Abstract
Lymphoma-associated hemophagocytic syndrome (LAHS) is a serious disorder, and its early diagnosis and treatment with appropriate chemotherapy are very important. However, reliable markers for early diagnosis of LAHS have not been identified. We screened serum cytokines using a newly introduced assay system, cytometric bead array (CBA), and identified interferon-inducible protein 10 (IP-10)/CXCL10 and monokine induced by interferon gamma (MIG)/CXCL9 as useful markers. Serum concentrations of IP-10 and MIG at the time of LAHS diagnosis were greater than 500 and 5,000 pg/ml, respectively. The sensitivity and specificity for LAHS diagnosis were 100 and 95 %, respectively, when we set the above values as the cut-off levels. Serum levels of these two chemokines were already elevated at the time of admission and significantly decreased after successful treatment, indicating their usefulness for both the diagnosis and therapeutic outcomes for LAHS. IP-10 and MIG were also useful in distinguishing severe from moderate/mild LAHS, and B-cell-type LAHS from T-cell/natural killer cell-type LAHS. Furthermore, IP-10 and MIG were of use to distinguish LAHS from sepsis in patients with hematologic malignancies. Rapid measurement of IP-10 and MIG by CBA appeared to be important for early diagnosis and treatment of LAHS.
Keywords: Lymphoma-associated hemophagocytic syndrome, IP-10, MIG, Cytometric bead array
Introduction
Hemophagocytic syndrome (HPS) is a serious disorder characterized by persistent high-grade fever, hepatosplenomegaly, pancytopenia, abnormal coagulopathy, and hemophagocytosis by macrophages in the bone marrow, spleen, liver, and lymph node [1–3]. The cause of HPS has been supposed to be hyper-activation of macrophages, T cells, and natural killer (NK) cells, hypercytokinemia caused by these activated cells, and further activation of these cells, that is, a malignant loop of excess cell activation and cytokine production [2, 4, 5]. Cytokines involved in HPS include interferon gamma (IFN-γ), macrophage colony-stimulating factor (M-CSF), tumor necrosis factor alpha (TNF-α), and interleukin-6 (IL-6), IL-10, IL-12, and IL-18 [4–10]. HPS mostly develops secondarily to various infections, malignancies including hematological neoplasm, and autoimmune diseases [2, 11].
Among these secondary HPS, the incidence of lymphoma-associated hemophagocytic syndrome (LAHS) is relatively high and its prognosis is poor compared with that of malignant lymphoma without HPS [11–16]. Although early diagnosis and appropriate chemotherapy for LAHS are important, LAHS at an early stage does not always fulfill the diagnostic criteria proposed by the International Histiocyte Society in 2004 (HLH-2004) [3]. In virus-associated hemophagocytic syndrome (VAHS), IFN-γ is a key cytokine that activates macrophages and transforms them into hemophagocytic cells [17–19]. The blood level of IFN-γ is elevated, and consequently, this cytokine could be a diagnostic marker for VAHS if rapid measurement is available. In LAHS, IFN-γ may also play an important role; however, the exact key cytokine in LAHS remains controversial [20–22]. Therefore, it is a pressing need to identify early markers for LAHS. For this purpose, we have screened multiple chemokines/cytokines in the blood of patients with LAHS.
Another problem in identifying these markers is the assay system for cytokines. Sandwich enzyme-linked immunosorbent assay (ELISA) has been a gold standard technique for the measurement of cytokines; however, ELISA has some disadvantages in terms of its employment as a routine laboratory test in a hospital. ELISA is time-consuming and expensive, and requires a large amount of serum. In recent years, a newly developed technique, cytometric bead array (CBA) [23–27], has been introduced in the field of clinical medicine [19, 28–32]. This assay system has resolved the majority of faults of ELISA. CBA has enabled us to screen cytokine markers for LAHS and rapidly obtain the result of multiple cytokine concentrations in a small amount of specimen.
Design and methods
Patients
Fifteen adult lymphoma patients with LAHS and 18 without LAHS were enrolled in the present study from May 2007 to January 2010 in Kobe City Medical Center General Hospital. The present study was a retrospective analysis of a single institutional clinical study designated as “Cytokine Profile in Lymphoma-Associated Hemophagocytic Syndrome,” which had been approved by an institutional review board. Written informed consent was obtained from all patients. The diagnosis of LAHS was made according to the criteria of Tsuda [1] with some modifications: (1) high fever for more than a week; (2) unexplained progressive cytopenia affecting at least two cell lineages; (3) bone marrow showing mature histiocytes comprising more than 3 % of nucleated cells with prominent hemophagocytosis and/or hemophagocytosis in the liver, spleen, or lymph node; and (4) definite diagnosis of malignant lymphoma (modified by us). Of the LAHS patients in the present study, nine had B-cell lymphomas (B-LAHS) and six had T-cell/natural killer cell lymphomas (T/NK-LAHS). As a control, 18 lymphoma patients without LAHS (non-LAHS) were analyzed. Of these patients, 16 had B-cell lymphomas and two T/NK-cell lymphomas. The diagnosis of malignant lymphoma was made according to the 2008 WHO Classification [33]. All patients diagnosed with LAHS during the above mentioned period were enrolled in the present study. Regarding non-LAHS patients, 18 patients admitted during the same period were randomly selected for the examination of the cytokine profile. Patient characteristics and laboratory data are shown in Table 1. Other controls included 20 healthy volunteers and nine sepsis patients with hematologic malignancies (three patients with B-cell type acute lymphoblastic leukemia, three myelodysplastic syndrome, one B-cell lymphoma, one T/NK-cell lymphoma, and one multiple myeloma).
Table 1.
