Abstract
Natural killer (NK) cells are innate lymphocytes that play important roles in the defense against microbial pathogens through secretion of IFN-γ and recognition and lysis of virally or bacterially infected host cells. A recently identified population of NK-like cells that shares characteristics of both NK cells and lymphoid tissue inducer (LTi) cells promotes innate immune responses in epithelial tissue through the secretion of IL-22. In contrast to classical NK cells, NK-like cells are localized preferentially at mucosal sites, such as the intestinal mucosa. In this review, we consider the function of NK and NK-like cells in anti-microbial defense as well as the maintenance of tissue integrity in the mucosal epithelium of the intestine, lung, and female reproductive tract. Current experimental evidence supports an important protective role for IL-22-producing NK-like cells during intestinal infections, whereas classical NK cells are crucial in the early defense against many pathogens in the respiratory tract. NK cells isolated from the pregnant uterus differ significantly in phenotype and function from those at other tissue locations. Uterine NK cells clearly contribute to the tissue remodeling that takes place during placentation, but their role in anti-microbial defense remains largely undefined.
Keywords: innate immune defense, intestinal epithelium, lung epithelium, mucosal epithelium, natural killer cells, NCR22, NK-22, uterine NK cells
Natural killer cells
Natural killer (NK) cells play a crucial role in the first line of defense against invading pathogens. The main functions of NK cells include the ability to rapidly secrete cytokines following their recognition of pathogens and following cues from antigen-presenting cells (APC) exposed to pathogen-derived components. NK cells also play a vital surveillance role in the innate immune response to pathogens by their ability to directly kill infected tissue cells.
Several subsets of NK cells have been identified based on their surface phenotype and functionality. In the most general sense, NK cells are classified as either “conventional” NK cells (cNK) or “NK-like” cells. Conventional NK cells are best known for their pro-inflammatory and cytotoxic functions upon recognition of virally infected or malignantly transformed cells. NK-like cells include NK cell receptor-expressing lymphocytes that bear resemblance to lymphoid-tissue inducer cells (LTi). These NK-like cells have little or no cytotoxic capacity and their cytokine secretion pattern has been shown to promote innate immune responses in the epithelium, tissue remodeling and wound healing.
This review will cover the known functions of both conventional NK cells and NK-like cells in the anti-microbial defense of mucosal tissues. We will focus on NK cell-mediated immune responses at the mucosal epithelium of the intestine and the lung, and we will briefly consider the known functions of NK cells found in the female reproductive tract.
Phenotype and functions of cNK cells
In the mouse, conventional NK cells are identified by the co-expression of the NK cell receptors NK1.1 (NKR-P1C, CD161) and NKp46, and by their lack of the T cell marker CD3. Human cNK cells are characterized by their expression of the NK cell receptors CD56 and NKp46, and their lack of the T cell marker CD3. cNK cells are found in many lymphoid and non-lymphoid tissues, such as blood, spleen, liver, lymph nodes, and skin. In addition, cNK cells have been described in mucosal-associated lymphoid tissues, such as the human tonsil and mouse Peyer’s patches, as well as in the intestinal and lung mucosa [1, 2].
cNK cells play important functions in the elimination of virally infected and malignantly transformed cells, as evidenced from experiments in mouse models that lack cNK cells, either through antibody-mediated cNK cell depletion or via genetic defects affecting NK cell development or function [3–6]. cNK cells can directly lyse infected and tumor cells through the release of cytotoxic granules containing granzymes and perforin, which causes apoptosis in the target cell. In addition, cytotoxicity mediated by NK cells can also be triggered through surface receptor interactions between NK cells and their target cells, e.g. through the cNK cell-expressed death receptor ligand TRAIL. Furthermore, cNK cells are an abundant source of cytokines, in particular IFN-γ, which promotes the killing of intracellular pathogens in phagocytes, as well as the skewing of adaptive immune responses toward a Th1 phenotype [4, 7].
In human blood, two major subsets of cNK cells are found based on their expression levels of CD56 and the IgG receptor CD16. The major population of CD56dim CD16+ cells has high cytolytic activity and can act as a rapid source of the pro-inflammatory cytokine IFN-γ. Around 10% of peripheral blood NK cells display the CD56bright CD16– phenotype; these cNK cells possess a low cytotoxic potential, but secrete large amounts of cytokines, such as IFN-γ, GM-CSF, and TNF-α upon in vitro stimulation with IL-12 and IL-18. The two cNK cell subsets show different tissue localizations, with CD56dim cells found mainly in the blood and spleen, and CD56bright cells predominantly in lymph nodes [8–10].
The direct equivalents of human CD56dim and CD56bright NK cells do not exist in the mouse due to their expression of different sets of surface receptors; however, several cNK cell subsets with different functions have been described in mice. The major three subsets differ in their expression of the surface markers CD11b (integrin αM) and CD27, with CD11bhi CD27hi cells representing mature cNK cells that are found predominantly in the spleen, liver, and lymph node, and displaying high potential for cytokine production and cytotoxicity. In contrast, CD11bhi CD27low cells are present in peripheral organs such as the lung and are more limited in their cytokine secretion and cytotoxic capacities. The third major subset represents immature NK cells with the surface phenotype CD11blow CD27hi. An additional subset of mouse NK cells has recently been identified that resembles human CD56bright NK cells in their production of ample amounts of cytokines. This subset appears to develop in the thymus, localizes predominantly to the lymph nodes, and is characterized by the expression of the IL-7 receptor (CD127). Also, enriched in lymph nodes is a further population of NK cells that in addition to NK1.1 expresses B220 and CD11c, two markers that are absent from the major NK cell subsets. These NK cells are potent producers of IFN-γ, have a high cytotoxic potential, and have been suggested to represent in vivo activated NK cells rather than a separate NK cell subset [2, 7, 11].
Development of human and mouse cNK cells from common lymphoid progenitors occurs in several stages, accompanied by the gradual acquisition of NK cell receptors and effector functions [12, 13]. cNK cell maturation is critically dependent on signaling through the IL-2R common gamma chain (γc) and the cytokine IL-15, as evidenced by mice deficient in either the Il2rg or the Il15 gene, which lack mature peripheral NK cells [12].
Phenotype and functions of IL-22-producing NK-like cells
We and other groups have recently identified a novel subset of NK-like cells that is found at mucosal-associated lymphoid tissues, such as the human tonsils and Peyer’s patches [14–18]. This NK-like cell population displays unconventional functions characterized by their low cytotoxic potential and the secretion of large amounts of the cytokine IL-22. IL-22 is a member of the IL-10 cytokine family; however, unlike IL-10, it targets cells outside of the immune system, such as intestinal epithelial cells. IL-22 induces the secretion of anti-microbial peptides from epithelial cells, thereby enhancing immune functions in the epithelium [19, 20]. In addition, IL-22 promotes epithelial cell survival and migration and thus enhances processes critical to wound healing [20–24]. In NK-like cells, IL-22 secretion is triggered by exposure to IL-23, a pro-inflammatory cytokine produced by APC, which have encountered pathogen-derived material [14, 17]. Thus, a model of innate mucosal responses has emerged, in which the recognition of pathogens by APC leads to IL-23 production, triggering rapid secretion of IL-22 by NK-like cells to promote anti-microbial functions at sites of pathogen invasion. This is accompanied by enhanced wound-healing activity leading to the restoration of tissue integrity [25].
Based on their cytokine secretion profile, we have suggested the term NK-22 cells for NK-like cells that produce IL-22. In the mouse, NK-22 cells can be identified as NK1.1– NKp46+ cells expressing the IL-7 receptor alpha chain (CD127) and the transcription factor RORγt, the latter of which is associated with their ability to produce IL-22 [16–18]. Human NK-22 cells express the NK cell receptors CD56, NKp46, and NKp44 and, as mouse NK-22 cells, stain positive for CD127 and the transcription factor RORγt [14, 15]. Interestingly, human peripheral blood cNK cells show increased transcript levels of IL-22 upon in vitro stimulation with IL-2 and IL-12 [26]. Although IL-22 production was not examined at the protein level in this study, and blood cNK cells do not express the transcription factor RORγt associated with IL-22 production, a potential role for cNK cells as a source of IL-22 cannot be excluded.
