Abstract
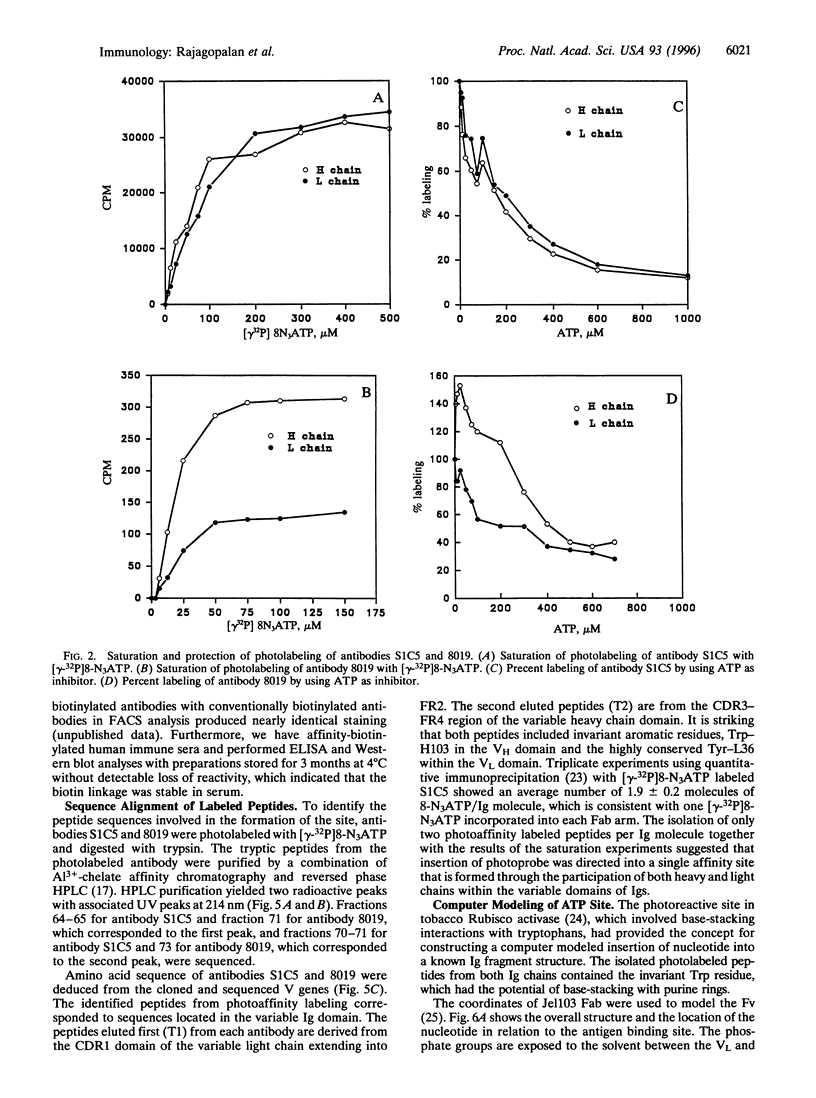
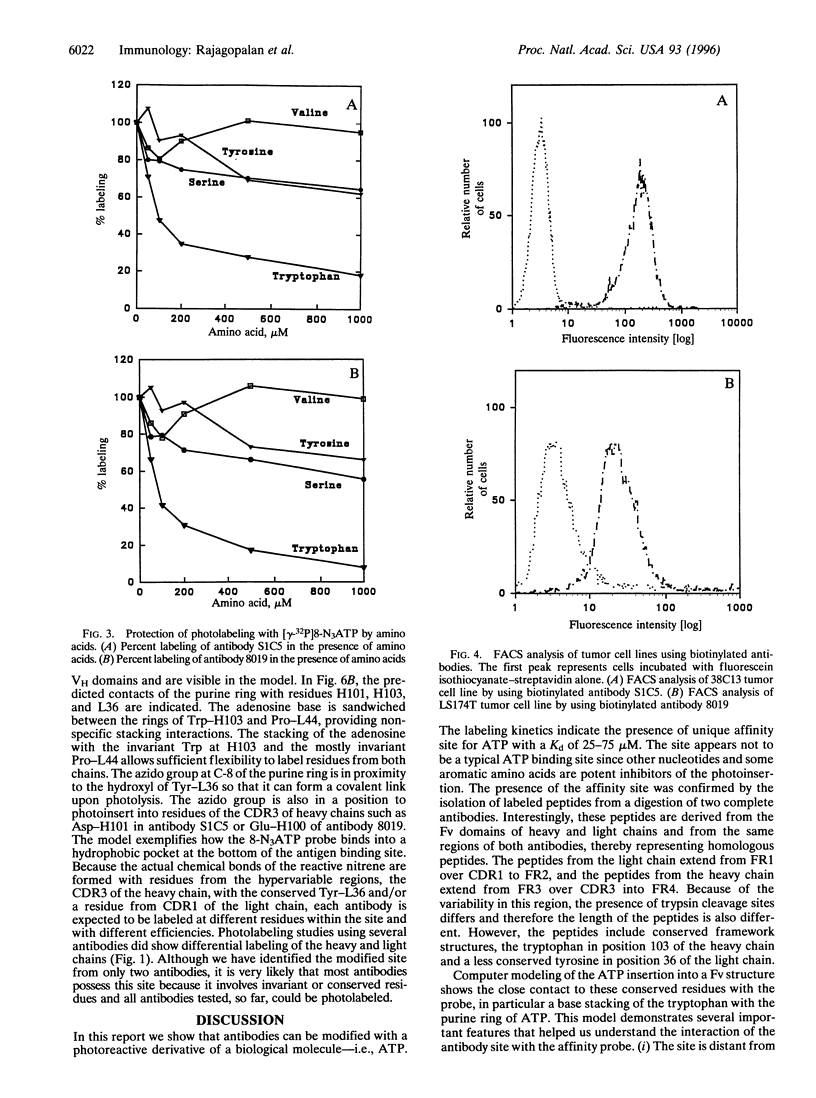
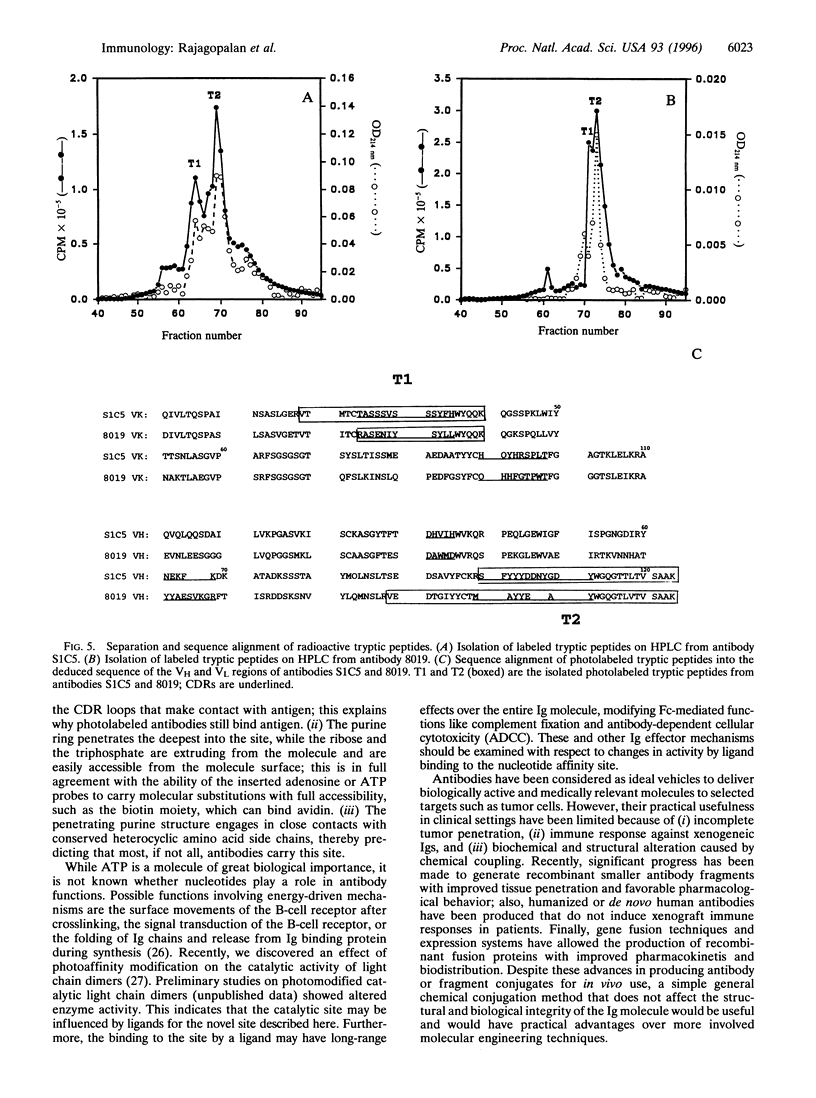
The variable immunoglobulin (Ig) domains contain hypervariable regions that are involved in the formation of the antigen binding site. Besides the canonical antigen binding site, so-called unconventional sites also reside in the variable region that bind bacterial and viral proteins. Docking to these unconventional sites does not typically interfere with antigen binding, which suggests that these sites may be a part of the biological functions of Igs. Herein, a novel unconventional binding site is described. The site is detected with 8-azidopurine nucleotide photoaffinity probes that label antibodies efficiently and under mild conditions. Tryptic peptides were isolated from photolabeled monoclonal antibodies and aligned with the variable antibody domains of heavy and light chains. The structure of a variable Ig fragment was used to model the binding of the purine nucleotide to invariant residues in a hydrophobic pocket of the Ig molecule at a location distant from the antigen binding site. Monoclonal and polyclonal antibodies were biotinylated with the photoaffinity linker and used in fluorescence-activated cell sorter and ELISA analyses. The data support the utility of this site for tethering diagnostic and therapeutic agents to the variable Ig fragment region without impairing the structural and functional integrity of antibodies.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andria M. L., Levy S., Benjamini E. Diverse VH and VL genes are used to produce antibodies against a defined protein epitope. J Immunol. 1990 Apr 1;144(7):2614–2619. [PubMed] [Google Scholar]
