Abstract
Adenoviruses are important infectious agents and also emerging vectors in different biomedical applications. These viruses elicit a strong innate and adaptive immune response, which influences both the course of disease and the success of the applied vectors. Several Toll-like Receptor (TLR)-dependent and -independent mechanisms contribute to these responses. Understanding of the involved viral and cellular factors is crucial for the treatment of various adenovirus diseases and the optimal design of adenovirus vector applications. Here we summarize our current understanding of the complex nature of adenovirus-induced innate immune mechanisms.
Keywords: adenovirus, endosomal escape, IL-1, innate response, IRF7, Toll-like receptors, type-I interferons, viral entry
Introduction
Adenoviruses are important human and animal pathogens. They are also promising vectors for human or veterinary clinical science applications and biotechnology. They can be grown to high titers, can be genetically manipulated to accept large pieces of foreign DNA, and infect many different cell types and tissues with high efficiency [1]. However, adenovirus infection and particularly the entry stages elicit a strong innate immune response, which affects the course of adenoviral disease in patients, and also the therapeutic efficiency of recombinant vectors [2, 3]. Understanding how these innate responses are generated is of key importance for the treatment of adenovirus disease and vector applications. Here we discuss the mechanisms that elicit innate responses during adenovirus entry into immune and non-immune cells.
Taxonomy and structure of human adenoviruses
Adenoviruses are non-enveloped, middle-sized viruses with an icosahedral symmetry containing a linear, double-stranded DNA genome [1, 4–6]. They belong to the genus of Mastadenovirus and are subdivided to seven Ad species (A–G). Currently, there are 68 sequenced HAdVs (see http://www.vmri.hu/~harrach/ADENOSEQ.HTM). All of these viruses are closely related, but may differ in their tropism, as some lead to infections of the respiratory tract while others infect eye, kidney, liver, or the gastrointestinal tract [7]. How the different tropisms precisely relate to the viral genetics is incompletely known [8].
Human illnesses associated with adenoviral infections
In individuals with functional immune system adenovirus infections can be asymptomatic, but often cause organ-restricted illnesses such as upper and lower respiratory tract infections (pharyngitis, bronchitis or pneumonia, species C, B and E), epidemic and follicular conjunctivitis (species D and B), and gastroenteritis (species A, E and F) [9]. The caused disease can be severe and even fatal (especially in infants) but most often self limiting with viral persistence and shedding [9].
In immuno-compromised patients such as bone marrow or solid organ transplant recipients or people suffering from AIDS, adenoviruses often cause systemic diseases with high mortality affecting various organs such as liver, heart, or brain [10].
Adenoviral vectors for the treatment and prevention of diseases
Adenoviruses are promising agents for viral gene therapy vectors because they can grow to high titres, infect various cell types, and can be easily manipulated to express relatively large genes [1, 9]. Potential applications include the delivery of curative genes (cystic fibrosis, cardiovascular and hepatic diseases), oncolytic viruses, and vaccine applications (recombinant subunit vaccines or virus-like particles) [11–13].
Importance of Ad-induced adaptive and innate responses in viral disease and vector applications
Adenoviruses, similar to other intracellular pathogens elicit an effective humoral and cellular immune response (neutralizing anti-viral capsid antibodies and the induction of CD8+ T cells) that prevents generalized disease and re-infection with the same Ad serotype [9]. Notably however, due to common exposure, most adult individuals already exhibit specific antibodies and cytotoxic T cells to prevalently used vectors of Ad species C. This prevents efficient organ targeting by such vectors and comprises major obstacles in systemic Ad gene therapy [14, 15].
In natural adenovirus infections, the elicited innate responses such as complement activation, phagocytosis and the induction of proinflammatory cytokines and interferons (IFNs) are beneficial in most cases as they result in the rapid inactivation of adenoviruses and adenovirus-producing cells and also help to build a proper antiviral-acquired immune response [9]. However, overproduction of certain proinflammatory mediators such as TNF, IL-6 and IL-8 can be harmful. In infections of lower respiratory tract such a mediator “storm” elicits symptoms analogous to septic shock caused by Gram-negative bacteria [2].
Ad vector-induced innate reactions can also be beneficial or harmful depending on the special type of therapeutic application. Using oncolytic vectors and recombinant vaccines innate responses elicited by the vector itself may be helpful to eliminate targeted tumour cells or drive an accelerated immune response to antigens of interest [16]. Nevertheless, overwhelming innate responses to the Ad vectors are harmful and constitute a major limitation in systemic gene therapy applications aiming to achieve prolonged gene expression as they lead to the destruction of the vector-transduced cells [17]. Moreover, depending on the amounts of applied vector, the induced innate reactions may even lead to life-threatening side effects [18]. Therefore, understanding and proper modification of Ad-induced innate responses is of primary interest for both adenoviral disease treatment and recombinant vector applications.
Ad infectious entry pathways in different cell types
Ad entry, the earliest step in the viral replication cycle seems to be predominantly responsible for the virus-induced innate responses [19]. As innate virus sensing and signalling can be initiated at various steps of this process, it is of crucial importance to understand the mechanisms of the initial events of virus–cell interaction during Ad infection.
Entry in non-immune cells
Adenovirus entry is relatively well characterized in non-immune cells (Fig. 1). HAdVs attach to high affinity receptors, such as Coxsackie Adenovirus Receptor (CAR, species A, C–F) [20], CD46 (species B2), or to low affinity high avidity receptors, such as desmoglein 2 and CD46 (species B1) [21–25], or very low affinity sialic acid residues [26, 27]. Additionally, association of the blood coagulation factor X with blood-delivered HAdVs mediates effective virus uptake into hepatocytes of the liver in mice [28]. The binding of species C and species B HAdV to integrins promotes endocytosis and signalling, which can result in macropinocytic stimulation, and enhances infection [29–33].
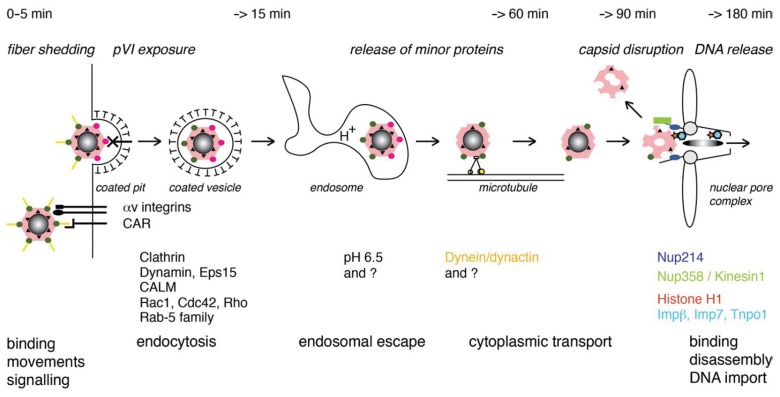
Fig. 1.
Ad entry into non-immune cells, HAdVs attached to CAR and to integrins promotes endocytosis. CAR receptors and alpha v integrin coreceptors trigger the initial uncoating, leading to exposure of protein VI that facilitates endosomal penetration. Ad receptor-mediated endocytosis also involves clathrin, dynamin, Eps15, Rab5 GTPase subfamily proteins, and actin dynamics by Rho GTPases. Ad particles that escaped are then transported by the dynein motor on microtubules to the nuclear pore complex, where viral DNA is injected in and transcription begins
The entry mechanisms of the species C HAdV (HAdV-C) is in part known at the mechanistic level. For example, the movement of CAR receptors by acto-myosin-mediated drifting motions and the stationary confinement of the alpha v integrin coreceptors trigger the initial uncoating steps for the incoming HAdV-C2 or C5 at the cell surface, the loss of the fibre proteins from the capsid [34–36]. This then leads to the exposure of the membrane lytic protein VI from the capsid interior, and thereby prepares the partly uncoated virus for membrane penetration [36–39]. HAdV-C2/5 are taken up by receptor-mediated endocytosis, and this involves clathrin, dynamin and the EGF receptor substrate Eps15, proteins from the Rab5 GTPase subfamily, and also actin dynamics indicated by the involvement of various Rho GTPases [34, 40–46].