The observation that NK-22 cells are not cytotoxic and do not secrete any substantial amounts of IFN-γ has sparked controversies as to whether these cells can be grouped as part of the NK cell lineage. NK-22 cells share several characteristics with another innate lymphoid cell type called lymphoid tissue inducer (LTi) cells. These cells, which play crucial roles in the fetal development of secondary lymphoid tissues such as lymph nodes, are also potent producers of IL-22 upon IL-23 stimulation [27–29]. However, unlike NK-22 cells, fetal LTi, as well as LTi-like cells found in adult tissues, produce not only IL-22 but also IL-17, a cytokine with potent pro-inflammatory functions. The development of both NK-22 cells and LTi cells, but not that of cNK cells, is dependent on RORγt and IL-7. In contrast, IL-15-deficient mice, which lack cNK cells, display normal numbers of LTi-like and NK-22 cells [17, 18, 27, 30]. Due to these shared developmental requirements, it has been proposed that NK-22 cells and LTi cells develop from the same precursor cell, which may be distinct from the precursor that gives rise to conventional NK cells [31–33]. However, there is evidence that NK-22 cells can give rise to cells with typical cNK features in vitro, such as IFN-γ secretion, suggesting that NK-22 cells may represent a precursor for cNK cells [33–35].
The elucidation of the lineage relationship of NK-22 cells is currently a subject of intense investigation. For the purpose of this review, we will refer to the group of IL-22-producing NK-like cells as NK-22 cells, although their relationship to the NK cell lineage is unclear at present. In the following sections, we summarize experimental evidence for the potential roles of both cNK cells and NK-22 cells during microbial infections at mucosal epithelia. We will focus on three mucosal tissues, each with similar, but also some distinct, characteristics: intestine, lung, and the female reproductive tract.
The intestinal mucosa
While the stomach and proximal small bowel are relatively sparsely colonized, the human distal small bowel and the large bowel contain a rich microbial flora reaching up to 1012 colony-forming units per gram of luminal content [36]. At the apical surface of intestinal epithelia, a thick mucus layer forms a physical barrier to prevent microbial invasion of the underlying epithelium. Beneath the mucus coating, a single layer of epithelial cells forms the second barrier between intestinal lumen and host tissue. These epithelial cells fulfill important functions by creating a physical barrier to pathogens and by responding to invading pathogens with the rapid production of anti-microbial peptides and chemoattractants to recruit immune cells [37, 38]. Intraepithelial lymphocytes (IEL), mainly consisting of CD8+ T cells, but also NK cells, reside within this epithelial layer, and a variety of leukocytes, including B cells, T cells, APC, and NK cells can be found in the underlying lamina propria (Fig. 1) [38–40]. Furthermore, the intestinal mucosa contains organized clusters of lymphoid tissue such as cryptopatches, isolated lymphoid follicles (ILF), and Peyer’s patches [29].
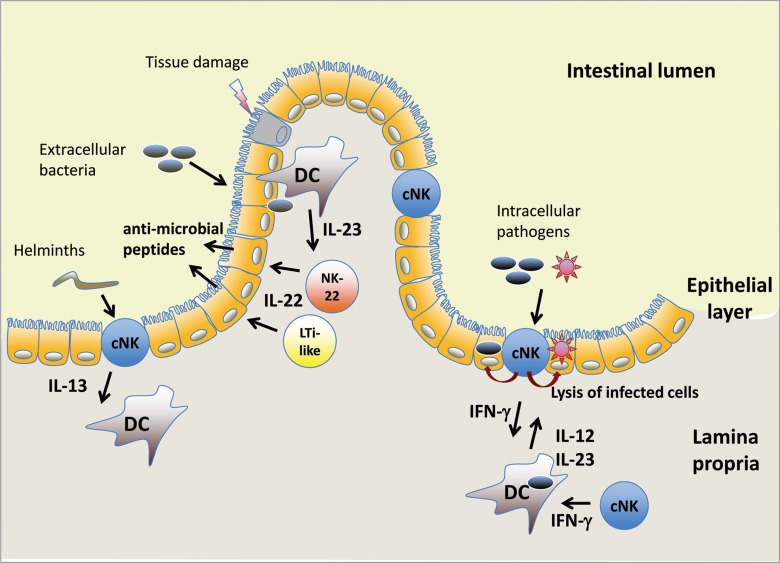
Fig. 1.
cNK cells and NK-22 cells in the intestinal mucosa. cNK cells are found in the intestinal epithelium and in the underlying lamina propria, while NK-22 cells and LTi-like cells reside in the lamina propria. Exposure of dendritic cells (DC) to pathogens triggers the release of IL-12 and/or IL-23, which induces IL-22 secretion by NK-22 and LTi-like cells and IFN-γ secretion by cNK cells. Helminth infections have been shown to cause IL-13 production by intraepithelial NK cells. DC, dendritic cell; cNK, conventional NK cell; LTi-like, adult equivalent of fetal lymphoid tissue inducer (LTi) cells
Pathogens typically enter the intestine via the ingestion of contaminated food. Infection occurs at varying sites within the alimentary tract depending on the pathogen and typically involves penetration of the epithelial layer, either by direct attachment and infection of epithelial cells, or by exploiting the antigen-sampling mechanisms of the immune system to be shuttled across the epithelium. Innate immune mechanisms play important roles in the rapid response to invading pathogens and in the initiation of appropriate adaptive immunity. As an innate source of cytokines, NK cells contribute to the immune defense of many different pathogens. In the sections below, we summarize some of the known functions of NK cells during gastrointestinal infections.
Roles of NK cells in intestinal anti-microbial immune responses
NK-22 cells
To elucidate the functions of NK-22 cells during microbial infections, mouse models deficient in innate and/or adaptive sources of IL-22 have often been utilized. However, in many cases, it is difficult or even impossible to differentiate between the roles that NK-22 cells and LTi cells play, since these cell types share high similarity in their developmental requirements, cell surface receptor and transcription factor expression and tissue location. When discussing roles of NK-22 cells in immune responses at mucosal tissues, this caveat has to be kept in mind.
Recent reports from our group and others have demonstrated that NK cells, in particular NK-22 cells, contribute to innate defense against the gastrointestinal pathogen Citrobacter rodentium [14, 18]. C. rodentium is a gram-negative bacterium that induces colitis in mice, its natural host. Protection from C. rodentium-induced mortality requires both innate and adaptive immune mechanisms. Rag-deficient mice, which lack adaptive immune cells (T cells, B cells), are able to contain infections with C. rodentium within the first several weeks of infection. This initial immune response is mediated by the cytokine IL-22, as demonstrated by studies using IL-22-deficient mice [41]. A role for NK-22 cells and LTi cells is inferred from the observation that these cell types accumulate during C. rodentium infection and are capable of producing IL-22. Furthermore, a role for NK cells in this bacterial infection was demonstrated by anti-NK1.1 antibody-mediated NK cell depletion in Rag-deficient mice, and by the infection of Rag–/– IL2rg–/– mice, which lack NK cells in addition to adaptive immune cells. In both models, NK cell deficiency caused an accelerated mortality upon C. rodentium infection [14, 18]. However, a recent report suggests that LTi, not NK-22 cells, provide the majority of the IL-22 required for early protection from C. rodentium [42]. Further studies and additional animal models are therefore required to more definitively elucidate the contributions of NK-22 cells during the host response to C. rodentium infection.
IL-22-producing NK cells and NK cell-like cells also appear to play protective roles in inflammatory bowel disease (IBD), a group of conditions characterized by chronic inflammation of the intestinal mucosa. In IBD, the immune system mounts aberrant responses to the intestinal microbial flora, leading to a breach of the epithelial barrier and a strong pro-inflammatory cytokine environment. IL-22-producing NK-like cells have been identified in experimental mouse models of colitis, and mice deficient in IL-22-producing cells (Rag1–/– Il2rg–/–; or Il22–/– Rag1–/–) display more severe colitis than Rag1–/– mice with intact IL-22 production [43]. Again, the exact contributions of NK cells and NK-22 cells versus LTi cells are still unknown.