Characteristics of patients and their laboratory data
| LAHS (n = 15) | Non-LAHS (n = 18) | P value | |
|---|---|---|---|
| Underlying NHL | B 9, T/NK 6 | B 16, T/NK 2 | NS |
| (DLBCL 5, IVL 4, AITL 1, ENKL 1, PTCL 4) | (DLBCL 9, FL 5, MCL 2, PTCL 2) | ||
| Age | 21–78 (61) | 50–86 (65) | NS |
| Stage | Stage IV 15 | Stage IV 11, stage I–III 7 | <0.05 |
| IPI | High 14, high-intermediate 1 | High 6, high-intermediate 4 | <0.05 |
| B symptom | (+) 15 | (+) 7, (−) 11 | <0.001 |
| BM invasion | (+) 15 | (+) 11 | <0.05 |
| WBC (×109/L) | 0.4–8.2 (4.5) | 2.1–11.2 (6.65) | <0.005 |
| Hb (g/dL) | 7.5–10.4 (8.3) | 7.7–17.0 (13.7) | <0.001 |
| PLT (×109/L) | 8–142 (56) | 84–499 (203) | <0.001 |
| LDH (IU/L) | 210–4,021 (838) | 109–3,985 (218) | <0.002 |
| sIL-2R (U/mL) | 795–53,900 (13,870) | 149–8,930 (1,385) | <0.001 |
| Ferritin (ng/mL) | 579–36,555 (7,287) | 80–3,038 (1,674) | <0.001 |
Abbreviations: LAHS lymphoma-associated hemophagocytic syndrome, NHL non-Hodgkin’s lymphoma, IPI International Prognostic Index, BM bone marrow, WBC white blood cell, Hb hemoglobin, PLT platelet, LDH lactate dehydrogenase, sIL-2R soluble interleukin-2 receptor, DLBCL diffuse large B-cell lymphoma, IVL intravascular large B-cell lymphoma, AITL angioimmunoblastic T-cell lymphoma, ENKL extranodal NK/T-cell lymphoma, PTCL peripheral T-cell lymphoma, FL follicular lymphoma, MCL mantle cell lymphoma, NS not significant
To determine the staging of lymphomas, a bone marrow aspirate was evaluated for possible lymphoma cell invasion in all lymphoma patients. Regarding the diagnosis of LAHS in the present study, persistent high fever and progressive cytopenia at presentation or on admission prompted us to evaluate the marrow aspirate. As shown in Table 1, abnormal lymphocytes and hemophagocytosis were concomitantly observed. These abnormal lymphocytes were determined as of lymphoid nature by flow cytometry. Because all these patients except for those with intravascular large B-cell lymphoma (IVL; Table 1) had superficial lymphadenopathy at presentation or on admission, a diagnosis of malignant lymphoma and its subtype was established based on pathologic examination of biopsied lymph nodes. Therefore, tentative diagnosis of malignant lymphoma was made based on flow cytometry of bone marrow cells before the final diagnosis by lymph node biopsy in about 50 % of LAHS patients. The final diagnosis of IVL with hemophagocytosis (Table 1) was made based on the presence of lymphoma cells in small vessels in the biopsy specimen of the skin, lung, or bone marrow in addition to the abnormal large lymphocytes in the bone marrow and their phenotype by flow cytometry as described above.
Cytokine assay by CBA
The sera were collected at the time of hospitalization, acute phase, remission after chemotherapy, and follow-up period and kept frozen at −80 °C until analysis. G-CSF, IFN-γ, IL-6, IL-8, IL-10, IP-10, MIG, MIP-1α, and TNF-α in the sera were measured using CBA Flex Set kits (BD Biosciences, San Jose, CA, USA). The minimum detection limits of these kits were 1.6 pg/ml for G-CSF, 0.3 pg/ml for IFN-γ, 1.6 pg/ml for IL-6, 1.2 pg/ml for IL-8, 0.1 pg/ml for IL-10, 0.5 pg/ml for IP-10, 0.2 pg/ml for MIG, 0.2 pg/ml for MIP-1α, and 0.7 pg/ml for TNF-α. The maximum detection limits of all kits were 2,500 pg/ml. When a cytokine concentration exceeded 2,500 pg/ml, diluted sera with an assay diluent were re-assayed. The data were analyzed by FCAP Array Software (BD Biosciences, San Jose, CA, USA).
Statistical analysis
Differences between two or three independent groups were evaluated by Mann–Whitney U test and Kruskal–Wallis tests, followed by Dunn’s multiple-comparison test, respectively. Analysis of multiple paired data was performed using Friedman’s test, followed by Tukey’s multiple comparison test. Rank correlation coefficient was determined using Spearman’s rank correlation coefficient method. All calculations were carried out using the program JMP 8.0 (SAS Institute Inc., Cary, NC, USA). P values of <0.05 were considered to be significant.
Results
Patients with or without LAHS
As shown in Table 1, reflecting severe clinical features, all indicated parameters (clinical stage, International Prognosis Index, B symptom, white blood cell count, hemoglobin concentration, platelet count, lactate dehydrogenase (LDH), concentration of soluble IL-2 receptor (sIL-2R), and ferritin level) except for subtype proportion of underlying lymphomas and patient’s age were significantly different between LAHS and non-LAHS groups. In addition, five of six non-LAHS patients with B symptom had a fever as LAHS patients.
Comparison of serum cytokine concentrations between LAHS, non-LAHS, and normal control groups
As shown in Fig. 1, serum concentrations of IP-10, MIG, IL-6, IL-10, IFN-γ, and MIP-1α in the LAHS group were significantly higher than those in the non-LAHS group. Of interest, concentrations of IP-10 and MIG in the LAHS group were extremely high, at more than 500 and 5,000 pg/ml, respectively. Meanwhile, serum levels of IL-8, TNF-α, and G-CSF did not significantly differ between the LAHS group and the non-LAHS group (data not shown).