Soon after endocytosis, the virus penetrates from an endosome to the cytosol [45, 47, 48]. Although the penetration mechanism is sensitive to lysosomotropic agents, such as ammonium chloride [34], it is independent of the protein ATPase inhibitor bafilomycin A1 [43, 49], and independent of microtubules [50] and the 19 °C block of early endosomal trafficking [43, 51]. This implies that virus penetration does not require late endosomes or lysosomes, in agreement with the observation that the membrane lytic activity of the viral protein VI is pH-independent [37]. Virus particles, which escaped from the endosome, are then transported by the dynein motor on microtubules to the nucleus, and they lead to infection [50, 52–55]. This is enhanced by the dynactin complex [50, 53, 56].
How the virus gets from the microtubules to the nuclear pore complex is still not completely understood, although this step is inhibited when nuclear export is blocked [57–59]. When the virus arrives near the nuclear envelope it docks the nuclear pore complex protein Nup214 [60]. The capsid is disassembled by the action of a kinesin motor protein, conventional kinesin-1, which binds in its inactive form to the virus particle [61]. The kinesin motor gets activated by a dual cue, the binding to the nuclear pore complex protein Nup358 and microtubules [62]. Microtubules also bind to the distal part of Nup358 [63], and hence provide a track for the displacement of the viral capsid fragments from the nuclear envelope to the periphery [61]. Surprisingly, this also leads to the disruption of the nuclear pore complex and thereby enhances the permeability of the nuclear pore. The enhanced permeability of the nuclear pore together with nuclear import factors importin beta, importin 7 and transportin, which bind to the viral DNA and associated proteins, then enhance import of the viral DNA genome into the nucleus [60, 64–66].
Ad entry in immune cells
Studies with various Ad vectors have shown that these viruses infect and activate mononuclear phagocytes very efficiently in vivo in a way that does not require the known high affinity virus-binding receptors (CAR, CD46 and HSPG) [19]. As these cells possess strong phagocytic activity, Ad entry into them is generally believed to be mediated by phagocytosis. Indeed, macrophage receptors, such as Fc receptor and DC-Sign have been shown to facilitate cellular binding requiring anti Ad antibodies and lactoferrin, respectively [67–69]. If modified by an Fc fusion protein, they can directly attach to the high affinity FcR CD64 [70]. In immature myeloid dendritic cells, adenoviruses escape from the phagolysosome and this requires the virion protease similarly to what was found in non-immune cells [71]; however, much less is known about additional steps of Ad entry into mononuclear phagocytes compared to other non-immune cells. Ads can also infect plasmacytoid dendritic cells (pDCs) that are non-phagocytic using CD46 as a receptor [72], but again, further details of Ad entry into these cells are not characterized.
Ad entry-induced cellular signalling and innate responses in non-immune cells
In non-immune cells signalling events activated by Ad entry are relatively well studied. High affinity receptors (e.g. CAR), necessary for cell surface binding of Ads, may initiate cellular signalling events, even in the absence of virus [73, 74]. Binding of RGD motifs of the Ad virion component penton base to cellular integrins triggers the activation of several important signalling molecules such as phosphatidyl-inositol 3-OH kinase (PI3K) and the Rho family of small GTPases and these events enhance virus internalization [8, 75]. Viruse uptake or endosomal escape of species C Ads has been shown to be associated with protein kinase C activity [35], and the p38 and ERK mitogen-activated protein kinases (MAPK) facilitate Ad movement along the microtubule network [76, 77]. The activation by the ERK and p38 MAPKs and nuclear factor kappa B (NF-κB) has also been shown upon species C Ad infection in renal epithelial cells [19]. Activation of these MAPKs and NFκB was responsible for the induction of chemokines such as IP-10 and IL-8 [78], which has also been observed in respiratory epithelial cells infected with species B Ads [79].
Ad entry-induced innate responses in immune cells
Although non-immune cells may contribute to certain Ad innate reactions, the majority of these responses stem from cells of the innate immune system such as various macrophage and dendritic cell populations. In the following sections, we discuss the major determinants of these responses.
Ad entry-elicited soluble mediators
In vivo studies with rodent and primate animal systems and in human clinical trials revealed the induction of a plethora of mostly pro-inflammatory mediators. These include pro-inflammatory cytokines (IL-6, TNF, IL-12, IL-1α and β), chemokines (IL-8, MIP-2, IP-10, RANTES, MIP-1α, MIP-1β and MCP-1), platelet-activating factor (PAF), and type-I interferons (IFN-αβ) [19, 71, 80–83]. Interestingly, certain Ad-induced mediators may in turn lead to the production of others, as it was shown recently that the production of Ad-induced chemokines depended on virus-induced IL-1 [84], and also that IFN-αβ signalling positively regulates the production of type I IFNs, IL-6 and IL-12 [71, 80].
Cellular sources of Ad-induced mediators
Ad entry-induced inflammatory mediators are predominantly produced by different kinds of mononuclear phagocytes [80]; however, epithelial cells and organ parenchymal cells also contribute to the production of chemokines [19]. Various Ad species have different organ tropism [85], and data available in this respect refer mostly to species C Ads. With these viruses, liver and spleen are the main targeted organs [85]. TNF has been shown to be produced by the liver resident macrophages, Kupffer cells [81], while the induction of chemokines, IL-1 and IL-6, has been demonstrated both in liver and spleen [71, 84, 86]. In the later organ, Ads induce the production of IL-1α and β in marginal zone and metallophilic macrophages [84]. Ads induce the production of IFN-αβ in various mononuclear phagocytes such as bone marrow-derived macrophages and dendritic cells or primary Kupffer cells [71, 80, 83] and in pDCs [71, 72, 80] in vitro. However, during systemic Ad infection, the vast majority of type I IFNs is made in spleen, predominantly in myeloid dendritic cells [71]. Interestingly, only a small part of IFN-αβ is made by pDCs [71] which are the main type I IFN producer cells in the course of infection with most other viruses [87].
Ad-induced LPS hypersensitivity
Importantly, experiments in mice revealed that infection with Ads modulates innate immune responses to other microbial agents. The best studied example is the induction of hypersensitivity to bacterial endotoxin (LPS) [88]. Other viruses such as Lymphocytic Choriomeningitis Virus (LCMV) have also been shown to induce LPS hypersensitivity. In these cases, early production of IFN-αβ 2–3 days after infection mediates a relatively weak (2–4-fold) hypersensitivity (or even downregulation of TNF production ) [92], while later (after 7 days) IFN- production mediates a strong hypersensitivity to LPS, characterized by the overproduction of TNF [89–93]. In contrast, Ad-induced early-type I IFN production mediates a very strong LPS hypersensitivity characterized by the dramatic overproduction of TNF (50–100-fold) and IL-6 (5–10-fold) [88]. Furthermore, there is a strong overproduction of nitric oxide (NO), mostly in spleen [88]. Interestingly, LPS injection of uninfected control animals elicits NO production mainly in liver. Ad-induced LPS hypersensitivity also results in enhanced, TNF-mediated lethality and thus represents an important mechanism of pathologies in mixed infections caused by both viruses and bacteria [71, 88] (Fig. 2).