In human intestinal tissues, NK cells with NK-22 characteristics are present and are capable of producing IL-22 upon in vitro treatment with IL-23 [14]. A recent study classified CD56+ NK cells in colonic lamina propria into NKp44+NKp46– and NKp44–NKp46+ subsets [44]. These subsets of NK cells differed in their cytokine production, with the NKp44+NKp46– representing the IL-22-producing RORγt+ NK-22 population, while the NKp44–NKp46+ subset produced IFN-γ and likely represents a conventional NK cell subset. Interestingly, in Crohn’s disease, an IBD characterized by Th17-mediated inflammation, the frequency of NK-22 cells in colonic lamina propria was decreased, while the frequency of cNK was markedly increased compared to healthy tissue [44]. Furthermore, NKp44–NKp46+ NK cells from Crohn’s disease patients were more potent in producing IFN-γ in response to IL-23 than the same NK cell subset from healthy individuals. A separate study found increased frequencies of IL-17-producing LTi-like cells in intestinal tissues from Crohn’s disease patients compared to healthy individuals, which resembled the innate cell type implicated in promoting colitis in mice [45, 46]. The above observations suggest that the different NK-like and LTi-like cell populations may have divergent roles in Crohn’s disease, with NK-22 cells potentially bearing beneficial roles, and LTi-like and cNK cell subsets playing pro-inflammatory roles, potentially exacerbating chronic inflammation.
The role of NK-22 cells in the intestinal immune defense to tissue-damaging pathogens other than C. rodentium has not been sufficiently studied. In humans, human immunodeficiency virus (HIV) is an important pathogen causing high viral burden in the gut, associated with intestinal pathology [47]. A recent study has explored whether NK-22 cells are present in non-primate intestinal tissue [48]. This study by Reeves et al. identified NK cells exhibiting an NK-22 phenotype in the gut of macaques, which expressed high transcript levels of IL-22 and, in contrast to human NK-22 cells, also produced IL-17. These NK-22 cells showed low cytotoxic potential. Upon infection with simian immunodeficiency virus (SIV), these gut NK-22-like cells were reduced in number and showed altered functions, resulting in a change of cytokine expression toward IFN-γ. Furthermore, these cells gained cytolytic potential, as evidenced by increased perforin expression and enhanced degranulation [48]. At present, the significance of the altered NK cell functions during SIV infection is unknown, but a loss of IL-17 and IL-22 contributes to the gut pathology seen in SIV infection [49], and NK-22 cell depletion during SIV infection may thus potentially contribute to the loss of gut integrity.
cNK cells
Conventional NK cells are known to contribute to the immune defense against various gastrointestinal pathogens, in particular intracellular bacteria and parasites. For example, during infections with Toxoplasma gondii, an intracellular parasite that initially infects the intestinal epithelium and causes tissue pathology, cNK cells are a major source of protective IFN-γ at early times of infection. IFN-γ is crucial for the development of an appropriate cytotoxic T cell response to the pathogen and induces macrophage activation. cNK cells may also contribute to the rapid elimination of infected cells by direct cytotoxicity. In the absence of cNK cells, impaired priming of CD4+ and CD8+ T cells was observed [50]. Recently, cNK cells have also been identified as a major source of IL-17 during T. gondii infection, a cytokine that confers resistance to this pathogen [51]. In contrast to what has been seen for C. rodentium, IL-22 has pathogenic roles during T. gondii infection, causing destruction of intestinal tissue integrity [52, 53]. A possible pathogenic role for NK-22 cells has not been described. T cells have been shown to be the major source of IL-22 in this experimental infection; thus, NK-22 cells may not contribute significantly to tissue pathology [52].
In a recent study by Tomasello and colleagues, oral infection of mice with Listeria monocytogenes led to rapid activation of both small intestinal cNK and NK-22 cells to produce IFN-γ and IL-22, respectively. However, protection from L. monocytogenes dissemination was dependent on IFN-γ, not IL-22, suggesting that cNK cells play a predominant role in limiting bacterial spread in this model [54].
In the host response to the gastrointestinal nematode Trichinella spiralis, cNK cells may perform surprising protective roles through their promotion of Th2-type responses, rather than their typical production of the Th1-type cytokine IFN-γ. In experimental infections with this helminth parasite, intestinal cNK cells produced IL-13 that contributed to the early immune response characterized by goblet cell hyperplasia and mucus overproduction, which facilitated expulsion of the parasite [55]. Thus, cNK cells are capable of contributing to the innate immune defense by secreting cytokines that either promote Th1-type or Th2-type immune responses, depending on the pathogen encountered. It is currently unclear whether different subsets of cNK provide the different cytokines seen in vivo, or whether the interaction of cNK with pathogens and pathogen-exposed APC determines the types of cytokines produced by NK cells.
The lung epithelium
In contrast to the situation in the distal bowel, the lung is not normally colonized by microbes and in the healthy state is almost sterile. Inhaled microbes are typically trapped in the thin mucus layer covering the lung epithelium and are readily cleared by a combination of mucociliary transport and coughing. Lung epithelial cells create a physical barrier against invading pathogens, but they also have direct roles in innate immune defense through the release of anti-microbial peptides [56, 57]. Yet, many pathogens have evolved mechanisms to invade the lung epithelium, necessitating the presence of immune mechanisms that can readily respond to invading microbes. During infection, lung resident lymphocytes, dendritic cells, and macrophages play important roles in limiting pathogen spread and in recruiting neutrophils and other immune cells from the blood to the site of infection [57].
cNK cells represent up to 10% of lung resident lymphocytes and are thought to play important roles as an early source of IFN-γ and other cytokines (Fig. 2). Inflammatory insults in the lung rapidly recruit blood NK cells [2, 58]. NK cells capable of producing IL-22 have recently been identified in mouse lung tissue [59], where they may contribute to tissue integrity and anti-microbial immune responses. Existing evidence for the roles of NK cell subsets in pathogen clearance and maintenance of tissue integrity in the lung is reviewed below.
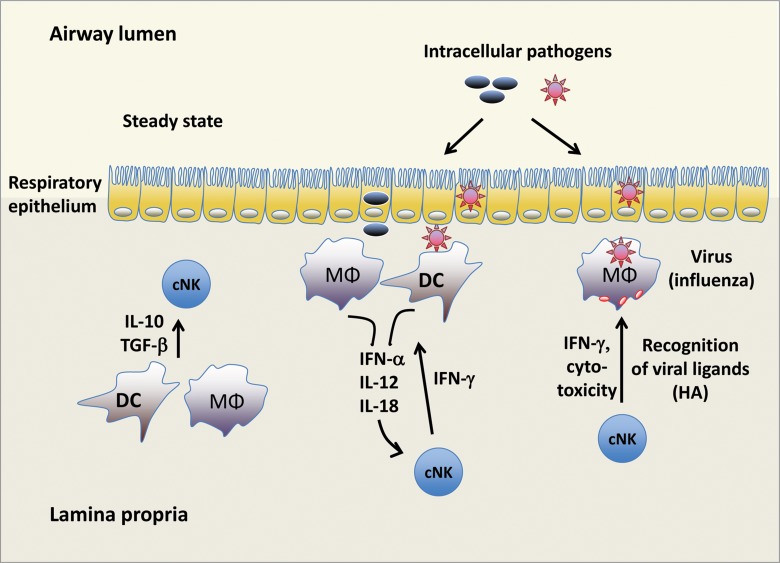
Fig. 2.