Fig. 1.
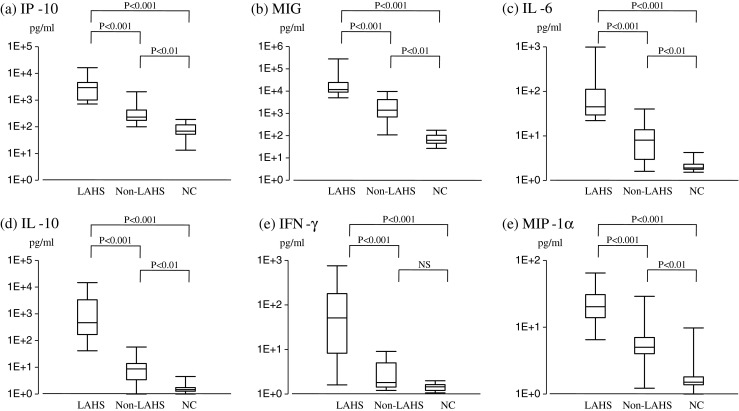
Serum cytokine profile in patients with LAHS, non-LAHS, and healthy controls. a IP-10, b MIG, c IL-6, d IL-10, e IFN-γ, f MIP-1α. NS not significant
Comparison of serum cytokine concentrations between LAHS and non-LAHS at similar clinical conditions
Because aggressive non-LAHS is clinically similar to LAHS, we compared cytokine profile between patients with LAHS and non-LAHS patients with advanced clinical stage (stage IV), high international prognostic index (IPI), B symptom, high LDH levels (more than two times of institutional upper limit; 460 IU/L), and bone marrow involvement of the lymphoma. As shown in Table 2, serum concentrations of IP-10 and MIG in LAHS patients were significantly higher than those in non-LAHS patients with these unfavorable parameters, while concentrations of IFNγ in LAHS patients with high LDH did not significantly differ from those in non-LAHS patients with similar parameter, LDH. These results indicate that high serum levels of IP-10 and MIG are characteristic of lymphoma-associated hemophagocytosis.
Table 2.
Comparison of serum concentrations of IP-10, MIG, IFNγ, and IL-6 between LAHS and non-LAHS in unfavorable clinical conditions
| Parameters | LAHS | Non-LAHS | IP-10: P value | MIG: P value | IFNγ: P value | IL-6 |
|---|---|---|---|---|---|---|
| Stage IV | n = 15 | n = 11 | <0.01 | <0.05 | <0.05 | NS |
| IPI | n = 14 | n = 6 | <0.01 | <0.05 | <0.05 | NS |
| B symptom | n = 15 | n = 7 | <0.01 | <0.05 | <0.05 | NS |
| LDH (>460 IU/L) | n = 10 | n = 4 | <0.05 | <0.05 | NS | NS |
| BM invasion | n = 15 | n = 11 | <0.01 | <0.05 | <0.05 | NS |
Concentrations of IP-10 and MIG in LAHS patients are significantly higher than those in non-LAHS patients. Concentrations of IFNγ in LAHS patients with high LDH did not significantly differ from those in non-LAHS patients with similar parameter, LDH
LAHS lymphoma-associated hemophagocytic syndrome, IPI International Prognostic Index, LDH lactate dehydrogenase, BM bone marrow, NS not significant
Serum concentrations of IP-10 and MIG at the diagnosis of LAHS and after chemotherapy
We then compared serum concentrations of IP-10, MIG, IL-6, IL-10, IFN-γ, and MIP-1α at the diagnosis of LAHS and after chemotherapy. The definition of the time when LAHS was diagnosed was the day when hemophagocytosis was confirmed by bone marrow tap. In addition, all patients with LAHS achieved complete or nearly complete remission after chemotherapy. As shown in Fig. 2, serum concentrations of IP-10, IL-6, IL-10, and MIP-1α, which were measured at the time of admission, were not significantly different when compared with those at the time of the diagnosis of LAHS, indicating that these levels had already reached a high level when the LAHS clinically manifested itself. Another analysis was the comparison of cytokine concentrations at the time of diagnosis with those at the time of remission after successful chemotherapy. As shown in Fig. 2, concentrations of IP-10, MIG, IL-10, and IFN-γ were significantly decreased compared with those at the time of diagnosis of LAHS, indicating that these cytokines are useful as markers of therapeutic outcomes. Serum concentrations of IP-10 and MIG in LAHS patients at the time of remission, however, were still significantly higher (P < 0.001 for both chemokines) than those in normal control presumably due to heterogeneous remission conditions including partial remission or post-remission chemotherapy. We could repeatedly measure serum concentrations of these two chemokines in three patients in complete remission after the completion of chemotherapy and found all results to be within normal levels.
Fig. 2.