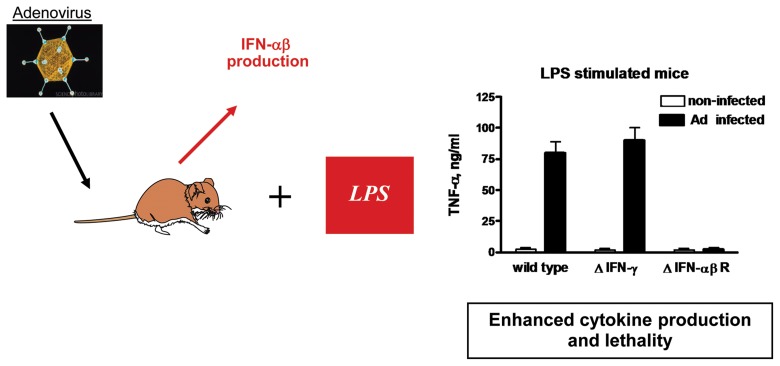
Fig. 2.
Adenovirus-induced LPS hypersensitivity is mediated by type I interferons. Human species B adenovirus injected into B6 mice intraperitoneally induces the production of IFN-αβ. Sixteen hours after virus infection, LPS challenge elicits strong overproduction in wild-type and IFN-γ-deficient mice but not in IRF7-deficient mice incapable of IFN-αβ production or in animals deficient in IFN-αβ receptor
Enhancement of Ad-induced monocyte-derived innate mediators on the infectious entry into non-immune cells
In confluent polarized epithelial cells, the high affinity species C receptor CAR and the integrin coreceptors are localized to the basolateral surface and tight intercellular junctions. They are inaccessible to incoming virus particles from the airway lumen on the apical side of the epithelium [94, 95]. This arrangement constitutes a “natural resistance” to viruses using these receptors, for example, coxsackie virus B3 [96]. A recent study showed that if monocytes are also present in the epithelium, they can take up virus particles without getting infected themselves, but they respond to the viruses by releasing cytokines, such as CXCL8 [97]. CXCL8 release then leads to the redistribution of CAR and alpha v beta 3 (but not beta 5) integrin to the apical surface, and allows the viruses in the apical airway lumen to directly infect the epithelial cells from the apical side (Fig. 3). Since macrophages can be pre-activated by bacterial infection, a bacterial and viral coinfection can potentially contribute to worsening the outcome of disease in mixed infections.
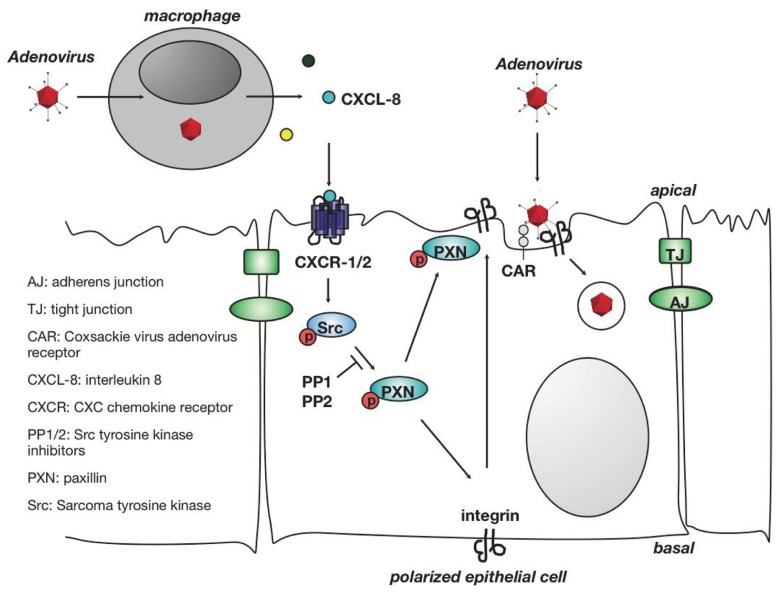
Fig. 3.
Ad-induced enhancement of monocyte-derived innate mediators on the infectious entry into epithelial cells. CAR and the integrin coreceptors are localized to the basolateral surface and tight intercellular junctions in confluent polarized epithelial cells, preventing species C Ad entry. Ad-infected monocytes produce IL-8 that induces redistribution of CAR and alpha v beta 3 integrin to the apical surface and allows virus infection from the apical airway lumen
Molecular mechanisms mediating the Ad triggered innate responses
Several receptors and signalling pathways have been shown to play important roles in the Ad-induced innate responses. However, not much attention was paid to possible differences in causative viral ligands, receptors involved, signalling and responses in different Ad-sensitive cell types. Actually, the inducing adenoviral ligand(s) and cellular receptors are largely unknown and multiple mechanisms seem to be involved. In the following, we discuss our current knowledge of the innate molecular sensing of Ads.
Binding to Ad surface receptors
Since cellular integrins elicit well-characterized downstream signalling events (e.g. PI3K activation) which can lead to NF-κB and MAPK activation, these receptors were thought to be involved in the induction of pro-inflammatory mediators for a long time. Indeed, RGD deleting Ads not capable of binding to cellular integrins elicit weaker responses [98]. However, since integrin mediated signalling events are also required for virus internalization many of these effects can reflect delayed activation of other sensors at later stages of Ad entry [8]. Nevertheless, at least in Ad infected spleen marginal zone macrophages integrin mediated signalling by itself is sufficient to elicit the production of IL-1α and β because Ad2 ts1, a mutant endocytosed normally but incapable of escaping from the endosome [43, 46, 99] induces IL-1 production requiring Ad RGD motifs and cellular integrins but not Toll-like receptors (TLRs) [84].
Ad-induced TLR activation
Cell surface TLRs 2 and 4 and endosomal TLR7, 8 and 9) are implicated in the innate sensing of different types of viruses [87, 100]. Also, in the case of Ads, TLRs have been shown to be involved in innate sensing. In pDCs, TLR9 plays a crucial role in Ad-induced type I IFN production [71, 72, 80]. Furthermore, TLR2, 4 and 9 were reported to play a role in the elicitation of IL-12, MCP-1 and RANTES responses in mononuclear phagocytes in Ad-infected mice or in vitro [86, 101]. However, the induction of IL-1, type I IFNs was predominantly independent of TLRs [71, 84].
Signalling and cytokine responses requiring Ad endosomal escape
IL-1β induction. Cell surface-initiated integrin signalling was shown to contribute to IL-1 production [84], but the same study has also shown that Ads capable of endosomal escape induce much more IL-1 than the escape-deficient ts1 mutant [84]. Two other studies also emphasized the crucial role of endosomal Ad escape in the induction of IL-1β. Muruve et al. demonstrated that cytosolic sensing of Ad DNA is capable of activating the inflammasomes in a manner requiring NALP3 and ASC and thus promoting IL-1β secretion [102]. As an alternative mechanism, the release of cathepsin B from phagolysosomes during Ad escape and the consequential activation of caspase-1 and the NLRP3 inflammasome has also been suggested for the production of this cytokine [103].
