin M, Eaton GM, Lauer MA, Sheldon WS, Grines CL, Halpern S, Crowe T, Blankenship JC, Kerensky R. Effect of recombinant apoa-i milano on coronary atherosclerosis in patients with acute coronary syndromes: A randomized controlled trial. Jama. 2003;290:2292–2300. doi: 10.1001/jama.290.17.2292. [DOI] [PubMed] [Google Scholar]
- 59.Tardif JC, Gregoire J, L'Allier PL, Ibrahim R, Lesperance J, Heinonen TM, Kouz S, Berry C, Basser R, Lavoie MA, Guertin MC, Rodes-Cabau J. Effects of reconstituted high-density lipoprotein infusions on coronary atherosclerosis: A randomized controlled trial. Jama. 2007;297:1675–1682. doi: 10.1001/jama.297.15.jpc70004. [DOI] [PubMed] [Google Scholar]
- 60.Shaw JA, Bobik A, Murphy A, Kanellakis P, Blombery P, Mukhamedova N, Woollard K, Lyon S, Sviridov D, Dart AM. Infusion of reconstituted high-density lipoprotein leads to acute changes in human atherosclerotic plaque. Circ Res. 2008;103:1084–1091. doi: 10.1161/CIRCRESAHA.108.182063. [DOI] [PubMed] [Google Scholar]
- 61.Digby JE, Ruparelia N, Choudhury RP. Niacin in cardiovascular disease: Recent preclinical and clinical developments. Arterioscler Thromb Vasc Biol. 2012;32:582–588. doi: 10.1161/ATVBAHA.111.236315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brown G, Albers JJ, Fisher LD, Schaefer SM, Lin JT, Kaplan C, Zhao XQ, Bisson BD, Fitzpatrick VF, Dodge HT. Regression of coronary artery disease as a result of intensive lipid-lowering therapy in men with high levels of apolipoprotein b. N Engl J Med. 1990;323:1289–1298. doi: 10.1056/NEJM199011083231901. [DOI] [PubMed] [Google Scholar]
- 63.Cashin-Hemphill L, Mack WJ, Pogoda JM, Sanmarco ME, Azen SP, Blankenhorn DH. Beneficial effects of colestipol-niacin on coronary atherosclerosis. A 4-year follow-up. Jama. 1990;264:3013–3017. [PubMed] [Google Scholar]
- 64.Brown BG, Zhao XQ, Chait A, Fisher LD, Cheung MC, Morse JS, Dowdy AA, Marino EK, Bolson EL, Alaupovic P, Frohlich J, Albers JJ. Simvastatin and niacin, antioxidant vitamins, or the combination for the prevention of coronary disease. N Engl J Med. 2001;345:1583–1592. doi: 10.1056/NEJMoa011090. [DOI] [PubMed] [Google Scholar]
- 65.Taylor AJ, Sullenberger LE, Lee HJ, Lee JK, Grace KA. Arterial biology for the investigation of the treatment effects of reducing cholesterol (arbiter) 2: A double-blind, placebo-controlled study of extended-release niacin on atherosclerosis progression in secondary prevention patients treated with statins. Circulation. 2004;110:3512–3517. doi: 10.1161/01.CIR.0000148955.19792.8D. [DOI] [PubMed] [Google Scholar]
- 66.Song WL, FitzGerald GA. Niacin, an old drug with a new twist. J Lipid Res. 2013;54:2586–2594. doi: 10.1194/jlr.R040592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Arai T, Wang N, Bezouevski M, Welch C, Tall AR. Decreased atherosclerosis in heterozygous low density lipoprotein receptor-deficient mice expressing the scavenger receptor bi transgene. J Biol Chem. 1999;274:2366–2371. doi: 10.1074/jbc.274.4.2366. [DOI] [PubMed] [Google Scholar]
- 68.Tall AR, Yvan-Charvet L, Terasaka N, Pagler T, Wang N. Hdl, abc transporters, and cholesterol efflux: Implications for the treatment of atherosclerosis. Cell Metab. 2008;7:365–375. doi: 10.1016/j.cmet.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 69.Zhong S, Sharp DS, Grove JS, Bruce C, Yano K, Curb JD, Tall AR. Increased coronary heart disease in japanese-american men with mutation in the cholesteryl ester transfer protein gene despite increased hdl levels. J Clin Invest. 1996;97:2917–2923. doi: 10.1172/JCI118751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Barter PJ, Caulfield M, Eriksson M, Grundy SM, Kastelein JJ, Komajda M, Lopez-Sendon J, Mosca L, Tardif JC, Waters DD, Shear CL, Revkin JH, Buhr KA, Fisher MR, Tall AR, Brewer B. Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med. 2007;357:2109–2122. doi: 10.1056/NEJMoa0706628. [DOI] [PubMed] [Google Scholar]
- 71.Barter P. Lessons learned from the investigation of lipid level management to understand its impact in atherosclerotic events (illuminate) trial. Am J Cardiol. 2009;104:10E–15E. doi: 10.1016/j.amjcard.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 72.Nicholls SJ, Tuzcu EM, Brennan DM, Tardif JC, Nissen SE. Cholesteryl ester transfer protein inhibition, high-density lipoprotein raising, and progression of coronary atherosclerosis: Insights from illustrate (investigation of lipid level management using coronary ultrasound to assess reduction of atherosclerosis by cetp inhibition and hdl elevation) Circulation. 2008;118:2506–2514. doi: 10.1161/CIRCULATIONAHA.108.790733. [DOI] [PubMed] [Google Scholar]