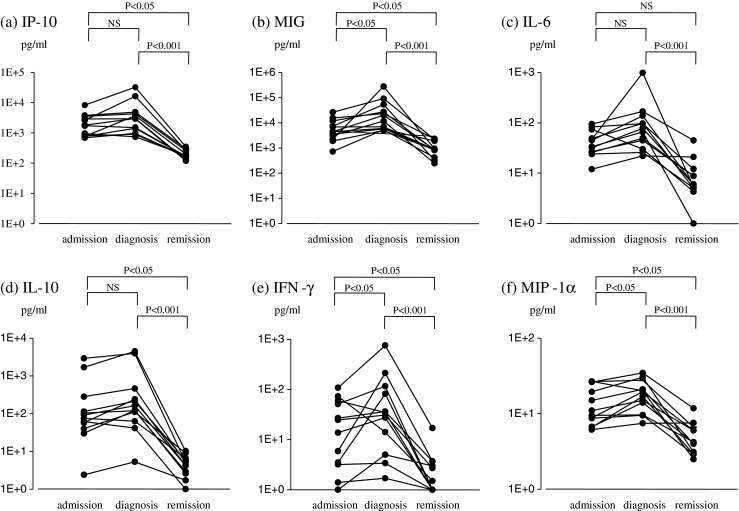
Changes in serum cytokine concentrations in 12 LAHS patients on three occasions: three time points are on admission, at the time of diagnosis, and during the remission of LAHS. The interval between the time of admission and diagnosis is around 3 days. a IP-10, b MIG, c IL-6, d IL-10, e IFN-γ, f MIP-1α. NS not significant
Correlation between serum concentration of IP-10 and MIG
From the findings in the preceding paragraph, we selected IP-10 and MIG as useful markers, and the sensitivity and specificity for the diagnosis of LAHS were 100 and 95 %, respectively, when we set respective values (IP-10 500 pg/ml and MIG 5,000 pg/ml) as the cut-off levels. In addition, we examined the correlation of these two chemokines in LAHS. The concentrations of these two strongly correlated (R = 0.732) in LAHS and non-LAHS patients.
The relationship of cytokine concentrations with severity of LAHS
We classified LAHS into two groups based on the severity of HPS [11]. In this setting, we defined severe LAHS as when a patient fulfilled all the following values and the remaining patients as having moderate/mild LAHS: platelet count: below 65 × 109/l, LDH: more than 750 IU/l, sIL-2R: more than 5,000 U/ml, and ferritin: more than 5,000 ng/ml. Figure 3 shows that only IP-10 and MIG could distinguish severe LAHS from moderate/mild LAHS.
Fig. 3.
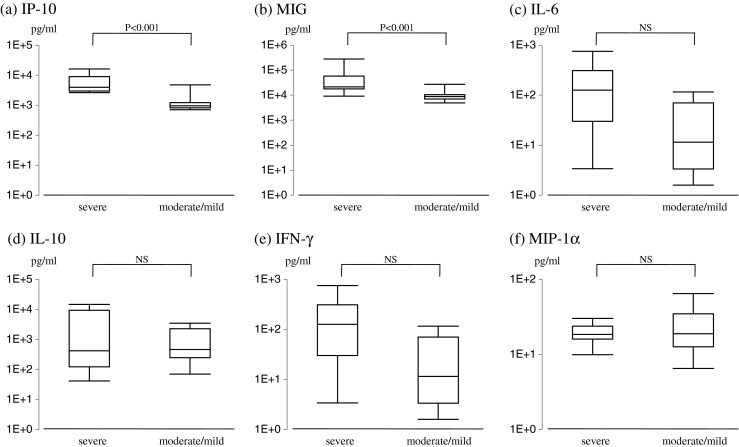
The relationship of serum cytokine levels to the disease severity in LAHS. a IP-10, b MIG, c IL-6, d IL-10, e IFN-γ, f MIP-1α. NS not significant
The difference of cytokine concentrations between B-LAHS and T/NK-LAHS
Next, we analyzed whether or not we can distinguish B-LAHS from T/NK-LAHS using cytokine concentrations. Serum concentrations of IP-10, MIG, and IFN-γ were significantly higher (P = 0.005, 0.005, and 0.05, respectively) in T/NK-LAHS than in B-LAHS, although the level of significance in IFN-γ was low (P = 0.05). Regarding IL-6, IL-10, and MIP-1α, serum levels of these cytokines did not significantly differ between the two groups.
IP-10 and MIG can distinguish LAHS from sepsis
Because of prominent inflammation in sepsis as in LAHS, we analyzed whether we can distinguish LAHS from sepsis by using blood levels of IP-10 and MIG. In this analysis, we compared serum concentrations of IP-10 and MIG in LAHS with those in nine patients with hematologic malignancies, who developed sepsis. As shown in Fig. 4, serum concentrations of IP-10 and MIG in sepsis patients were significantly low compared with those in LAHS (P < 0.001).
Fig. 4.
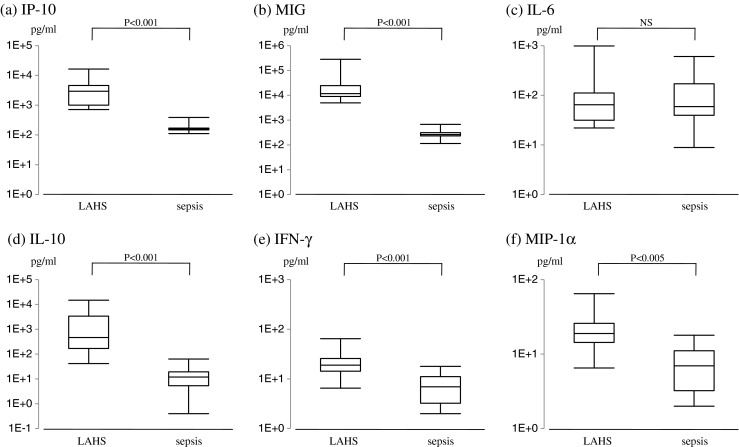
Comparison between serum concentrations of IP-10 and MIG in LAHS patients and those in patients with hematologic malignancies complicated with sepsis. a IP-10, b MIG, c IL-6, d IL-10, e IFN-γ, f MIP-1α. NS not significant
Discussion
In the present study, we identified IP-10 and MIG as being useful markers for both the diagnosis of LAHS and therapeutic outcomes for the following reasons: (1) serum concentrations of these two chemokines at the time of LAHS diagnosis were extremely high compared with those of other examined cytokines; (2) the concentrations of these two chemokines significantly decreased after successful chemotherapy; (3) importantly, the scattering amplitude of values for these two chemokines at three occasions was small (Fig. 2) compared with that in other cytokines, including IFN-γ and IL-10, which varied widely; and (4) the sensitivity and specificity for the diagnosis of LAHS were 100 and 95 %, respectively, when we set the cut-off values of IP-10 (500 pg/ml) and MIG (5,000 pg/ml) at the LAHS diagnosis. Thus, these two chemokines could be reliable markers for both diagnosis of LAHS and its therapeutic outcomes. In the present study, we included five patients with follicular lymphoma (FL) in non-LAHS group that is one of controls to LAHS group. Although FL is an indolent lymphoma, mean serum concentrations of IP-10 (434.2 pg) and MIG (3,221.1 pg) in patients with FL were comparable to those of IP-10 (417.0 pg) and MIG (2,580.1) in patients with non-FL (n = 13), respectively. Therefore, it is unlikely that FL affected cytokine levels of IP-10 and MIG in non-LAHS group because of its indolence.