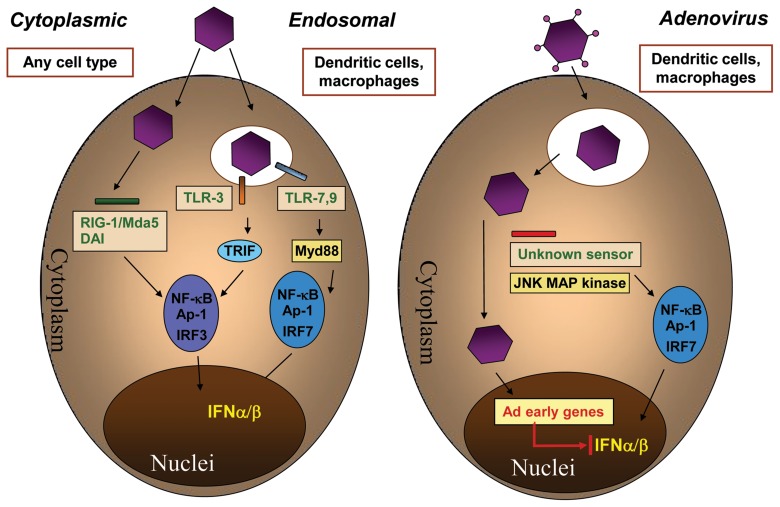
IFN-αβ induction. The Ad entry-mediated production of type I IFNs has been recognized only recently. The importance of IFN-αβ production was indicated by clinical trials with oncolytic Ads, where a better prognosis was associated with situations in which recombinant Ad vectors induced robust IFN-αβ responses [104]. While pDCs can recognize Ad DNA via TLR9 in vitro [71, 72, 80], in vivo studies in Ad-infected mice have shown that the majority of IFN-αβ is produced in myeloid DCs (Fig. 4) in a TLR-independent manner [71]. So far reported, cytosolic induction of IFN-αβ by bacterial and viral DNA strictly requires IRF3 but not the activation of MAP kinases [105, 106]. In contrast, Ad-induced type I IFN production strictly requires endosomal escape-mediated IRF7 (but not necessarily IRF3), JNK MAP kinase and TBK1 activation. But the cytosolic DNA sensor DAI, the RNA helicases RIG-I/MDA-5 [71, 107] are not required for IFN-αβ induction and adenoviral gene expression inhibits their production [71]. Therefore, Ad-induced type I IFN production appear to represent a novel, distinct viral induction pathway, besides the previously described ones mediated by TLRs, RIG-I/MDA5 and free cytosolic DNA recognition (Fig. 5).
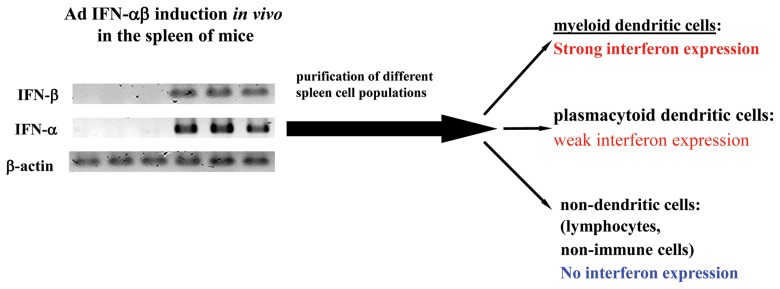
Fig. 4.
Splenic myeloid dendritic cells are the major cellular sources of type-I interferons in response to Ad infection in vivo. IFN-αβ is produced in the spleen of Ad-infected mice. Detection of IFN-αβ in FACS-sorted splenocytes reveals weak induction in pDCs, strong production in myeloid DCs and the absence of IFN-αβ synthesis in all other spleen cell populations
Fig. 5.
Adenovirus elicits IFN-αβ production by a novel viral induction pathway. Left: Type-I IFN induction pathways by viruses other than Ads. Recognition of viral RNAs or DNA in the cytoplasm leads to IFN-αβ production via the RNA helicases RIG-I/MDA-5 and the DNA sensor DAI requiring IRF3 in any infected cell type. Alternatively, in dendritic cells and macrophages, endosomal TLR3 or TLR7-9 activation leads to the induction of IFN-αβ by activating IRF3 or IRF7, respectively. Right: Adenoviruses are recognized in mononuclear phagocytes after endosomal escape in the cytoplasm by a so far unknown receptor requiring JNK MAP kinases and IRF7. This activates type-I IFN production independent of TLRs, RIG-I/MDA-5, DAI, and IRF3. Ad-induced IFN-αβ production is inhibited by viral early gene expression
Concluding remarks and further questions
The adenovirus entry-elicited innate responses represent a very complex example of virus-induced immune reactions. Although many individual aspects of the mechanisms involved are known, our understanding is still rather incomplete. We need more information about the key viral and host elements involved such as the main cellular sensors of Ads and the inducing cognate viral ligands. By studying these questions, further experiments will probably increase our understanding not only on adenovirus-generated responses, but also on general aspects of host-pathogen relationship.
Acknowledgments
We acknowledge Verena Lütschg and Dominic van Essen for their help in making Fig. 3 and for the critical reading of the manuscript, respectively.
Contributor Information
G. Fejer, 1Max Planck Institute of Immunobiology and Epigenetics, Freiburg, Germany; 2Peninsula College of Medicine and Dentistry, Plymouth, Truro, UK.
M. Freudenberg, 1Max Planck Institute of Immunobiology and Epigenetics, Freiburg, Germany.
U. F. Greber, 3Institute of Molecular Life Sciences, University of Zurich, Zurich, Switzerland.
I. Gyory, 1Max Planck Institute of Immunobiology and Epigenetics, Freiburg, Germany.
References
- 1.Shenk T. In: Adenoviridae: The Viruses and Their Replication. Fields BN DMK, Howley PM, editors. New York: Raven Press; 1996. pp. 979–1016. [Google Scholar]
- 2.Mistchenko AS, Diez RA, Mariani AL, Robaldo J, Maffey AF, Bayley-Bustamante G, Grinstein S. Cytokines in adenoviral disease in children: association of interleukin-6, interleukin-8, and tumor necrosis factor alpha levels with clinical outcome. J Pediatr. 1994 May;124(5 Pt 1):714–720. doi: 10.1016/s0022-3476(05)81360-5. [DOI] [PubMed] [Google Scholar]
- 3.Bangari DS, Mittal SK. Current strategies and future directions for eluding adenoviral vector immunity. Curr Gene Ther. 2006 Apr;6(2):215–226. doi: 10.2174/156652306776359478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Horwitz MS. Adenoviruses. In: Knipe DM, Howley PM, editors. Fields Virology. 4. Philadelphia, PA: Raven Press; 2001. pp. 2301–2326. [Google Scholar]
- 5.Reddy VS, Natchiar SK, Stewart PL, Nemerow GR. Crystal structure of human adenovirus at 3.5 A resolution. Science. 2010 Aug 27;329(5995):1071–1075. doi: 10.1126/science.1187292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu H, Jin L, Koh SB, Atanasov I, Schein S, Wu L, Zhou ZH. Atomic structure of human adenovirus by cryo-EM reveals interactions among protein networks. Science. 2010 Aug 27;329(5995):1038–1043. doi: 10.1126/science.1187433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Horwitz MS. Adenovirus immunoregulatory genes and their cellular targets. Virology. 2001 Jan 5;279(1):1–8. doi: 10.1006/viro.2000.0738. [DOI] [PubMed] [Google Scholar]
- 8.Smith JG, Wiethoff CM, Stewart PL, Nemerow GR. Adenovirus. Curr Top Microbiol Immunol. 2010;343:195–224. doi: 10.1007/82_2010_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Horwitz MS. Adenoviruses. In: Knipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B, Straus E, editors. Fields Virology. Philadelphia: Lippincott Williams & Wilkins; 2001. pp. 2313–2316. [Google Scholar]
- 10.Kojaoghlanian T, Flomenberg P, Horwitz MS. The impact of adenovirus infection on the immunocompromised host. Rev Med Virol. 2003 May-Jun;13(3):155–171. doi: 10.1002/rmv.386. [DOI] [PubMed] [Google Scholar]
- 11.Nicklin SA, Wu E, Nemerow GR, Baker AH. The influence of adenovirus fiber structure and function on vector development for gene therapy. Mol Ther. 2005 Sep;12(3):384–393. doi: 10.1016/j.ymthe.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 12.Chen CY, Weaver EA, Khare R, May SM, Barry MA. Mining the adenovirus virome for oncolytics against multiple solid tumor types. Cancer Gene Ther. 2011 Oct;18(10):744–750. doi: 10.1038/cgt.2011.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khare R, Chen CY, Weaver EA, Barry MA. Advances and future challenges in adenoviral vector pharmacology and targeting. Curr Gene Ther. 2011 Aug;11(4):241–258. doi: 10.2174/156652311796150363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moffatt S, Hays J, HogenEsch H, Mittal SK. Circumvention of vector-specific neutralizing antibody response by alternating use of human and non-human adenoviruses: implications in gene therapy. Virology. 2000 Jun 20;272(1):159–167. doi: 10.1006/viro.2000.0350. [DOI] [PubMed] [Google Scholar]