- Berberian L., Goodglick L., Kipps T. J., Braun J. Immunoglobulin VH3 gene products: natural ligands for HIV gp120. Science. 1993 Sep 17;261(5128):1588–1591. doi: 10.1126/science.7690497. [DOI] [PubMed] [Google Scholar]
- Bernstein F. C., Koetzle T. F., Williams G. J., Meyer E. F., Jr, Brice M. D., Rodgers J. R., Kennard O., Shimanouchi T., Tasumi M. The Protein Data Bank: a computer-based archival file for macromolecular structures. J Mol Biol. 1977 May 25;112(3):535–542. doi: 10.1016/s0022-2836(77)80200-3. [DOI] [PubMed] [Google Scholar]
- Cleary P., Retnoningrum D. Group A streptococcal immunoglobulin-binding proteins: adhesins, molecular mimicry or sensory proteins? Trends Microbiol. 1994 Apr;2(4):131–136. doi: 10.1016/0966-842x(94)90600-9. [DOI] [PubMed] [Google Scholar]
- Evans S. V. SETOR: hardware-lighted three-dimensional solid model representations of macromolecules. J Mol Graph. 1993 Jun;11(2):134-8, 127-8. doi: 10.1016/0263-7855(93)87009-t. [DOI] [PubMed] [Google Scholar]
- Fields B. A., Goldbaum F. A., Ysern X., Poljak R. J., Mariuzza R. A. Molecular basis of antigen mimicry by an anti-idiotope. Nature. 1995 Apr 20;374(6524):739–742. doi: 10.1038/374739a0. [DOI] [PubMed] [Google Scholar]