IP-10 and MIG appeared also to be useful in distinguishing both severe LAHS from moderate/mild LAHS and T/NK-LAHS from B-LAHS. It was interesting that serum levels of these two chemokines in T/NK-LAHS were higher than those in B-LAHS, possibly reflecting higher cytokine production in T/NK neoplasms than in B-cell tumors. Furthermore, these two chemokines were also useful to distinguish LAHS from sepsis in which blood levels of multiple inflammatory cytokines are elevated [34–39]. From the findings described above, both IP-10 and MIG are considered to be specific to many aspects of LAHS.
The major producers of IP-10 and MIG are IFN-γ-activated macrophages [40–42]. Among these activated macrophages, resident macrophages more strongly express IP-10 than those derived from monocytes [43]. Neutrophils produce these two chemokines [44], and dendritic cells and endothelial cells also express IP-10 and MIG, respectively [45, 46]. Importantly, IP-10 and MIG are chemo-attractants for activated T cells, especially Th1 cells that produce IFN-γ [47–49]. Therefore, IP-10 and MIG, which are produced by IFN-γ-activated macrophages, attract Th1 cells through CXCR3 toward activated macrophages [48–50]. These mobilized Th1 cells produce IFN-γ and further activate macrophages to produce both IP-10 and MIG. This loop may explain the high blood levels of IP-10 and MIG during LAHS.
IFN-γ has been supposed to be one of the major mediators of LAHS [20, 22]. However, its blood level was low during LAHS in the present study. Furthermore, IFN-γ was not a good marker for the diagnosis of LAHS. In the loop supposed in the previous paragraph, IFN-γ production by Th1 cells should be enhanced by persistent recruitment of Th1 cells toward macrophages. Although the reason for this remains to be elucidated, migrated Th1 cells might be able to activate the loop even with a small amount of IFN-γ because Th1 cells are mobilized close to macrophages. In other words, IFN-γ may play a role as a trigger for the loop. Therefore, the present study does not rule out the possible central role of IFN-γ in LAHS. Rather, it is possible to assert that the initiator of LAHS is not always a good marker for LAHS.
LAHS may be initiated by cytokines derived from lymphoma cells, which activate macrophages in a direct or indirect fashion. As a direct mediator, IFN-γ is the most likely cytokine. As an indirect mediator, TNF-α, IL-1, or IL-2 is a possibility. In this fashion, these cytokines activate T cells to produce IFN-γ. However, the production of these cytokines by lymphoma cells in LAHS has not been studied. In Hodgkin’s lymphoma, the expression of IP-10, MIG, or IFN-γ was previously reported [50]. Although this is an interesting finding, it is unclear whether these cytokines are directly produced by tumor cells.
In conclusion, we have shown specifically high blood levels of IP-10 and MIG in LAHS as well as a strong correlation between concentrations of these chemokines and clinical status for the first time. We also demonstrated the usefulness of CBA in terms of rapid measurement of these chemokines, which leads to early and proper diagnosis of LAHS. Further studies are needed to elucidate the exact role of IP-10 and MIG in the pathophysiology of LAHS.
Acknowledgments
The authors would like to thank all the physicians in Kobe City Medical Center General Hospital for many useful comments and help in general. We would also like to thank Kyoko Tanaka, Kobe City Medical Center General Hospital, for technical assistance.
Funding
This work was supported by Kasahara Foundation for the Promotion of Cancer Research, Kobe City Medical Center General Hospital.
Disclosures
The authors have no conflict of interest to disclose. The authors also do not have a financial relationship with the Kasahara Foundation for the Promotion of Cancer Research that financially supported the present study.