NK cell responses in the lung. Under homeostatic conditions, cNK cell functions are suppressed via IL-10 and TGF-β produced by macrophages and DC. During microbial infections, cytokines released by macrophages and DC promote IFN-γ production by cNK cells, which aids in limiting pathogen spread. During infection with influenza virus, cNK cells can directly recognize viral hemagglutinin (HA) expressed on the surface of infected phagocytes, resulting in IFN-γ production and cytotoxicity toward the infected cell. MΦ, macrophage
Roles of NK cells in anti-microbial immune responses in the lung
NK cells can be found as resident lymphocytes within the healthy lung. Lung epithelial cells produce IL-15 and may thus contribute to the survival of NK cells within the lung mucosa. In the steady state, inflammatory mechanisms in the lung are dampened by cytokines produced by alveolar macrophages, such as IL-10 and TGF-β, and NK cells isolated from healthy lungs show an impaired cytotoxic potential. Upon exposure to infection or in vitro culture with type I interferons, these NK cells become readily activated and acquire cytotoxic functions [58].
In mouse lungs, tissue-resident NK cells have been described as almost uniformly expressing markers of mature cNK, such as NK1.1, DX5 (CD49b), CD11b, NKG2D, and Ly49 receptor family members [59, 60]. While an NK-22-like cell subset has not been described in lung tissues, there is some evidence that a subset of conventional lung NK cells are capable of producing IL-22 in vitro and in vivo upon IL-23 stimulation and viral infection, respectively [59].
Microbial infections of the lung lead to a rapid recruitment of NK cells from the blood, as seen, for instance, in lung tissues from humans and mice with acute influenza infection that contain increased numbers of cNK cells [58]. In experimental influenza infections in mice, lung resident NK cells were responsible for IFN-γ production during the first few days after infection, while by day 4, most IFN-γ was produced by NK cells recruited from the blood [59]. Mice depleted of NK cells by anti-NK1.1 or anti-asialo-GM1 antibody injection show enhanced susceptibility for severe influenza infection [61, 62]. NK cells can recognize viral hemagglutinin (HA) present on influenza virus-infected cells via their NKp46 receptor, and this interaction is thought to trigger cytotoxicity toward infected target cells [63, 64]. In accordance with this, NKp46-deficient mice show enhanced susceptibility to lethal influenza infection [65].
In mouse models of pulmonary bacterial and fungal infections, NK cells were found to play protective roles against several different pathogens, such as Staphylococcus aureus, Legionella pneumophila, Pseudomonas aeruginosa, Bordetella pertussis, and the fungal pathogen Aspergillus fumigatus [66–72]. In most of these studies, NK cell-derived IFN-γ was identified as critical for the early anti-microbial response and promoted pathogen clearance. The role of NK cells in mycobacterial infection is less clear. In vitro, human NK cells recognize and respond to Mycobacterium tuberculosis-infected monocytes and macrophages by inducing intracellular bacterial killing or lysis of the infected phagocyte. This response to M. tuberculosis appears to be mediated by NK cell receptor recognition of an unknown, possibly bacterial, ligand binding to NKp44, as well as NKp46 and NKG2D binding to host cell ligands [73–76]. In a recent study, inhibition of bacterial growth in macrophages was in vitro partially mediated by IL-22 production, implicating an unexpected role for cNK-derived IL-22 in anti-microbial protection [77]. Animal models of M. tuberculosis infection, however, have resulted in controversial results. While NK cell activation and IFN-γ production have been clearly demonstrated during infection, NK cell depletion experiments suggest a redundant or even detrimental role of NK cells during M. tuberculosis infection [58].
In summary, lung-resident NK cells play important roles in the early response to many pathogens. Protective effects of NK cells in lung infections are in most cases mediated by cNK through their production of IFN-γ and by their cytotoxicity toward infected host cells. Potential roles for NK-like cells in lung infections have not sufficiently been explored yet, but unlike intestinal NK cells, resident lung NK cells display a fairly homogeneous phenotype typical of cNK cells [59]. Thus, it is conceivable that lung tissue does not harbor NK-22 cells in steady state or during the experimental models tested to date. However, as mentioned before, cNK cells represent a possible source of IL-22 during infection with intracellular pathogens in the lung [59, 77]. IL-22 plays important roles in the protection of mice from mortality caused by the pneumonia-inducing pathogen Klebsiella pneumoniae; however, the contribution of innate cell types to IL-22 production in the lung has not been addressed yet [78].
NK cells in the female reproductive tract
NK cells are present in humans and mice in the decidua, the mucosa lining the uterus during pregnancy. In humans, but not mice, NK cells can also be found in the endometrium, the mucosa of the non-pregnant uterus, where they increase in numbers during the later stages of the menstrual cycle [79, 80]. During pregnancy, immune cells residing within the decidua play important roles in the development of the placenta and in the maintenance of tolerance to the developing fetus. In particular, decidual NK cells, which can make up to 70% of decidual lymphocytes, modulate placentation, neovascularization, and trophoblast invasion through their secretion of cytokines and angiogenic factors (Fig. 3) [79–82].
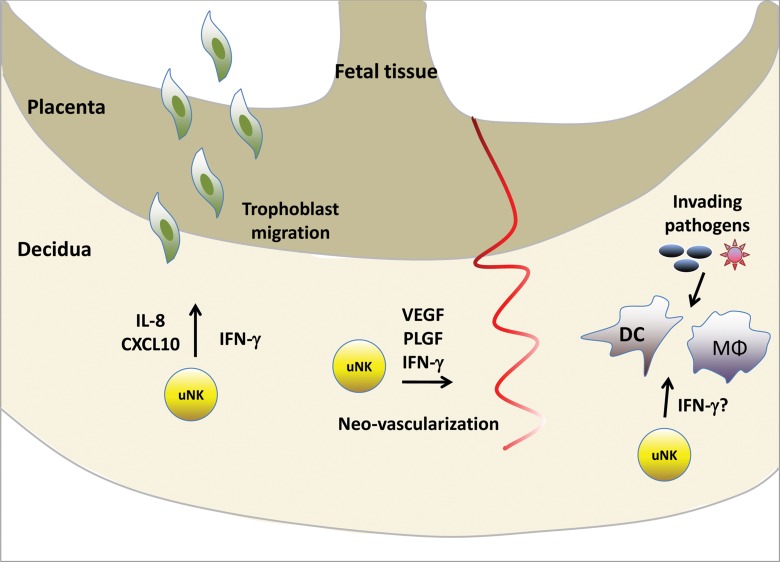
Fig. 3.
Uterine NK cells have unique functions during pregnancy. In the pregnant uterus, NK cells in the decidua contribute to the tissue remodeling that takes place during placentation. uNK cells mediate trophoblast migration through the secretion of cytokines and chemokines, and they promote vascularization through the release of angiogenic factors. The role of uNK cells during microbial infections is still unclear. VEGF, vascular endothelial growth factor; PLGF, placental growth factor
NK cells found in the human uterus resemble the CD56bright NK cell subset found in peripheral blood, due to their high expression of CD56, their expression of CD94/NKG2A, and lack of CD16. Uterine NK cells (uNK) also display a variety of cell surface receptors not found on blood NK cells, such as CD9, the tetraspanin CD151 and the activation marker CD69. NK cells with similar phenotypes have also been described in the human cervix, ectocervix, and in the fallopian tubes [79, 81, 83].
In contrast to CD56bright cells, but similar to CD56dim blood NK cells, uNK also show abundant intracellular granzyme and perforin expression. Despite this, uNK cells exhibit only low cytotoxicity to classical NK cell targets, which may in part be mediated by a higher expression of inhibitory NK cell receptors by uNK than blood NK cells. uNK cells are a potent source of a variety of cytokines, such as IFN-γ, TNF-α, GM-CSF, and IL-10. Additionally, they secrete IL-8, vascular endothelial growth factor (VEGF), CXCL10, and SDF-1 that play roles in vascularization, tissue remodeling, and trophoblast migration during placental development [80–82].