- Haas I. G., Wabl M. Immunoglobulin heavy chain binding protein. Nature. 1983 Nov 24;306(5941):387–389. doi: 10.1038/306387a0. [DOI] [PubMed] [Google Scholar]
- Jones T. A., Zou J. Y., Cowan S. W., Kjeldgaard M. Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogr A. 1991 Mar 1;47(Pt 2):110–119. doi: 10.1107/s0108767390010224. [DOI] [PubMed] [Google Scholar]
- Kang C. Y., Brunck T. K., Kieber-Emmons T., Blalock J. E., Kohler H. Inhibition of self-binding antibodies (autobodies) by a VH-derived peptide. Science. 1988 May 20;240(4855):1034–1036. doi: 10.1126/science.3368787. [DOI] [PubMed] [Google Scholar]
- Koprowski H., Steplewski Z., Mitchell K., Herlyn M., Herlyn D., Fuhrer P. Colorectal carcinoma antigens detected by hybridoma antibodies. Somatic Cell Genet. 1979 Nov;5(6):957–971. doi: 10.1007/BF01542654. [DOI] [PubMed] [Google Scholar]
- Lenert P., Kroon D., Spiegelberg H., Golub E. S., Zanetti M. Human CD4 binds immunoglobulins. Science. 1990 Jun 29;248(4963):1639–1643. doi: 10.1126/science.2363051. [DOI] [PubMed] [Google Scholar]
- Maloney D. G., Kaminski M. S., Burowski D., Haimovich J., Levy R. Monoclonal anti-idiotype antibodies against the murine B cell lymphoma 38C13: characterization and use as probes for the biology of the tumor in vivo and in vitro. Hybridoma. 1985 Fall;4(3):191–209. doi: 10.1089/hyb.1985.4.191. [DOI] [PubMed] [Google Scholar]
- Paul S., Li L., Kalaga R., Wilkins-Stevens P., Stevens F. J., Solomon A. Natural catalytic antibodies: peptide-hydrolyzing activities of Bence Jones proteins and VL fragment. J Biol Chem. 1995 Jun 23;270(25):15257–15261. doi: 10.1074/jbc.270.25.15257. [DOI] [PubMed] [Google Scholar]