References
- 1.Tsuda H. Hemophagocytic syndrome (HPS) in children and adults. Int J Hematol. 1997;65:215–226. doi: 10.1016/S0925-5710(96)00560-9. [DOI] [PubMed] [Google Scholar]
- 2.Imashuku S. Differential diagnosis of hemophagocytic syndrome: underlying disorders and selection of the most effective treatment. Int J Hematol. 1997;66:135–151. doi: 10.1016/S0925-5710(97)00584-7. [DOI] [PubMed] [Google Scholar]
- 3.Henter JI, Horne A, Aricó M, Egeler RM, Filipovich AH, Imashuku S, Ladisch S, McClain K, Webb D, Winiarski J, Janka G. HLH-2004: diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Canc. 2007;48:124–131. doi: 10.1002/pbc.21039. [DOI] [PubMed] [Google Scholar]
- 4.Henter JI, Elinder G, Söder O, Hansson M, Andersson B, Andersson U. Hypercytokinemia in familial hemophagocytic lymphohistiocytosis. Blood. 1991;78:2918–2922. [PubMed] [Google Scholar]
- 5.Fujiwara F, Hibi S, Imashuku S. Hypercytokinemia in hemophagocytic syndrome. Am J Pediatr Hematol Oncol. 1993;15:92–98. doi: 10.1097/00043426-199302000-00012. [DOI] [PubMed] [Google Scholar]
- 6.Akashi K, Hayashi S, Gondo H, Mizuno S, Harada M, Tamura K, Yamasaki K, Uike N, Okamura T, Miyamoto T, Niho Y. Involvement of interferon-gamma and macrophage colony-stimulating factor in pathogenesis of haemophagocytic lymphohistiocytosis in adults. Br J Haematol. 1994;87:243–250. doi: 10.1111/j.1365-2141.1994.tb04905.x. [DOI] [PubMed] [Google Scholar]
- 7.Osugi Y, Hara J, Tagawa S, Takai K, Hosoi G, Matsuda Y, Ohta H, Fujisaki H, Kobayashi M, Sakata N, Kawa-Ha K, Okada S, Tawa A. Cytokine production regulating Th1 and Th2 cytokines in hemophagocytic lymphohistiocytosis. Blood. 1997;89:4100–4103. [PubMed] [Google Scholar]
- 8.Takada H, Nomura A, Ohga S, Hara T. Interleukin-18 in hemophagocytic lymphohistiocytosis. Leuk Lymphoma. 2001;42:21–28. doi: 10.3109/10428190109097673. [DOI] [PubMed] [Google Scholar]
- 9.Mazodier K, Marin V, Novick D, Farnarier C, Robitail S, Schleinitz N, Veit V, Paul P, Rubinstein M, Dinarello CA, Harlé JR, Kaplanski G. Severe imbalance of IL-18/IL-18BP in patients with secondary hemophagocytic syndrome. Blood. 2005;106:3483–3489. doi: 10.1182/blood-2005-05-1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murohashi I, Yoshida K, Ihara N, Wakao D, Yagasaki F, Nakamura Y, Kawai N, Matsuda A, Jinnai I, Bessho M. Serum levels of Th1/Th2 cytokines, angiogenic growth factors, and other prognostic factors in young adult patients with hemophagocytic syndrome. Lab Hematol. 2006;12:71–74. doi: 10.1532/LH96.05035. [DOI] [PubMed] [Google Scholar]
- 11.Ishii E, Ohga S, Imashuku S, Yasukawa M, Tsuda H, Miura I, Yamamoto K, Horiuchi H, Takada K, Ohshima K, Nakamura S, Kinukawa N, Oshimi K, Kawa K. Nationwide survey of hemophagocytic lymphohistiocytosis in Japan. Int J Hematol. 2007;86:58–65. doi: 10.1532/IJH97.07012. [DOI] [PubMed] [Google Scholar]
- 12.Shimazaki C, Inaba T, Nakagawa M. B-cell lymphoma-associated hemophagocytic syndrome. Leuk Lymphoma. 2000;38:121–130. doi: 10.3109/10428190009060325. [DOI] [PubMed] [Google Scholar]
- 13.Takahashi N, Chubachi A, Kume M, Hatano Y, Komatsuda A, Kawabata Y, Yanagiya N, Ichikawa Y, Miura AB, Miura I. A clinical analysis of 52 adult patients with hemophagocytic syndrome: the prognostic significance of the underlying diseases. Int J Hematol. 2001;74:209–213. doi: 10.1007/BF02982007. [DOI] [PubMed] [Google Scholar]
- 14.Go RS, Wester SM. Immunophenotypic and molecular features, clinical outcomes, treatments, and prognostic factors associated with subcutaneous panniculitis-like T-cell lymphoma. Cancer. 2004;101:1404–1413. doi: 10.1002/cncr.20502. [DOI] [PubMed] [Google Scholar]
- 15.Han AR, Lee HR, Park BB, Hwang IG, Park S, Lee SC, Kim K, Lim HY, Ko YH, Kim SH, Kim WS. Lymphoma-associated hemophagocytic syndrome: clinical features and treatment outcome. Ann Hematol. 2007;86:493–498. doi: 10.1007/s00277-007-0278-6. [DOI] [PubMed] [Google Scholar]
- 16.Tong H, Ren Y, Liu H, Xiao F, Mai W, Meng H, Qian W, Huang J, Mao L, Tong Y, Wang L, Qian J, Jin J. Clinical characteristics of T-cell lymphoma associated with hemophagocytic syndrome: comparison of T-cell lymphoma with and without hemophagocytic syndrome. Leuk Lymphoma. 2008;49:81–87. doi: 10.1080/10428190701713630. [DOI] [PubMed] [Google Scholar]
- 17.Ohga S, Matsuzaki A, Nishizaki M, Nagashima T, Kai T, Suda M, Ueda K. Inflammatory cytokines in virus-associated hemophagocytic syndrome. Interferon-gamma as a sensitive indicator of disease activity. Am J Pediatr Hematol Oncol. 1993;15:291–298. [PubMed] [Google Scholar]