The presence of immature NK cells with NK-22 characteristics (IL-22 producing, CD56+, CD127+, RORC+) in the human uterus has recently been described. Upon in vitro culture, these cells acquire CD94 and increase CD56 expression, suggesting that they are cells of the NK cell lineage [84]. Their function at this site is not clear to date, but these NK-22 cells may represent an NK cell precursor population that differentiates into mature uNK cells in the uterine mucosa. Their production of IL-22 may play functions in enhancing innate immunity and maintaining tissue integrity at this site.
In vivo, cytotoxicity of NK cells in the human pregnant uterus is suppressed through an anti-inflammatory cytokine milieu, as well as through the interaction of the inhibitory NK cell receptors NKG2A and ILT2 with HLA-E and HLA-G, respectively, expressed on trophoblasts [82]. Infections of the uterine epithelia or the fetus may activate uNK cells to acquire cytolytic potential and to secrete pro-inflammatory cytokines. This is evident from in vitro experiments with uNK cells, whereby NKp46 ligation led to potent cytotoxicity. Furthermore, ligation of NKp30, as well as cytokine stimulation with IL-12 and IL-15, led to robust IFN-γ and TNF-α production [83, 85, 86].
The contributions of uNK cells in the female reproductive tract to the innate immune defense are unclear at present. The large number of uNK cells in both the pregnant and non-pregnant uterus suggest a potential role of these cells as an innate source of pro-inflammatory cytokines, such as IFN-γ and TNF-α, in response to infection. However, a lack of uNK cells during experimental L. monocytogenes infection did not result in increased bacterial burden in the placenta of pregnant mice [87]. A potential role for uNK cells in anti-viral immune responses was recently described, where uNK cells, but not peripheral blood NK cells, were shown to limit HIV-1 replication in vitro through their secretion of CXCL12 [88].
Thus, in the uterus, NK cells represent a unique cell population with versatile functions ranging from maintenance of tolerance to the developing fetus in pregnancy, the modulation of tissue remodeling to ensure proper blood exchange between placenta and fetus, and possible functions in anti-microbial defense.
Conclusions
NK cells have traditionally been identified as innate immune cells with cytotoxic capacities toward tumor cells or microbially infected cells. However, it is becoming increasingly clear that NK cells have divergent functions depending on the tissue environment and their differentiation or maturation stage. NK-like cells that secrete IL-22 and lack cytolytic functions may represent either a separate lineage of cells, or may be a precursor to conventional NK cells. Additionally, precursors of cNK cells may, depending on the tissue location, differentiate into either cytolytic or non-cytolytic NK cells as seen in the uterine mucosa.
The mucosal epithelia found in gut, lung, and uterus differ considerably from each other in their tissue architecture and functions, as well as in their different magnitudes of microbial colonization. The intestine has a rich commensal microflora and is thus constantly exposed to pathogen-derived material. In contrast, the lung epithelium is under healthy conditions not exposed to microbial stimuli, while the female reproductive tract exhibits low levels of microbial colonization. Considering these differences, it seems apparent that different mucosal tissues would harbor unique types of resident innate immune effector cells with divergent activation thresholds and different cytokine patterns to respond appropriately to the inflammatory and pathogenic stimuli encountered in these tissues. Fully elucidating the functions of these NK cell subsets will aid our understanding of innate immune defenses and will prove crucial for the development of future effective anti-microbial vaccines and therapeutics.
Acknowledgments
The authors would like to thank Marina Cella for critical reading of this manuscript.
Contributor Information
A. Fuchs, Department of Pathology and Immunology, Washington University School of Medicine, St. Louis, Missouri 63110, USA.
M. Colonna, Department of Pathology and Immunology, Washington University School of Medicine, St. Louis, Missouri 63110, USA
References
- 1.Grégoire C, Chasson L, Luci C, Tomasello E, Geissmann F, Vivier E, Walzer T. The trafficking of natural killer cells. Immunol Rev. 2007 Dec;220:169–182. doi: 10.1111/j.1600-065X.2007.00563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shi FD, Ljunggren HG, La Cava A, Van Kaer L. Organ-specific features of natural killer cells. Nat Rev Immunol. 2011 Sep 23;11(10):658–671. doi: 10.1038/nri3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim S, Iizuka K, Aguila HL, Weissman IL, Yokoyama WM. In vivo natural killer cell activities revealed by natural killer cell-deficient mice. Proc Natl Acad Sci U S A. 2000 Mar 14;97(6):2731–2736. doi: 10.1073/pnas.050588297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lodoen MB, Lanier LL. Natural killer cells as an initial defense against pathogens. Curr Opin Immunol. 2006 Aug;18(4):391–398. doi: 10.1016/j.coi.2006.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol. 2008 May;9(5):503–510. doi: 10.1038/ni1582. [DOI] [PubMed] [Google Scholar]
- 6.Wallace ME, Smyth MJ. The role of natural killer cells in tumor control-effectors and regulators of adaptive immunity. Springer Semin Immunopathol. 2005 Jun;27(1):49–64. doi: 10.1007/s00281-004-0195-x. [DOI] [PubMed] [Google Scholar]
- 7.Strowig T, Brilot F, Münz C. Noncytotoxic functions of NK cells: direct pathogen restriction and assistance to adaptive immunity. J Immunol. 2008 Jun 15;180(12):7785–7791. doi: 10.4049/jimmunol.180.12.7785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferlazzo G, Thomas D, Lin SL, Goodman K, Morandi B, Muller WA, Moretta A, Münz C. The abundant NK cells in human secondary lymphoid tissues require activation to express killer cell Ig-like receptors and become cytolytic. J Immunol. 2004 Feb 1;172(3):1455–1462. doi: 10.4049/jimmunol.172.3.1455. [DOI] [PubMed] [Google Scholar]
- 9.Caligiuri MA. Human natural killer cells. Blood. 2008 Aug 1;112(3):461–469. doi: 10.1182/blood-2007-09-077438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Maria A, Bozzano F, Cantoni C, Moretta L. Revisiting human natural killer cell subset function revealed cytolytic CD56(dim)CD16+ NK cells as rapid producers of abundant IFN-gamma on activation. Proc Natl Acad Sci U S A. 2011 Jan 11;108(2):728–732. doi: 10.1073/pnas.1012356108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hayakawa Y, Huntington ND, Nutt SL, Smyth MJ. Functional subsets of mouse natural killer cells. Immunol Rev. 2006 Dec;214:47–55. doi: 10.1111/j.1600-065X.2006.00454.x. [DOI] [PubMed] [Google Scholar]
- 12.Yokoyama WM, Kim S, French AR. The dynamic life of natural killer cells. Annu Rev Immunol. 2004;22:405–429. doi: 10.1146/annurev.immunol.22.012703.104711. [DOI] [PubMed] [Google Scholar]