- 15.Sailaja G, HogenEsch H, North A, Hays J, Mittal SK. Encapsulation of recombinant adenovirus into alginate microspheres circumvents vector-specific immune response. Gene Ther. 2002 Dec;9(24):1722–1729. doi: 10.1038/sj.gt.3301858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dhar D, Toth K, Wold WS. Syrian hamster tumor model to study oncolytic Ad5-based vectors. Methods Mol Biol. 2012;797:53–63. doi: 10.1007/978-1-61779-340-0_4. [DOI] [PubMed] [Google Scholar]
- 17.Wold WS, Doronin K, Toth K, Kuppuswamy M, Lichtenstein DL, Tollefson AE. Immune responses to adenoviruses: viral evasion mechanisms and their implications for the clinic. Curr Opin Immunol. 1999 Aug;11(4):380–386. doi: 10.1016/S0952-7915(99)80064-8. [DOI] [PubMed] [Google Scholar]
- 18.Raper SE, Chirmule N, Lee FS, Wivel NA, Bagg A, Gao GP, Wilson JM, Batshaw ML. Fatal systemic inflammatory response syndrome in a ornithine transcarbamylase deficient patient following adenoviral gene transfer. Mol Genet Metab. 2003 Sep-Oct;80(1-2):148–158. doi: 10.1016/j.ymgme.2003.08.016. [DOI] [PubMed] [Google Scholar]
- 19.Muruve DA. The innate immune response to adenovirus vectors. Hum Gene Ther. 2004 Dec;15(12):1157–1166. doi: 10.1089/hum.2004.15.1157. [DOI] [PubMed] [Google Scholar]
- 20.Bergelson JM, Cunningham JA, Droguett G, Kurt-Jones EA, Krithivas A, Hong JS, Horwitz MS, Crowell RL, Finberg RW. Isolation of a common receptor for Coxsackie B viruses and adenoviruses 2 and 5. Science. 1997 Feb 28;275(5304):1320–1323. doi: 10.1126/science.275.5304.1320. [DOI] [PubMed] [Google Scholar]
- 21.Segerman A, Atkinson JP, Marttila M, Dennerquist V, Wadell G, Arnberg N. Adenovirus type 11 uses CD46 as a cellular receptor. J Virol. 2003 Sep;77(17):9183–9191. doi: 10.1128/JVI.77.17.9183-9191.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gaggar A, Shayakhmetov DM, Lieber A. CD46 is a cellular receptor for group B adenoviruses. Nat Med. 2003 Nov;9(11):1408–1412. doi: 10.1038/nm952. [DOI] [PubMed] [Google Scholar]
- 23.Sirena D, Lilienfeld B, Eisenhut M, Kälin S, Boucke K, Beerli RR, Vogt L, Ruedl C, Bachmann MF, Greber UF, Hemmi S. The human membrane cofactor CD46 is a receptor for species B adenovirus serotype 3. J Virol. 2004 May;78(9):4454–4462. doi: 10.1128/JVI.78.9.4454-4462.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu E, Trauger SA, Pache L, Mullen TM, von Seggern DJ, Siuzdak G, Nemerow GR. Membrane cofactor protein is a receptor for adenoviruses associated with epidemic keratoconjunctivitis. J Virol. 2004 Apr;78(8):3897–3905. doi: 10.1128/JVI.78.8.3897-3905.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang H, Li ZY, Liu Y, Persson J, Beyer I, Möller T, Koyuncu D, Drescher MR, Strauss R, Zhang XB, Wahl JK, 3rd, Urban N, Drescher C, Hemminki A, Fender P, Lieber A. Desmoglein 2 is a receptor for adenovirus serotypes 3, 7, 11 and 14. Nat Med. 2011 Jan;17(1):96–104. doi: 10.1038/nm.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arnberg N, Pring-Akerblom P, Wadell G. Adenovirus type 37 uses sialic acid as a cellular receptor on Chang C cells. J Virol. 2002 Sep;76(17):8834–8841. doi: 10.1128/JVI.76.17.8834-8841.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nilsson EC, Storm RJ, Bauer J, Johansson SM, Lookene A, Ångström J, Hedenström M, Eriksson TL, Frängsmyr L, Rinaldi S, Willison HJ, Pedrosa Domellöf F, Stehle T, Arnberg N. The GD1a glycan is a cellular receptor for adenoviruses causing epidemic keratoconjunctivitis. Nat Med. 2011 Jan;17(1):105–109. doi: 10.1038/nm.2267. [DOI] [PubMed] [Google Scholar]
- 28.Waddington SN, McVey JH, Bhella D, Parker AL, Barker K, Atoda H, Pink R, Buckley SM, Greig JA, Denby L, Custers J, Morita T, Francischetti IM, Monteiro RQ, Barouch DH, van Rooijen N, Napoli C, Havenga MJ, Nicklin SA, Baker AH. Adenovirus serotype 5 hexon mediates liver gene transfer. Cell. 2008 Feb 8;132(3):397–409. doi: 10.1016/j.cell.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 29.Wickham TJ, Mathias P, Cheresh DA, Nemerow GR. Integrins alpha v beta 3 and alpha v beta 5 promote adenovirus internalization but not virus attachment. Cell. 1993 Apr 23;73(2):309–319. doi: 10.1016/0092-8674(93)90231-e. [DOI] [PubMed] [Google Scholar]
- 30.Nemerow GR, Stewart PL. Role of alpha(v) integrins in adenovirus cell entry and gene delivery. Microbiol Mol Biol Rev. 1999 Sep;63(3):725–734. doi: 10.1128/mmbr.63.3.725-734.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Greber UF. Signalling in viral entry. Cell Mol Life Sci. 2002 Apr;59(4):608–626. doi: 10.1007/s00018-002-8453-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Amstutz B, Gastaldelli M, Kälin S, Imelli N, Boucke K, Wandeler E, Mercer J, Hemmi S, Greber UF. Subversion of CtBP1-controlled macropinocytosis by human adenovirus serotype 3. EMBO J. 2008 Apr 9;27(7):956–969. doi: 10.1038/emboj.2008.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kälin S, Amstutz B, Gastaldelli M, Wolfrum N, Boucke K, Havenga M, DiGennaro F, Liska N, Hemmi S, Greber UF. Macropinocytotic uptake and infection of human epithelial cells with species B2 adenovirus type 35. J Virol. 2010 May;84(10):5336–5350. doi: 10.1128/JVI.02494-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Greber UF, Willetts M, Webster P, Helenius A. Stepwise dismantling of adenovirus 2 during entry into cells. Cell. 1993 Nov 5;75(3):477–486. doi: 10.1016/0092-8674(93)90382-z. [DOI] [PubMed] [Google Scholar]