- 18.Imashuku S, Hibi S, Tabata Y, Sako M, Sekine Y, Hirayama K, Sakazaki H, Maeda N, Kito H, Shichino H, Mugishima H. Biomarker and morphological characteristics of Epstein-Barr virus-related hemophagocytic lymphohistiocytosis. Med Pediatr Oncol. 1998;31:131–137. doi: 10.1002/(SICI)1096-911X(199809)31:3<131::AID-MPO1>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 19.Tang Y, Xu X, Song H, Yang S, Shi S, Wei J, Pan B, Zhao F, Liao C, Luo C. Early diagnostic and prognostic significance of a specific Th1/Th2 cytokine pattern in children with haemophagocytic syndrome. Br J Haematol. 2008;143:84–91. doi: 10.1111/j.1365-2141.2008.07298.x. [DOI] [PubMed] [Google Scholar]
- 20.Miyahara M, Sano M, Shibata K, Matsuzaki M, Ibaraki K, Shimamoto Y, Tokunaga O. B-cell lymphoma-associated hemophagocytic syndrome: clinicopathological characteristics. Ann Hematol. 2000;79:378–388. doi: 10.1007/s002770000155. [DOI] [PubMed] [Google Scholar]
- 21.Murase T, Nakamura S, Kawauchi K, Matsuzaki H, Sakai C, Inaba T, Nasu K, Tashiro K, Suchi T, Saito H. An Asian variant of intravascular large B-cell lymphoma: clinical, pathological and cytogenetic approaches to diffuse large B-cell lymphoma associated with haemophagocytic syndrome. Br J Hematol. 2000;111:826–834. [PubMed] [Google Scholar]
- 22.Ohno T, Ueda Y, Nagai K, Takahashi T, Konaka Y, Takamatsu T, Suzuki T, Sasada M, Uchiyama T. The serum cytokine profiles of lymphoma-associated hemophagocytic syndrome: a comparative analysis of B-cell and T-cell/Natural killer cell lymphomas. Int J Hematol. 2003;77:286–294. doi: 10.1007/BF02983788. [DOI] [PubMed] [Google Scholar]
- 23.Chen R, Lowe L, Wilson JD, Crowther E, Tzeggai K, Bishop JE, Varro R. Simultaneous quantification of six human cytokines in a single sample using microparticle-based flow cytometric technology. Clin Chem. 1999;45:1693–1694. [PubMed] [Google Scholar]
- 24.Cook EB, Stahl JL, Lowe L, Chen R, Morgan E, Wilson J, Varro R, Chan A, Graziano FM, Barney NP. Simultaneous measurement of six cytokines in a single sample of human tears using microparticle-based flow cytometry: allergics vs non-allergics. J Immunol Methods. 2001;254:109–118. doi: 10.1016/S0022-1759(01)00407-0. [DOI] [PubMed] [Google Scholar]
- 25.Tárnok A, Hambsch J, Chen R, Varro R. Cytometric bead array to measure six cytokines in twenty-five microliters of serum. Clin Chem. 2003;49:1000–1002. doi: 10.1373/49.6.1000. [DOI] [PubMed] [Google Scholar]
- 26.Morgan E, Varro R, Sepulveda H, Ember JA, Apgar J, Wilson J, Lowe L, Chen R, Shivraj L, Agadir A, Campos R, Ernst D, Gaur A. Cytometric bead array: a multiplexed assay platform with applications in various areas of biology. Clin Immunol. 2004;110:252–266. doi: 10.1016/j.clim.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 27.Varro R, Chen R, Sepulveda H, Apgar J. Bead-based multianalyte flow immunoassays: the cytometric bead array system. Methods Mol Biol. 2007;378:125–152. doi: 10.1007/978-1-59745-323-3_9. [DOI] [PubMed] [Google Scholar]
- 28.Fujii N, Hiraki A, Aoe K, Murakami T, Ikeda K, Masuda K, Matsuo K, Shinagawa K, Ishimaru F, Sugi K, Darzynkiewicz Z, Tanimoto M. Serum cytokine concentrations and acute graft-versus-host disease after allogeneic peripheral blood stem cell transplantation: concurrent measurement of ten cytokines and their respective ratios using cytometric bead array. Int J Mol Med. 2006;17:881–885. [PubMed] [Google Scholar]
- 29.Maier R, Weger M, Haller-Schober EM, EI-Shabrawi Y, Theisl A, Barth A, Aigner R, Haas A. Application of multiplex cytometric bead array technology for the measurement of angiogenic factors in the vitreous. Mol Vis. 2006;12:1143–1147. [PubMed] [Google Scholar]
- 30.Falasca K, Vecchiet F, Ucciferri C, Vignale F, Conti P, Pizzigallo A, Piatteli A, Vecchiet J. Periodontitis and cytokine patterns in HIV positive patients. Eur J Med Res. 2008;13:163–168. [PubMed] [Google Scholar]
- 31.Ichiyama T, Shoji H, Takahashi Y, Matsushige T, Kajimoto M, Inuzuka T, Furukawa S. Cerebrospinal fluid levels of cytokines in non-herpetic acute limbic encephalitis: comparison with herpes simplex encephalitis. Cytokine. 2008;44:149–153. doi: 10.1016/j.cyto.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 32.Tang Y, Liao C, Xu X, Song H, Shi S, Yang S, Zhao F, Xu W, Chen X, Mao J, Zhang L, Pan B. Evaluation of Th1/Th2 cytokines as a rapid diagnostic tool for severe infection in paediatric haematology/oncology patients by the use of cytometric bead array technology. Clin Microbiol Infect. 2011;17:1666–1673. doi: 10.1111/j.1469-0691.2011.03490.x. [DOI] [PubMed] [Google Scholar]
- 33.Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Vardiman JW. WHO classification of tumours of haematopoietic and lymphoid tissues. 4. IARC: Lyon; 2008. [Google Scholar]
- 34.Pinsky MR, Vincent JL, Deviere J, Alegre M, Kahn RJ, Dupont E. Serum cytokine levels in human septic shock. Relation to multiple-system organ failure and mortality. Chest. 1993;103:565–575. doi: 10.1378/chest.103.2.565. [DOI] [PubMed] [Google Scholar]