- 13.Freud AG, Caligiuri MA. Human natural killer cell development. Immunol Rev. 2006 Dec;214:56–72. doi: 10.1111/j.1600-065X.2006.00451.x. [DOI] [PubMed] [Google Scholar]
- 14.Cella M, Fuchs A, Vermi W, Facchetti F, Otero K, Lennerz JK, Doherty JM, Mills JC, Colonna M. A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature. 2009 Feb 5;457(7230):722–725. doi: 10.1038/nature07537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cupedo T, Crellin NK, Papazian N, Rombouts EJ, Weijer K, Grogan JL, Fibbe WE, Cornelissen JJ, Spits H. Human fetal lymphoid tissue-inducer cells are interleukin 17-producing precursors to RORC+ CD127+ natural killer-like cells. Nat Immunol. 2009 Jan;10(1):66–74. doi: 10.1038/ni.1668. [DOI] [PubMed] [Google Scholar]
- 16.Luci C, Reynders A, Ivanov II, Cognet C, Chiche L, Chasson L, Hardwigsen J, Anguiano E, Banchereau J, Chaussabel D, Dalod M, Littman DR, Vivier E, Tomasello E. Influence of the transcription factor RORgammat on the development of NKp46+ cell populations in gut and skin. Nat Immunol. 2009 Jan;10(1):75–82. doi: 10.1038/ni.1681. [DOI] [PubMed] [Google Scholar]
- 17.Sanos SL, Bui VL, Mortha A, Oberle K, Heners C, Johner C, Diefenbach A. RORgammat and commensal microflora are required for the differentiation of mucosal interleukin 22-producing NKp46+ cells. Nat Immunol. 2009 Jan;10(1):83–91. doi: 10.1038/ni.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Satoh-Takayama N, Vosshenrich CA, Lesjean-Pottier S, Sawa S, Lochner M, Rattis F, Mention JJ, Thiam K, Cerf-Bensussan N, Mandelboim O, Eberl G, Di Santo JP. Microbial flora drives interleukin 22 production in intestinal NKp46+ cells that provide innate mucosal immune defense. Immunity. 2008 Dec 19;29(6):958–970. doi: 10.1016/j.immuni.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 19.Ouyang W. Distinct roles of IL-22 in human psoriasis and inflammatory bowel disease. Cytokine Growth Factor Rev. 2010 Dec;21(6):435–441. doi: 10.1016/j.cytogfr.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 20.Wolk K, Witte E, Witte K, Warszawska K, Sabat R. Biology of interleukin-22. Semin Immunopathol. 2010 Mar;32(1):17–31. doi: 10.1007/s00281-009-0188-x. [DOI] [PubMed] [Google Scholar]
- 21.Aujla SJ, Kolls JK. IL-22: a critical mediator in mucosal host defense. J Mol Med (Berl) 2009 May;87(5):451–454. doi: 10.1007/s00109-009-0448-1. [DOI] [PubMed] [Google Scholar]
- 22.Zenewicz LA, Flavell RA. IL-22 and inflammation: leukin' through a glass onion. Eur J Immunol. 2008 Dec;38(12):3265–3268. doi: 10.1002/eji.200838655. [DOI] [PubMed] [Google Scholar]
- 23.Sonnenberg GF, Fouser LA, Artis D. Border patrol: regulation of immunity, inflammation and tissue homeostasis at barrier surfaces by IL-22. Nat Immunol. 2011 May;12(5):383–390. doi: 10.1038/ni.2025. [DOI] [PubMed] [Google Scholar]
- 24.Ouyang W, Rutz S, Crellin NK, Valdez PA, Hymowitz SG. Regulation and functions of the IL-10 family of cytokines in inflammation and disease. Annu Rev Immunol. 2011;29:71–109. doi: 10.1146/annurev-immunol-031210-101312. [DOI] [PubMed] [Google Scholar]
- 25.Cooper MA, Colonna M, Yokoyama WM. Hidden talents of natural killers: NK cells in innate and adaptive immunity. EMBO Rep. 2009 Oct;10(10):1103–1110. doi: 10.1038/embor.2009.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wolk K, Kunz S, Asadullah K, Sabat R. Cutting edge: immune cells as sources and targets of the IL-10 family members? J Immunol. 2002 Jun 1;168(11):5397–5402. doi: 10.4049/jimmunol.168.11.5397. [DOI] [PubMed] [Google Scholar]
- 27.Spits H, Di Santo JP. The expanding family of innate lymphoid cells: regulators and effectors of immunity and tissue remodeling. Nat Immunol. 2011 Jan;12(1):21–27. doi: 10.1038/ni.1962. [DOI] [PubMed] [Google Scholar]
- 28.Takatori H, Kanno Y, Watford WT, Tato CM, Weiss G, Ivanov II, Littman DR, O'Shea JJ. Lymphoid tissue inducer-like cells are an innate source of IL-17 and IL-22. J Exp Med. 2009 Jan 16;206(1):35–41. doi: 10.1084/jem.20072713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ivanov II, Diehl GE, Littman DR. Lymphoid tissue inducer cells in intestinal immunity. Curr Top Microbiol Immunol. 2006;308:59–82. doi: 10.1007/3-540-30657-9_3. [DOI] [PubMed] [Google Scholar]
- 30.Sanos SL, Vonarbourg C, Mortha A, Diefenbach A. Control of epithelial cell function by interleukin-22-producing RORγt+ innate lymphoid cells. Immunology. 2011 Apr;132(4):453–465. doi: 10.1111/j.1365-2567.2011.03410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crellin NK, Trifari S, Kaplan CD, Cupedo T, Spits H. Human NKp44+IL-22+ cells and LTi-like cells constitute a stable RORC+ lineage distinct from conventional natural killer cells. J Exp Med. 2010 Feb 15;207(2):281–290. doi: 10.1084/jem.20091509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Satoh-Takayama N, Lesjean-Pottier S, Vieira P, Sawa S, Eberl G, Vosshenrich CA, Di Santo JP. IL-7 and IL-15 independently program the differentiation of intestinal CD3-NKp46+ cell subsets from Id2-dependent precursors. J Exp Med. 2010 Feb 15;207(2):273–280. doi: 10.1084/jem.20092029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vonarbourg C, Mortha A, Bui VL, Hernandez PP, Kiss EA, Hoyler T, Flach M, Bengsch B, Thimme R, Hölscher C, Hönig M, Pannicke U, Schwarz K, Ware CF, Finke D, Diefenbach A. Regulated expression of nuclear receptor RORγt confers distinct functional fates to NK cell receptor-expressing RORγt(+) innate lymphocytes. Immunity. 2010 Nov 24;33(5):736–751. doi: 10.1016/j.immuni.2010.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cella M, Otero K, Colonna M. Expansion of human NK-22 cells with IL-7, IL-2, and IL-1beta reveals intrinsic functional plasticity. Proc Natl Acad Sci U S A. 2010 Jun 15;107(24):10961–10966. doi: 10.1073/pnas.1005641107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hughes T, Becknell B, Freud AG, McClory S, Briercheck E, Yu J, Mao C, Giovenzana C, Nuovo G, Wei L, Zhang X, Gavrilin MA, Wewers MD, Caligiuri MA. Interleukin-1beta selectively expands and sustains interleukin-22+ immature human natural killer cells in secondary lymphoid tissue. Immunity. 2010 Jun 25;32(6):803–814. doi: 10.1016/j.immuni.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guarner F. Enteric flora in health and disease. Digestion. 2006;73(Suppl 1):5–12. doi: 10.1159/000089775. [DOI] [PubMed] [Google Scholar]
- 37.Maloy KJ, Powrie F. Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature. 2011 Jun 15;474(7351):298–306. doi: 10.1038/nature10208. [DOI] [PubMed] [Google Scholar]
- 38.Hooper LV, Macpherson AJ. Immune adaptations that maintain homeostasis with the intestinal microbiota. Nat Rev Immunol. 2010 Mar;10(3):159–169. doi: 10.1038/nri2710. [DOI] [PubMed] [Google Scholar]
- 39.Kinoshita N, Hiroi T, Ohta N, Fukuyama S, Park EJ, Kiyono H. Autocrine IL-15 mediates intestinal epithelial cell death via the activation of neighboring intraepithelial NK cells. J Immunol. 2002 Dec 1;169(11):6187–6192. doi: 10.4049/jimmunol.169.11.6187. [DOI] [PubMed] [Google Scholar]