- 35.Nakano MY, Boucke K, Suomalainen M, Stidwill RP, Greber UF. The first step of adenovirus type 2 disassembly occurs at the cell surface, independently of endocytosis and escape to the cytosol. J Virol. 2000 Aug;74(15):7085–7095. doi: 10.1128/jvi.74.15.7085-7095.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Burckhardt CJ, Suomalainen M, Schoenenberger P, Boucke K, Hemmi S, Greber UF. Drifting motions of the adenovirus receptor CAR and immobile integrins initiate virus uncoating and membrane lytic protein exposure. Cell Host Microbe. 2011 Aug 18;10(2):105–117. doi: 10.1016/j.chom.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 37.Wiethoff CM, Wodrich H, Gerace L, Nemerow GR. Adenovirus protein VI mediates membrane disruption following capsid disassembly. J Virol. 2005 Feb;79(4):1992–2000. doi: 10.1128/JVI.79.4.1992-2000.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wodrich H, Henaff D, Jammart B, Segura-Morales C, Seelmeir S, Coux O, Ruzsics Z, Wiethoff CM, Kremer EJ. A capsid-encoded PPxY-motif facilitates adenovirus entry. PLoS Pathog. 2010 Mar 19;6(3):e1000808. doi: 10.1371/journal.ppat.1000808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moyer CL, Wiethoff CM, Maier O, Smith JG, Nemerow GR. Functional genetic and biophysical analyses of membrane disruption by human adenovirus. J Virol. 2011 Mar;85(6):2631–2641. doi: 10.1128/JVI.02321-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li E, Stupack D, Bokoch GM, Nemerow GR. Adenovirus endocytosis requires actin cytoskeleton reorganization mediated by Rho family GTPases. J Virol. 1998 Nov;72(11):8806–8812. doi: 10.1128/jvi.72.11.8806-8812.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang K, Huang S, Kapoor-Munshi A, Nemerow G. Adenovirus internalization and infection require dynamin. J Virol. 1998 Apr;72(4):3455–3458. doi: 10.1128/jvi.72.4.3455-3458.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rauma T, Tuukkanen J, Bergelson JM, Denning G, Hautala T. rab5 GTPase regulates adenovirus endocytosis. J Virol. 1999 Nov;73(11):9664–9668. doi: 10.1128/jvi.73.11.9664-9668.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gastaldelli M, Imelli N, Boucke K, Amstutz B, Meier O, Greber UF. Infectious adenovirus type 2 transport through early but not late endosomes. Traffic. 2008 Dec;9(12):2265–2278. doi: 10.1111/j.1600-0854.2008.00835.x. [DOI] [PubMed] [Google Scholar]
- 44.Meier O, Boucke K, Hammer SV, Keller S, Stidwill RP, Hemmi S, Greber UF. Adenovirus triggers macropinocytosis and endosomal leakage together with its clathrin-mediated uptake. J Cell Biol. 2002 Sep 16;158(6):1119–1131. doi: 10.1083/jcb.200112067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pastan I, Seth P, FitzGerald D, Willingham M. In: Adenovirus Entry into Cells: Some New Observations on an Old Problem. Notkins AL, Oldstone MBA, editors. New York: Springer Verlag; 1986. pp. 141–146. [Google Scholar]
- 46.Imelli N, Ruzsics Z, Puntener D, Gastaldelli M, Greber UF. Genetic reconstitution of the human adenovirus type 2 temperature-sensitive 1 mutant defective in endosomal escape. Virol J. 2009 Oct 27;6:174. doi: 10.1186/1743-422X-6-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.FitzGerald DJ, Padmanabhan R, Pastan I, Willingham MC. Adenovirus-induced release of epidermal growth factor and pseudomonas toxin into the cytosol of KB cells during receptor-mediated endocytosis. Cell. 1983 Feb;32(2):607–617. doi: 10.1016/0092-8674(83)90480-4. [DOI] [PubMed] [Google Scholar]
- 48.Seth P, Willingham MC, Pastan I. Adenovirus-dependent release of 51Cr from KB cells at an acidic pH. J Biol Chem. 1984 Dec 10;259(23):14350–14353. [PubMed] [Google Scholar]
- 49.Brabec M, Schober D, Wagner E, Bayer N, Murphy RF, Blaas D, Fuchs R. Opening of size-selective pores in endosomes during human rhinovirus serotype 2 in vivo uncoating monitored by single-organelle flow analysis. J Virol. 2005 Jan;79(2):1008–1016. doi: 10.1128/JVI.79.2.1008-1016.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Suomalainen M, Nakano MY, Keller S, Boucke K, Stidwill RP, Greber UF. Microtubule-dependent plus- and minus end-directed motilities are competing processes for nuclear targeting of adenovirus. J Cell Biol. 1999 Feb 22;144(4):657–672. doi: 10.1083/jcb.144.4.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dunn WA, Hubbard AL, Aronson NN., Jr. Low temperature selectively inhibits fusion between pinocytic vesicles and lysosomes during heterophagy of 125I-asialofetuin by the perfused rat liver. J Biol Chem. 1980 Jun 25;255(12):5971–5978. [PubMed] [Google Scholar]
- 52.Leopold PL, Kreitzer G, Miyazawa N, Rempel S, Pfister KK, Rodriguez-Boulan E, Crystal RG. Dynein- and microtubule-mediated translocation of adenovirus serotype 5 occurs after endosomal lysis. Hum Gene Ther. 2000 Jan 1;11(1):151–165. doi: 10.1089/10430340050016238. [DOI] [PubMed] [Google Scholar]
- 53.Bremner KH, Scherer J, Yi J, Vershinin M, Gross SP, Vallee RB. Adenovirus transport via direct interaction of cytoplasmic dynein with the viral capsid hexon subunit. Cell Host Microbe. 2009 Dec 17;6(6):523–535. doi: 10.1016/j.chom.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Warren JC, Cassimeris L. The contributions of microtubule stability and dynamic instability to adenovirus nuclear localization efficiency. Cell Motil Cytoskeleton. 2007 Sep;64(9):675–689. doi: 10.1002/cm.20215. [DOI] [PubMed] [Google Scholar]
- 55.Warren JC, Rutkowski A, Cassimeris L. Infection with replication-deficient adenovirus induces changes in the dynamic instability of host cell microtubules. Mol Biol Cell. 2006 Aug;17(8):3557–3568. doi: 10.1091/mbc.E05-09-0850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Engelke MF, Burckhardt CJ, Morf MK, Greber UF. The dynactin complex enhances the speed of microtubule-dependent motions of adenovirus both towards and away from the nucleus. Viruses. 2011 Mar;3(3):233–253. doi: 10.3390/v3030233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Strunze S, Trotman LC, Boucke K, Greber UF. Nuclear targeting of adenovirus type 2 requires CRM1-mediated nuclear export. Mol Biol Cell. 2005 Jun;16(6):2999–3009. doi: 10.1091/mbc.E05-02-0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Smith JG, Cassany A, Gerace L, Ralston R, Nemerow GR. Neutralizing antibody blocks adenovirus infection by arresting microtubule-dependent cytoplasmic transport. J Virol. 2008 Jul;82(13):6492–6500. doi: 10.1128/JVI.00557-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Puntener D, Engelke MF, Ruzsics Z, Strunze S, Wilhelm C, Greber UF. Stepwise loss of fluorescent core protein V from human adenovirus during entry into cells. J Virol. 2011 Jan;85(1):481–496. doi: 10.1128/JVI.01571-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Trotman LC, Mosberger N, Fornerod M, Stidwill RP, Greber UF. Import of adenovirus DNA involves the nuclear pore complex receptor CAN/Nup214 and histone H1. Nat Cell Biol. 2001 Dec;3(12):1092–1100. doi: 10.1038/ncb1201-1092. [DOI] [PubMed] [Google Scholar]