- 73.Cannon CP, Shah S, Dansky HM, Davidson M, Brinton EA, Gotto AM, Stepanavage M, Liu SX, Gibbons P, Ashraf TB, Zafarino J, Mitchel Y, Barter P. Safety of anacetrapib in patients with or at high risk for coronary heart disease. N Engl J Med. 2010;363:2406–2415. doi: 10.1056/NEJMoa1009744. [DOI] [PubMed] [Google Scholar]
- 74.Nicholls SJ, Brewer HB, Kastelein JJ, Krueger KA, Wang MD, Shao M, Hu B, McErlean E, Nissen SE. Effects of the cetp inhibitor evacetrapib administered as monotherapy or in combination with statins on hdl and ldl cholesterol: A randomized controlled trial. Jama. 2011;306:2099–2109. doi: 10.1001/jama.2011.1649. [DOI] [PubMed] [Google Scholar]
- 75.Nicholls SJ, Tuzcu EM, Sipahi I, Grasso AW, Schoenhagen P, Hu T, Wolski K, Crowe T, Desai MY, Hazen SL, Kapadia SR, Nissen SE. Statins, high-density lipoprotein cholesterol, and regression of coronary atherosclerosis. Jama. 2007;297:499–508. doi: 10.1001/jama.297.5.499. [DOI] [PubMed] [Google Scholar]
- 76.Yancey PG, Bortnick AE, Kellner-Weibel G, de la Llera-Moya M, Phillips MC, Rothblat GH. Importance of different pathways of cellular cholesterol efflux. Arterioscler Thromb Vasc Biol. 2003;23:712–719. doi: 10.1161/01.ATV.0000057572.97137.DD. [DOI] [PubMed] [Google Scholar]
- 77.Asztalos BF, Roheim PS, Milani RL, Lefevre M, McNamara JR, Horvath KV, Schaefer EJ. Distribution of apoa-i-containing hdl subpopulations in patients with coronary heart disease. Arterioscler Thromb Vasc Biol. 2000;20:2670–2676. doi: 10.1161/01.atv.20.12.2670. [DOI] [PubMed] [Google Scholar]
- 78.Wang X, Collins HL, Ranalletta M, Fuki IV, Billheimer JT, Rothblat GH, Tall AR, Rader DJ. Macrophage abca1 and abcg1, but not sr-bi, promote macrophage reverse cholesterol transport in vivo. J Clin Invest. 2007;117:2216–2224. doi: 10.1172/JCI32057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Calabresi L, Franceschini G, Sirtori CR, De Palma A, Saresella M, Ferrante P, Taramelli D. Inhibition of vcam-1 expression in endothelial cells by reconstituted high density lipoproteins. Biochemical and biophysical research communications. 1997;238:61–65. doi: 10.1006/bbrc.1997.7236. [DOI] [PubMed] [Google Scholar]
- 80.Mineo C, Shaul PW. Role of high-density lipoprotein and scavenger receptor b type i in the promotion of endothelial repair. Trends Cardiovasc Med. 2007;17:156–161. doi: 10.1016/j.tcm.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 81.Undurti A, Huang Y, Lupica JA, Smith JD, DiDonato JA, Hazen SL. Modification of high density lipoprotein by myeloperoxidase generates a pro-inflammatory particle. J Biol Chem. 2009;284:30825–30835. doi: 10.1074/jbc.M109.047605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fisher EA, Feig JE, Hewing B, Hazen SL, Smith JD. High-density lipoprotein function, dysfunction, and reverse cholesterol transport. Arterioscler Thromb Vasc Biol. 2012;32:2813–2820. doi: 10.1161/ATVBAHA.112.300133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Didonato JA, Huang Y, Aulak KS, Even-Or O, Gerstenecker G, Gogonea V, Wu Y, Fox PL, Tang WH, Plow EF, Smith JD, Fisher EA, Hazen SL. Function and distribution of apolipoprotein a1 in the artery wall are markedly distinct from those in plasma. Circulation. 2013;128:1644–1655. doi: 10.1161/CIRCULATIONAHA.113.002624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Parolini C, Marchesi M, Lorenzon P, Castano M, Balconi E, Miragoli L, Chaabane L, Morisetti A, Lorusso V, Martin BJ, Bisgaier CL, Krause B, Newton RS, Sirtori CR, Chiesa G. Dose-related effects of repeated etc-216 (recombinant apolipoprotein a-i milano/1-palmitoyl-2-oleoyl phosphatidylcholine complexes) administrations on rabbit lipid-rich soft plaques: In vivo assessment by intravascular ultrasound and magnetic resonance imaging. J Am Coll Cardiol. 2008;51:1098–1103. doi: 10.1016/j.jacc.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 85.Cho KH. A reconstituted high density lipoprotein containing the v156e mutant of apolipoprotein a-i exhibits anti-atherosclerotic activity in apo-e deficient mice. J Atheroscler Thromb. 2009;16:217–229. doi: 10.5551/jat.509. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.