- 40.León F, Roldán E, Sanchez L, Camarero C, Bootello A, Roy G. Human small-intestinal epithelium contains functional natural killer lymphocytes. Gastroenterology. 2003 Aug;125(2):345–356. doi: 10.1016/s0016-5085(03)00886-2. [DOI] [PubMed] [Google Scholar]
- 41.Zheng Y, Valdez PA, Danilenko DM, Hu Y, Sa SM, Gong Q, Abbas AR, Modrusan Z, Ghilardi N, de Sauvage FJ, Ouyang W. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat Med. 2008 Mar;14(3):282–289. doi: 10.1038/nm1720. [DOI] [PubMed] [Google Scholar]
- 42.Sonnenberg GF, Monticelli LA, Elloso MM, Fouser LA, Artis D. CD4(+) lymphoid tissue-inducer cells promote innate immunity in the gut. Immunity. 2011 Jan 28;34(1):122–134. doi: 10.1016/j.immuni.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zenewicz LA, Yancopoulos GD, Valenzuela DM, Murphy AJ, Stevens S, Flavell RA. Innate and adaptive interleukin-22 protects mice from inflammatory bowel disease. Immunity. 2008 Dec 19;29(6):947–957. doi: 10.1016/j.immuni.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Takayama T, Kamada N, Chinen H, Okamoto S, Kitazume MT, Chang J, Matuzaki Y, Suzuki S, Sugita A, Koganei K, Hisamatsu T, Kanai T, Hibi T. Imbalance of NKp44(+)NKp46(-) and NKp44(-)NKp46(+) natural killer cells in the intestinal mucosa of patients with Crohn's disease. Gastroenterology. 2010 Sep;139(3):882–892. doi: 10.1053/j.gastro.2010.05.040. 892.e1-3. [DOI] [PubMed] [Google Scholar]
- 45.Geremia A, Arancibia-Cárcamo CV, Fleming MP, Rust N, Singh B, Mortensen NJ, Travis SP, Powrie F. IL-23-responsive innate lymphoid cells are increased in inflammatory bowel disease. J Exp Med. 2011 Jun 6;208(6):1127–1133. doi: 10.1084/jem.20101712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Buonocore S, Ahern PP, Uhlig HH, Ivanov II, Littman DR, Maloy KJ, Powrie F. Innate lymphoid cells drive interleukin-23-dependent innate intestinal pathology. Nature. 2010 Apr 29;464(7293):1371–1375. doi: 10.1038/nature08949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brenchley JM, Douek DC. HIV infection and the gastrointestinal immune system. Mucosal Immunol. 2008 Jan;1(1):23–30. doi: 10.1038/mi.2007.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reeves RK, Rajakumar PA, Evans TI, Connole M, Gillis J, Wong FE, Kuzmichev YV, Carville A, Johnson RP. Gut inflammation and indoleamine deoxygenase inhibit IL-17 production and promote cytotoxic potential in NKp44+ mucosal NK cells during SIV infection. Blood. 2011 Sep 22;118(12):3321–3330. doi: 10.1182/blood-2011-04-347260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Raffatellu M, Santos RL, Verhoeven DE, George MD, Wilson RP, Winter SE, Godinez I, Sankaran S, Paixao TA, Gordon MA, Kolls JK, Dandekar S, Bäumler AJ. Simian immunodeficiency virus-induced mucosal interleukin-17 deficiency promotes Salmonella dissemination from the gut. Nat Med. 2008 Apr;14(4):421–428. doi: 10.1038/nm1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Miller CM, Boulter NR, Ikin RJ, Smith NC. The immunobiology of the innate response to Toxoplasma gondii. Int J Parasitol. 2009 Jan;39(1):23–39. doi: 10.1016/j.ijpara.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 51.Passos ST, Silver JS, O'Hara AC, Sehy D, Stumhofer JS, Hunter CA. IL-6 promotes NK cell production of IL-17 during toxoplasmosis. J Immunol. 2010 Feb 15;184(4):1776–1783. doi: 10.4049/jimmunol.0901843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Muñoz M, Heimesaat MM, Danker K, Struck D, Lohmann U, Plickert R, Bereswill S, Fischer A, Dunay IR, Wolk K, Loddenkemper C, Krell HW, Libert C, Lund LR, Frey O, Hölscher C, Iwakura Y, Ghilardi N, Ouyang W, Kamradt T, Sabat R, Liesenfeld O. Interleukin (IL)-23 mediates Toxoplasma gondii-induced immunopathology in the gut via matrixmetalloproteinase-2 and IL-22 but independent of IL-17. J Exp Med. 2009 Dec 21;206(13):3047–3059. doi: 10.1084/jem.20090900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wilson MS, Feng CG, Barber DL, Yarovinsky F, Cheever AW, Sher A, Grigg M, Collins M, Fouser L, Wynn TA. Redundant and pathogenic roles for IL-22 in mycobacterial, protozoan, and helminth infections. J Immunol. 2010 Apr 15;184(8):4378–4390. doi: 10.4049/jimmunol.0903416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Reynders A, Yessaad N, Vu Manh TP, Dalod M, Fenis A, Aubry C, Nikitas G, Escalière B, Renauld JC, Dussurget O, Cossart P, Lecuit M, Vivier E, Tomasello E. Identity, regulation and in vivo function of gut NKp46+RORγt+ and NKp46+RORγt- lymphoid cells. EMBO J. 2011 Jun 17;30(14):2934–2947. doi: 10.1038/emboj.2011.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McDermott JR, Humphreys NE, Forman SP, Donaldson DD, Grencis RK. Intraepithelial NK cell-derived IL-13 induces intestinal pathology associated with nematode infection. J Immunol. 2005 Sep 1;175(5):3207–3213. doi: 10.4049/jimmunol.175.5.3207. [DOI] [PubMed] [Google Scholar]
- 56.Evans SE, Xu Y, Tuvim MJ, Dickey BF. Inducible innate resistance of lung epithelium to infection. Annu Rev Physiol. 2010;72:413–435. doi: 10.1146/annurev-physiol-021909-135909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Holt PG, Strickland DH, Wikström ME, Jahnsen FL. Regulation of immunological homeostasis in the respiratory tract. Nat Rev Immunol. 2008 Feb;8(2):142–152. doi: 10.1038/nri2236. [DOI] [PubMed] [Google Scholar]
- 58.Culley FJ. Natural killer cells in infection and inflammation of the lung. Immunology. 2009 Oct;128(2):151–163. doi: 10.1111/j.1365-2567.2009.03167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Guo H, Topham DJ. Interleukin-22 (IL-22) production by pulmonary Natural Killer cells and the potential role of IL-22 during primary influenza virus infection. J Virol. 2010 Aug;84(15):7750–7759. doi: 10.1128/JVI.00187-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Junqueira-Kipnis AP, Kipnis A, Jamieson A, Juarrero MG, Diefenbach A, Raulet DH, Turner J, Orme IM. NK cells respond to pulmonary infection with Mycobacterium tuberculosis, but play a minimal role in protection. J Immunol. 2003 Dec 1;171(11):6039–6045. doi: 10.4049/jimmunol.171.11.6039. [DOI] [PubMed] [Google Scholar]
- 61.Kos FJ, Engleman EG. Role of natural killer cells in the generation of influenza virus-specific cytotoxic T cells. Cell Immunol. 1996 Oct 10;173(1):1–6. doi: 10.1006/cimm.1996.0245. [DOI] [PubMed] [Google Scholar]
- 62.Stein-Streilein J, Guffee J, Fan W. Locally and systemically derived natural killer cells participate in defense against intranasally inoculated influenza virus. Reg Immunol. 1988 Sep-Oct;1(2):100–105. [PubMed] [Google Scholar]
- 63.Arnon TI, Achdout H, Lieberman N, Gazit R, Gonen-Gross T, Katz G, Bar-Ilan A, Bloushtain N, Lev M, Joseph A, Kedar E, Porgador A, Mandelboim O. The mechanisms controlling the recognition of tumor- and virus-infected cells by NKp46. Blood. 2004 Jan 15;103(2):664–672. doi: 10.1182/blood-2003-05-1716. [DOI] [PubMed] [Google Scholar]
- 64.Mandelboim O, Lieberman N, Lev M, Paul L, Arnon TI, Bushkin Y, Davis DM, Strominger JL, Yewdell JW, Porgador A. Recognition of haemagglutinins on virus-infected cells by NKp46 activates lysis by human NK cells. Nature. 2001 Feb 22;409(6823):1055–1060. doi: 10.1038/35059110. [DOI] [PubMed] [Google Scholar]