- 61.Strunze S, Engelke MF, Wang IH, Puntener D, Boucke K, Schleich S, Way M, Schoenenberger P, Burckhardt CJ, Greber UF. Kinesin-1-mediated capsid disassembly and disruption of the nuclear pore complex promote virus infection. Cell Host Microbe. 2011 Sep 15;10(3):210–223. doi: 10.1016/j.chom.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 62.Cho KI, Yi H, Desai R, Hand AR, Haas AL, Ferreira PA. RANBP2 is an allosteric activator of the conventional kinesin-1 motor protein, KIF5B, in a minimal cell-free system. EMBO Rep. 2009 May;10(5):480–486. doi: 10.1038/embor.2009.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Joseph J, Dasso M. The nucleoporin Nup358 associates with and regulates interphase microtubules. FEBS Lett. 2008 Jan 23;582(2):190–196. doi: 10.1016/j.febslet.2007.11.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Walkiewicz MP, Morral N, Engel DA. Accurate single-day titration of adenovirus vectors based on equivalence of protein VII nuclear dots and infectious particles. J Virol Methods. 2009 Aug;159(2):251–258. doi: 10.1016/j.jviromet.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wodrich H, Cassany A, D'Angelo MA, Guan T, Nemerow G, Gerace L. Adenovirus core protein pVII is translocated into the nucleus by multiple import receptor pathways. J Virol. 2006 Oct;80(19):9608–9618. doi: 10.1128/JVI.00850-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hindley CE, Lawrence FJ, Matthews DA. A role for transportin in the nuclear import of adenovirus core proteins and DNA. Traffic. 2007 Oct;8(10):1313–1322. doi: 10.1111/j.1600-0854.2007.00618.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zaiss AK, Vilaysane A, Cotter MJ, Clark SA, Meijndert HC, Colarusso P, Yates RM, Petrilli V, Tschopp J, Muruve DA. Antiviral antibodies target adenovirus to phagolysosomes and amplify the innate immune response. J Immunol. 2009 Jun 1;182(11):7058–7068. doi: 10.4049/jimmunol.0804269. [DOI] [PubMed] [Google Scholar]
- 68.Ebbinghaus C, Al-Jaibaji A, Operschall E, Schöffel A, Peter I, Greber UF, Hemmi S. Functional and selective targeting of adenovirus to high-affinity Fcgamma receptor I-positive cells by using a bispecific hybrid adapter. J Virol. 2001 Jan;75(1):480–489. doi: 10.1128/JVI.75.1.480-489.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Adams WC, Bond E, Havenga MJ, Holterman L, Goudsmit J, Karlsson Hedestam GB, Koup RA, Loré K. Adenovirus serotype 5 infects human dendritic cells via a coxsackievirus-adenovirus receptor-independent receptor pathway mediated by lactoferrin and DC-SIGN. J Gen Virol. 2009 Jul;90(Pt 7):1600–1610. doi: 10.1099/vir.0.008342-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Meier O, Gastaldelli M, Boucke K, Hemmi S, Greber UF. Early steps of clathrin-mediated endocytosis involved in phagosomal escape of Fcgamma receptor-targeted adenovirus. J Virol. 2005 Feb;79(4):2604–2613. doi: 10.1128/JVI.79.4.2604-2613.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fejer G, Drechsel L, Liese J, Schleicher U, Ruzsics Z, Imelli N, Greber UF, Keck S, Hildenbrand B, Krug A, Bogdan C, Freudenberg MA. Key role of splenic myeloid DCs in the IFN-alphabeta response to adenoviruses in vivo. PLoS Pathog. 2008 Nov;4(11):e1000208. doi: 10.1371/journal.ppat.1000208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Iacobelli-Martinez M, Nemerow GR. Preferential activation of Toll-like receptor nine by CD46-utilizing adenoviruses. J Virol. 2007 Feb;81(3):1305–1312. doi: 10.1128/JVI.01926-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Farmer C, Morton PE, Snippe M, Santis G, Parsons M. Coxsackie adenovirus receptor (CAR) regulates integrin function through activation of p44/42 MAPK. Exp Cell Res. 2009 Sep 10;315(15):2637–2647. doi: 10.1016/j.yexcr.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 74.Yuen S, Smith J, Caruso L, Balan M, Opavsky MA. The coxsackie-adenovirus receptor induces an inflammatory cardiomyopathy independent of viral infection. J Mol Cell Cardiol. 2011 May;50(5):826–840. doi: 10.1016/j.yjmcc.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 75.Meier O, Greber UF. Adenovirus endocytosis. J Gene Med. 2004 Feb;6(Suppl 1):S152–S163. doi: 10.1002/jgm.553. [DOI] [PubMed] [Google Scholar]
- 76.Suomalainen M, Nakano MY, Boucke K, Keller S, Greber UF. Adenovirus-activated PKA and p38/MAPK pathways boost microtubule-mediated nuclear targeting of virus. EMBO J. 2001 Mar 15;20(6):1310–1319. doi: 10.1093/emboj/20.6.1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tibbles LA, Spurrell JC, Bowen GP, Liu Q, Lam M, Zaiss AK, Robbins SM, Hollenberg MD, Wickham TJ, Muruve DA. Activation of p38 and ERK signaling during adenovirus vector cell entry lead to expression of the C-X-C chemokine IP-10. J Virol. 2002 Feb;76(4):1559–1568. doi: 10.1128/JVI.76.4.1559-1568.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bruder JT, Kovesdi I. Adenovirus infection stimulates the Raf/MAPK signaling pathway and induces interleukin-8 expression. J Virol. 1997 Jan;71(1):398–404. doi: 10.1128/jvi.71.1.398-404.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Booth JL, Coggeshall KM, Gordon BE, Metcalf JP. Adenovirus type 7 induces interleukin-8 in a lung slice model and requires activation of Erk. J Virol. 2004 Apr;78(8):4156–4164. doi: 10.1128/JVI.78.8.4156-4164.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhu J, Huang X, Yang Y. Innate immune response to adenoviral vectors is mediated by both Toll-like receptor-dependent and -independent pathways. J Virol. 2007 Apr;81(7):3170–3180. doi: 10.1128/JVI.02192-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lieber A, He CY, Meuse L, Schowalter D, Kirillova I, Winther B, Kay MA. The role of Kupffer cell activation and viral gene expression in early liver toxicity after infusion of recombinant adenovirus vectors. J Virol. 1997 Nov;71(11):8798–8807. doi: 10.1128/jvi.71.11.8798-8807.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Xu Z, Smith JS, Tian J, Byrnes AP. Induction of shock after intravenous injection of adenovirus vectors: a critical role for platelet-activating factor. Mol Ther. 2010 Mar;18(3):609–616. doi: 10.1038/mt.2009.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nociari M, Ocheretina O, Schoggins JW, Falck-Pedersen E. Sensing infection by adenovirus: Toll-like receptor-independent viral DNA recognition signals activation of the interferon regulatory factor 3 master regulator. J Virol. 2007 Apr;81(8):4145–4157. doi: 10.1128/JVI.02685-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Di Paolo NC, Miao EA, Iwakura Y, Murali-Krishna K, Aderem A, Flavell RA, Papayannopoulou T, Shayakhmetov DM. Virus binding to a plasma membrane receptor triggers interleukin-1 alpha-mediated proinflammatory macrophage response in vivo. Immunity. 2009 Jul 17;31(1):110–121. doi: 10.1016/j.immuni.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.DiPaolo N, Ni S, Gaggar A, Strauss R, Tuve S, Li ZY, Stone D, Shayakhmetov D, Kiviat N, Touré P, Sow S, Horvat B, Lieber A. Evaluation of adenovirus vectors containing serotype 35 fibers for vaccination. Mol Ther. 2006 Apr;13(4):756–765. doi: 10.1016/j.ymthe.2005.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Appledorn DM, Patial S, McBride A, Godbehere S, Van Rooijen N, Parameswaran N, Amalfitano A. Adenovirus vector-induced innate inflammatory mediators, MAPK signaling, as well as adaptive immune responses are dependent upon both TLR2 and TLR9 in vivo. J Immunol. 2008 Aug 1;181(3):2134–2144. doi: 10.4049/jimmunol.181.3.2134. [DOI] [PubMed] [Google Scholar]