- Pokkuluri P. R., Bouthillier F., Li Y., Kuderova A., Lee J., Cygler M. Preparation, characterization and crystallization of an antibody Fab fragment that recognizes RNA. Crystal structures of native Fab and three Fab-mononucleotide complexes. J Mol Biol. 1994 Oct 21;243(2):283–297. doi: 10.1006/jmbi.1994.1654. [DOI] [PubMed] [Google Scholar]
- Salvucci M. E., Chavan A. J., Klein R. R., Rajagopolan K., Haley B. E. Photoaffinity labeling of the ATP binding domain of Rubisco activase and a separate domain involved in the activation of ribulose-1,5-bisphosphate carboxylase/oxygenase. Biochemistry. 1994 Dec 13;33(49):14879–14886. doi: 10.1021/bi00253a027. [DOI] [PubMed] [Google Scholar]
- Sasso E. H., Silverman G. J., Mannik M. Human IgM molecules that bind staphylococcal protein A contain VHIII H chains. J Immunol. 1989 Apr 15;142(8):2778–2783. [PubMed] [Google Scholar]
- Shokri F., Mageed R. A., Maziak B. R., Jefferis R. Expression of VHIII-associated cross-reactive idiotype on human B lymphocytes. Association with staphylococcal protein A binding and Staphylococcus aureus Cowan I stimulation. J Immunol. 1991 Feb 1;146(3):936–940. [PubMed] [Google Scholar]
- Silverman G. J., Roben P., Bouvet J. P., Sasano M. Superantigen properties of a human sialoprotein involved in gut-associated immunity. J Clin Invest. 1995 Jul;96(1):417–426. doi: 10.1172/JCI118051. [DOI] [PMC free article] [PubMed] [Google Scholar]