- 65.Gazit R, Gruda R, Elboim M, Arnon TI, Katz G, Achdout H, Hanna J, Qimron U, Landau G, Greenbaum E, Zakay-Rones Z, Porgador A, Mandelboim O. Lethal influenza infection in the absence of the natural killer cell receptor gene Ncr1. Nat Immunol. 2006 May;7(5):517–523. doi: 10.1038/ni1322. [DOI] [PubMed] [Google Scholar]
- 66.Small CL, McCormick S, Gill N, Kugathasan K, Santosuosso M, Donaldson N, Heinrichs DE, Ashkar A, Xing Z. NK cells play a critical protective role in host defense against acute extracellular Staphylococcus aureus bacterial infection in the lung. J Immunol. 2008 Apr 15;180(8):5558–5568. doi: 10.4049/jimmunol.180.8.5558. [DOI] [PubMed] [Google Scholar]
- 67.Spörri R, Joller N, Albers U, Hilbi H, Oxenius A. MyD88-dependent IFN-gamma production by NK cells is key for control of Legionella pneumophila infection. J Immunol. 2006 May 15;176(10):6162–6171. doi: 10.4049/jimmunol.176.10.6162. [DOI] [PubMed] [Google Scholar]
- 68.Borchers MT, Harris NL, Wesselkamper SC, Zhang S, Chen Y, Young L, Lau GW. The NKG2D-activating receptor mediates pulmonary clearance of Pseudomonas aeruginosa. Infect Immun. 2006 May;74(5):2578–2586. doi: 10.1128/IAI.74.5.2578-2586.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wesselkamper SC, Eppert BL, Motz GT, Lau GW, Hassett DJ, Borchers MT. NKG2D is critical for NK cell activation in host defense against Pseudomonas aeruginosa respiratory infection. J Immunol. 2008 Oct 15;181(8):5481–5489. doi: 10.4049/jimmunol.181.8.5481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Byrne P, McGuirk P, Todryk S, Mills KH. Depletion of NK cells results in disseminating lethal infection with Bordetella pertussis associated with a reduction of antigen-specific Th1 and enhancement of Th2, but not Tr1 cells. Eur J Immunol. 2004 Sep;34(9):2579–2588. doi: 10.1002/eji.200425092. [DOI] [PubMed] [Google Scholar]
- 71.Morrison BE, Park SJ, Mooney JM, Mehrad B. Chemokine-mediated recruitment of NK cells is a critical host defense mechanism in invasive aspergillosis. J Clin Invest. 2003 Dec;112(12):1862–1870. doi: 10.1172/JCI18125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Park SJ, Hughes MA, Burdick M, Strieter RM, Mehrad B. Early NK cell-derived IFN-{gamma} is essential to host defense in neutropenic invasive aspergillosis. J Immunol. 2009 Apr 1;182(7):4306–4312. doi: 10.4049/jimmunol.0803462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Garg A, Barnes PF, Porgador A, Roy S, Wu S, Nanda JS, Griffith DE, Girard WM, Rawal N, Shetty S, Vankayalapati R. Vimentin expressed on Mycobacterium tuberculosis-infected human monocytes is involved in binding to the NKp46 receptor. J Immunol. 2006 Nov 1;177(9):6192–6198. doi: 10.4049/jimmunol.177.9.6192. [DOI] [PubMed] [Google Scholar]
- 74.Vankayalapati R, Garg A, Porgador A, Griffith DE, Klucar P, Safi H, Girard WM, Cosman D, Spies T, Barnes PF. Role of NK cell-activating receptors and their ligands in the lysis of mononuclear phagocytes infected with an intracellular bacterium. J Immunol. 2005 Oct 1;175(7):4611–4617. doi: 10.4049/jimmunol.175.7.4611. [DOI] [PubMed] [Google Scholar]
- 75.Esin S, Batoni G, Counoupas C, Stringaro A, Brancatisano FL, Colone M, Maisetta G, Florio W, Arancia G, Campa M. Direct binding of human NK cell natural cytotoxicity receptor NKp44 to the surfaces of mycobacteria and other bacteria. Infect Immun. 2008 Apr;76(4):1719–1727. doi: 10.1128/IAI.00870-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Esin S, Batoni G, Pardini M, Favilli F, Bottai D, Maisetta G, Florio W, Vanacore R, Wigzell H, Campa M. Functional characterization of human natural killer cells responding to Mycobacterium bovis bacille Calmette-Guérin. Immunology. 2004 May;112(1):143–152. doi: 10.1111/j.1365-2567.2004.01858.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dhiman R, Indramohan M, Barnes PF, Nayak RC, Paidipally P, Rao LV, Vankayalapati R. IL-22 produced by human NK cells inhibits growth of Mycobacterium tuberculosis by enhancing phagolysosomal fusion. J Immunol. 2009 Nov 15;183(10):6639–6645. doi: 10.4049/jimmunol.0902587. [DOI] [PubMed] [Google Scholar]
- 78.Aujla SJ, Chan YR, Zheng M, Fei M, Askew DJ, Pociask DA, Reinhart TA, McAllister F, Edeal J, Gaus K, Husain S, Kreindler JL, Dubin PJ, Pilewski JM, Myerburg MM, Mason CA, Iwakura Y, Kolls JK. IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nat Med. 2008 Mar;14(3):275–281. doi: 10.1038/nm1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Manaster I, Mandelboim O. The unique properties of human NK cells in the uterine mucosa. Placenta. 2008 Mar;29(Suppl A):S60–S66. doi: 10.1016/j.placenta.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 80.Zhang J, Chen Z, Smith GN, Croy BA. Natural killer cell-triggered vascular transformation: maternal care before birth? Cell Mol Immunol. 2011 Jan;8(1):1–11. doi: 10.1038/cmi.2010.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Vacca P, Moretta L, Moretta A, Mingari MC. Origin, phenotype and function of human natural killer cells in pregnancy. Trends Immunol. 2011 Nov;32(11):517–523. doi: 10.1016/j.it.2011.06.013. [DOI] [PubMed] [Google Scholar]
- 82.Moffett-King A. Natural killer cells and pregnancy. Nat Rev Immunol. 2002 Sep;2(9):656–663. doi: 10.1038/nri886. [DOI] [PubMed] [Google Scholar]
- 83.Mselle TF, Meadows SK, Eriksson M, Smith JM, Shen L, Wira CR, Sentman CL. Unique characteristics of NK cells throughout the human female reproductive tract. Clin Immunol. 2007 Jul;124(1):69–76. doi: 10.1016/j.clim.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 84.Male V, Hughes T, McClory S, Colucci F, Caligiuri MA, Moffett A. Immature NK cells, capable of producing IL-22, are present in human uterine mucosa. J Immunol. 2010 Oct 1;185(7):3913–3918. doi: 10.4049/jimmunol.1001637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.El Costa H, Tabiasco J, Berrebi A, Parant O, Aguerre-Girr M, Piccinni MP, Le Bouteiller P. Effector functions of human decidual NK cells in healthy early pregnancy are dependent on the specific engagement of natural cytotoxicity receptors. J Reprod Immunol. 2009 Nov;82(2):142–147. doi: 10.1016/j.jri.2009.06.123. [DOI] [PubMed] [Google Scholar]
- 86.Eriksson M, Meadows SK, Wira CR, Sentman CL. Unique phenotype of human uterine NK cells and their regulation by endogenous TGF-beta. J Leukoc Biol. 2004 Sep;76(3):667–675. doi: 10.1189/jlb.0204090. [DOI] [PubMed] [Google Scholar]
- 87.Barber EM, Pollard JW. The uterine NK cell population requires IL-15 but these cells are not required for pregnancy nor the resolution of a Listeria monocytogenes infection. J Immunol. 2003 Jul 1;171(1):37–46. doi: 10.4049/jimmunol.171.1.37. [DOI] [PubMed] [Google Scholar]
- 88.Mselle TF, Howell AL, Ghosh M, Wira CR, Sentman CL. Human uterine natural killer cells but not blood natural killer cells inhibit human immunodeficiency virus type 1 infection by secretion of CXCL12. J Virol. 2009 Nov;83(21):11188–11195. doi: 10.1128/JVI.00562-09. [DOI] [PMC free article] [PubMed] [Google Scholar]