- 87.Blasius AL, Beutler B. Intracellular toll-like receptors. Immunity. 2010 Mar 26;32(3):305–315. doi: 10.1016/j.immuni.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 88.Fejér G, Szalay K, Gyory I, Fejes M, Kúsz E, Nedieanu S, Páli T, Schmidt T, Siklódi B, Lázár G, Jr., Lázár G, Sr., Duda E. Adenovirus infection dramatically augments lipopolysaccharide-induced TNF production and sensitizes to lethal shock. J Immunol. 2005 Aug 1;175(3):1498–1506. doi: 10.4049/jimmunol.175.3.1498. [DOI] [PubMed] [Google Scholar]
- 89.Doughty L, Nguyen K, Durbin J, Biron C. A role for IFN-alpha beta in virus infection-induced sensitization to endotoxin. J Immunol. 2001 Feb 15;166(4):2658–2664. doi: 10.4049/jimmunol.166.4.2658. [DOI] [PubMed] [Google Scholar]
- 90.Freudenberg MA, Kalis C, Chvatchko Y, Merlin T, Gumenscheimer M, Galanos C. Role of interferons in LPS hypersensitivity. J Endotoxin Res. 2003;9(5):308–312. doi: 10.1179/096805103225002566. [DOI] [PubMed] [Google Scholar]
- 91.Nansen A, Randrup Thomsen A. Viral infection causes rapid sensitization to lipopolysaccharide: central role of IFN-alpha beta. J Immunol. 2001 Jan 15;166(2):982–988. doi: 10.4049/jimmunol.166.2.982. [DOI] [PubMed] [Google Scholar]
- 92.Le-Thi-Phuong T, Dumoutier L, Renauld JC, Van Snick J, Coutelier JP. Divergent roles of IFNs in the sensitization to endotoxin shock by lactate dehydrogenase-elevating virus. Int Immunol. 2007 Nov;19(11):1303–1311. doi: 10.1093/intimm/dxm101. [DOI] [PubMed] [Google Scholar]
- 93.Gumenscheimer M, Balkow S, Simon MM, Jirillo E, Galanos C, Freudenberg MA. Stage of primary infection with lymphocytic choriomeningitis virus determines predisposition or resistance of mice to secondary bacterial infections. Med Microbiol Immunol. 2007 Jun;196(2):79–88. doi: 10.1007/s00430-006-0030-1. [DOI] [PubMed] [Google Scholar]
- 94.Pickles RJ, Fahrner JA, Petrella JM, Boucher RC, Bergelson JM. Retargeting the coxsackievirus and adenovirus receptor to the apical surface of polarized epithelial cells reveals the glycocalyx as a barrier to adenovirus-mediated gene transfer. J Virol. 2000 Jul;74(13):6050–6057. doi: 10.1128/jvi.74.13.6050-6057.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Walters RW, Freimuth P, Moninger TO, Ganske I, Zabner J, Welsh MJ. Adenovirus fiber disrupts CAR-mediated intercellular adhesion allowing virus escape. Cell. 2002 Sep 20;110(6):789–799. doi: 10.1016/s0092-8674(02)00912-1. [DOI] [PubMed] [Google Scholar]
- 96.Coyne CB, Bergelson JM. Virus-induced Abl and Fyn kinase signals permit coxsackievirus entry through epithelial tight junctions. Cell. 2006 Jan 13;124(1):119–131. doi: 10.1016/j.cell.2005.10.035. [DOI] [PubMed] [Google Scholar]
- 97.Lütschg V, Boucke K, Hemmi S, Greber UF. Chemotactic antiviral cytokines promote infectious apical entry of human adenovirus into polarized epithelial cells. Nat Commun. 2011 Jul 12;2:391. doi: 10.1038/ncomms1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Philpott NJ, Nociari M, Elkon KB, Falck-Pedersen E. Adenovirus-induced maturation of dendritic cells through a PI3 kinase-mediated TNF-alpha induction pathway. Proc Natl Acad Sci U S A. 2004 Apr 20;101(16):6200–6205. doi: 10.1073/pnas.0308368101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Weber J. Genetic analysis of adenovirus type 2 III. Temperature sensitivity of processing viral proteins. J Virol. 1976 Feb;17(2):462–471. doi: 10.1128/jvi.17.2.462-471.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kurt-Jones EA, Popova L, Kwinn L, Haynes LM, Jones LP, Tripp RA, Walsh EE, Freeman MW, Golenbock DT, Anderson LJ, Finberg RW. Pattern recognition receptors TLR4 and CD14 mediate response to respiratory syncytial virus. Nat Immunol. 2000 Nov;1(5):398–401. doi: 10.1038/80833. [DOI] [PubMed] [Google Scholar]
- 101.Yamaguchi T, Kawabata K, Koizumi N, Sakurai F, Nakashima K, Sakurai H, Sasaki T, Okada N, Yamanishi K, Mizuguchi H. Role of MyD88 and TLR9 in the innate immune response elicited by serotype 5 adenoviral vectors. Hum Gene Ther. 2007 Aug;18(8):753–762. doi: 10.1089/hum.2007.016. [DOI] [PubMed] [Google Scholar]
- 102.Muruve DA, Pétrilli V, Zaiss AK, White LR, Clark SA, Ross PJ, Parks RJ, Tschopp J. The inflammasome recognizes cytosolic microbial and host DNA and triggers an innate immune response. Nature. 2008 Mar 6;452(7183):103–107. doi: 10.1038/nature06664. [DOI] [PubMed] [Google Scholar]
- 103.Moyer CL, Wiethoff CM, Maier O, Smith JG, Nemerow GR. Functional genetic and biophysical analyses of membrane disruption by human adenovirus. J Virol. 2011 Mar;85(6):2631–2641. doi: 10.1128/JVI.02321-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Urosevic M, Fujii K, Calmels B, Laine E, Kobert N, Acres B, Dummer R. Type I IFN innate immune response to adenovirus-mediated IFN-gamma gene transfer contributes to the regression of cutaneous lymphomas. J Clin Invest. 2007 Oct;117(10):2834–2846. doi: 10.1172/JCI32077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Stetson DB, Medzhitov R. Recognition of cytosolic DNA activates an IRF3-dependent innate immune response. Immunity. 2006 Jan;24(1):93–103. doi: 10.1016/j.immuni.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 106.Ishii KJ, Coban C, Kato H, Takahashi K, Torii Y, Takeshita F, Ludwig H, Sutter G, Suzuki K, Hemmi H, Sato S, Yamamoto M, Uematsu S, Kawai T, Takeuchi O, Akira S. A Toll-like receptor-independent antiviral response induced by double-stranded B-form DNA. Nat Immunol. 2006 Jan;7(1):40–48. doi: 10.1038/ni1282. [DOI] [PubMed] [Google Scholar]
- 107.Nociari M, Ocheretina O, Murphy M, Falck-Pedersen E. Adenovirus induction of IRF3 occurs through a binary trigger targeting Jun N-terminal kinase and TBK1 kinase cascades and type I interferon autocrine signaling. J Virol. 2009 May;83(9):4081–4091. doi: 10.1128/JVI.02591-08. [DOI] [PMC free article] [PubMed] [Google Scholar]