- 35.Bossink AW, Paemen L, Jansen PM, Hack CE, Thijs LG, Van Damme J. Plasma levels of the chemokines monocyte chemotactic proteins-1 and -2 are elevated in human sepsis. Blood. 1995;86:3841–3847. [PubMed] [Google Scholar]
- 36.Taniguchi T, Koido Y, Aiboshi J, Yamashita T, Suzaki S, Kurokawa A. Change in the ratio of interleukin-6 to interleukin-10 predicts a poor outcome in patients with systemic inflammatory response syndrome. Crit Care Med. 1999;27:1262–1264. doi: 10.1097/00003246-199907000-00005. [DOI] [PubMed] [Google Scholar]
- 37.Bozza FA, Gomes RN, Japiassú AM, Soares M, Castro-Faria-Neto HC, Bozza PT, Bozza MT. Macrophage migration inhibitory factor levels correlate with fatal outocome in sepsis. Shock. 2004;22:309–313. doi: 10.1097/01.shk.0000140305.01641.c8. [DOI] [PubMed] [Google Scholar]
- 38.Oberholzer A, Souza SM, Tschoeke SK, Oberholzer C, Abouhamze A, Pribble JP, Moldawer LL. Plasma cytokine measurements augment prognostic scores as indicator of outcome in patients with severe sepsis. Shock. 2005;23:488–493. [PubMed] [Google Scholar]
- 39.Kellum JA, Kong L, Fink MP, Weissfeld LA, Yealy DM, Pinsky MR, Fine J, Krichevsky A, Delude RL, Angus DC. Understanding the inflammatory cytokine response in pneumonia and sepsis: results of the Genetic and Inflammatory Markers of Sepsis (GenIMS) Study. Arch Intern Med. 2007;167:1655–1663. doi: 10.1001/archinte.167.15.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Luster AD, Unkeless JC, Ravetch JV. Gamma-interferon transcriptionally regulates an early-response gene containing homology to platelet proteins. Nature. 1985;315:672–676. doi: 10.1038/315672a0. [DOI] [PubMed] [Google Scholar]
- 41.Ohmori Y, Hamilton TA. A macrophage LPS-inducible early gene encodes the murine homologue of IP-10. Biochem Biophys Res Commun. 1990;168:1261–1267. doi: 10.1016/0006-291X(90)91164-N. [DOI] [PubMed] [Google Scholar]
- 42.Farber JM. A collection of mRNA species that are inducible in the RAW 264.7 mouse macrophage cell line by gamma interferon and other agents. Mol Cell Biol. 1992;12:1535–1545. doi: 10.1128/mcb.12.4.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Narumi S, Hamilton TA. Inducible expression of murine IP-10 mRNA varies with the state of macrophage inflammation activity. J Immunol. 1991;146:3038–3044. [PubMed] [Google Scholar]
- 44.Gasperini S, Marchi M, Calzetti F, Laudanna C, Vicentini L, Olsen H, Murphy M, Liao F, Farber J, Cassatella MA. Gene expression and production of the monokine induced by IFN-gamma (MIG), IFN-inducible T cell alpha chemoattractant (I-TAC) and IFN-gamma-inducible protein-10 (IP-10) chemokines by human neutrophils. J Immunol. 1999;162:4928–4937. [PubMed] [Google Scholar]
- 45.Yoneyama H, Narumi S, Zhang Y, Murai M, Baggiolini M, Lanzavecchia A, Ichida T, Asakura H, Matsushima K. Pivotal role of dendritic cell-derived CXCL10 in the retention of T helper cell 1 lymphocytes in secondary lymph nodes. J Exp Med. 2002;195:1257–1266. doi: 10.1084/jem.20011983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Janatpour MJ, Hudak S, Sathe M, Sedgwick JD, McEvoy LM. Tumor necrosis factor-dependent segmental control of MIG expression by high endothelial venules in inflamed lymph nodes regulates monocyte recruitment. J Exp Med. 2001;194:1375–1384. doi: 10.1084/jem.194.9.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bonecchi R, Bianchi G, Bordignon PP, D’Ambrosio D, Lang R, Borsatti A, Sozzani S, Allavena P, Gray PA, Mantovani A, Sinigaglia F. Differential expression of chemokine receptors and chemotactic responsiveness of type 1 T helper cells (Th1s) and Th2s. J Exp Med. 1998;187:129–134. doi: 10.1084/jem.187.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sallusto F, Lenig D, Mackay CR, Lanzavecchia A. Flexible programs of chemokine receptor expression on human polarized T helper 1 and 2 lymphocytes. J Exp Med. 1998;187:875–883. doi: 10.1084/jem.187.6.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Meyer M, Hensbergen PJ, van der Raaij-Helmer EM, Brandacher G, Margreiter R, Heufler C, Koch F, Narumi S, Werner ER, Colvin R, Luster AD, Tensen CP, Werner- Felmayer G. Cross reactivity of three T cell attracting murine chemokines stimulating the CXC chemokine receptor CXCR3 and their induction in cultured cells and during allograft rejection. Eur J Immunol. 2001;31:2521–2527. doi: 10.1002/1521-4141(200108)31:8<2521::AID-IMMU2521>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 50.Teruya-Feldstein J, Jaffe ES, Burd PR, Kingma DW, Setsuda JE, Tosato G. Differential chemokine expression in tissues involved by Hodgkin’s disease: direct correlation of eotaxin expression and tissue eosinophilia. Blood. 1999;93:2463–2470. [PubMed] [Google Scholar]


