Abstract
Expression of gelatinases A and B, also referred to matrixmetalloproteinases (MMP)-2 and -9, respectively, is increased in inflamed tissues of experimental intestinal inflammation and humans with inflammatory bowel disease (IBDs). Given that we recently reported that treatment with the selective gelatinase inhibitor RO28-2653 ameliorates acute dextrane sulfate sodium (DSS) colitis, we asked whether gelatinase A or B expression is pivotal in mediating large intestinal inflammation. Results from our study reveal that symptoms of acute DSS colitis as well as histopathological colonic changes were ameliorated in MMP-2-, but not MMP-9-deficient mice, and were paralleled by a diminished influx of immune cells. In MMP-2-deficient mice, we observed lower expression of pro-inflammatory cytokines including interferon-γ (IFN-γ), tumor necrosis factor-α (TNF-α), and IL-6 in colonic biopsies and less overgrowth of the colonic lumen by potentially pro-inflammatory enterobacteria from the commensal gut microbiota. We conclude that rather MMP-2 than MMP-9 is causative for the establishment of DSS colitis in mice. The discrepancy of these data to prior reports might be due to substantial differences in the intestinal microbiota composition of the mice bred at different animal facilities impacting susceptibility to inflammatory stimuli. Consequently, a detailed survey of the gut microbiota should be implemented in immunological/inflammatory studies in the future in order to allow comparison of data from different facilities.
Keywords: acute DSS colitis, E. coli, gelatinases A and B, gut microbiota, host responses to inflammation, innate immunity, matrixmetalloproteinases
Introduction
Matrixmetalloproteinases (MMPs) comprise a large family of zinc- and calcium-dependent extracellular matrix degrading endopeptidases, that are under tight control of endogenous tissue inhibitors of matrixmetalloproteinases (TIMPs) [1–3]. With respect to their sequence molecules and substrate specificity, MMPs can be categorized into collagenases (MMP-1, -8, -13), gelatinases (MMP-2, -9), stromelysins (MMP-3, -10, -12), matrilysin (MMP-7), and membrane-type matrix metalloproteinases (MT-MMP-1 through -5) [1–4]. MMPs have pivotal functions in embryo- and organogenesis, as well as in tissue proliferation and regeneration [1, 3]. An imbalance of activators (e.g. pro-inflammatory cytokines such as IL-1, IL-6, tumor necrosis factor-α (TNF-α)) and inhibitors (e.g. anti-inflammatory molecules IL-4, IL-10, TGF-β besides TIMPs) of MMP expression and function leads to tissue-destruction or cancerogenesis [5, 6]. In experimental models of Th1-type inflammation (e.g. rheumatoid arthritis, atherosclerosis and colitis) [7–10] as well as in humans with inflammatory bowel diseases (IBDs) such as ulcerative colitis and Crohn’s disease [11–16], levels of gelatinases A (MMP-2) and B (MMP-9) are increased in inflamed tissue sites.
We have recently shown that treatment with the synthetic selective gelatinase blocker RO28-2653 prevented Toxoplasma gondii-infected mice from hyper-acute Th1-type-driven pan-ileitis, a model mimicking immunopathological key features of Crohn’s disease [17]. Furthermore, MMP-2-, but not MMP-9-deficient mice were protected from ileitis development [17]. In addition, acute dextrane sulfate sodium (DSS) colitis was ameliorated by preventive use of RO28-2653 [18]. Thus, we were interested whether gelatinase A, B, or both are essential in mediating acute colonic inflammation. To our knowledge, we report here for the first time that MMP-2, but not MMP-9 is essential in the immunopathogenesis of acute DSS colitis as indicated by (1) ameliorated clinical colitis pathology, (2) less histopathological changes in the colon mucosa, (3) reduced immune cell influx into mucosa and submucosa, (4) lower expression of pro-inflammatory cytokines such as interferon-γ (IFN-γ), TNF-α and IL-6, and finally, (5) less overgrowth of the colon lumen by potentially pro-inflammatory Escherichia coli in MMP-2-, but not MMP-9-, deficient mice as compared to wildtype (wt) controls.
Materials and methods
Ethics statement
All animal experiments were conducted according to the European guidelines for animal welfare (2010/63/EU) with approval of the commission for animal experiments headed by the “Landesamt für Gesundheit und Soziales” (LaGeSo, Berlin, Germany). Animal welfare was monitored twice daily by assessment of clinical conditions.
Mice, colitis induction and determination of clinical scores
Breeding stocks of MMP-2–/– and MMP-9–/– mice (all in C57BL/6 background) were provided by Prof. Claude Libert (University of Ghent, Belgium) and Prof. Leif R. Lund (University of Copenhagen, Denmark), respectively. Female MMP-2–/–, MMP-9–/–, and respective C57BL/6 wildtype (wt) control mice used in the experiments were bred and maintained within the same specific pathogen-free (SPF) unit, in the same room and under identical hygiene conditions in the Forschungsinstitut für Experimentelle Medizin (FEM, Charité, Berlin, Germany). To confirm the absence of MMP-2 or MMP-9 gene expression, genomic DNA was isolated and disruption of either gene confirmed by polymerase chain reaction (PCR). For colitis induction, mice 3 months of age were treated with 3.5% (wt/vol) DSS (40 kDa, MP Biomedicals, Illkirch, France) in drinking water ad libitum for 7 days followed by 1 day of normal drinking water. Mice were monitored daily for occult blood in stool (as determined by the guajak method using Haemoccult™, Beckman Coulter/PCD, Krefeld, Germany), stool consistence and body weight. Total clinical scores with a maximum of 12 were calculated by combined scores of weight loss, occurence of blood in stool, and stool consistence, as described previously [18–20].
Sampling procedures and histologic scoring
Mice were sacrificed by isofluran treatment (Abbott, Germany) on day 8 after induction of colitis. Colon samples from each mouse were isolated under sterile conditions and collected in parallel for histopathological, immunohistochemical, and microbiological analyses, as well as for detection of cytokines (mRNA and protein level). For immunohistochemical stainings, colon samples were immediately fixed in 5% formalin and embedded in paraffin, and sections (5 µm) were stained with the respective antibodies as described below. Histopathology was investigated in hematoxylin eosin (HE)-stained tissue sections. A published standardized histologic score ranging from 0 to 6 was used for blinded evaluation of the inflammatory processes in the colon [19].
Immunohistochemistry
In situ immunohistochemical analysis of colon paraffin sections was performed as described previously [18, 21, 22]. Primary antibodies against CD3 (#N1580, Dako, Denmark, dilution 1:10), FOXP-3 (FJK-16s, eBioscience, 1:100), B220 (eBioscience, San Diego, CA, USA, 1:200), myeloperoxidase-7 (MPO-7, # A0398, Dako, 1:10000), and F4/80 (#14-4801, clone BM8, eBioscience, 1:50) were used. For each animal, the average number of positively stained cells within at least six high power fields (HPF, ×400 magnification) was determined by light microscopy.
Real-time PCR
RNA was isolated from organs, reverse transcribed and analyzed as described previously [17]. Mouse IFN-γ and TNF-α mRNA expressions were detected and analyzed using LightCycler™ Data Analysis Software (Roche). Expression levels were calculated relative to the hypoxanthine-guanine-phosphoribosyltransferase (HPRT) expression and indicated as “Arbitrary Units”.
Cytokine detection in colon culture supernatants
Colon biopsies were cut longitudinally, washed with phosphate-buffered saline (PBS) and strips of 1 cm2 placed in 24-flat-bottom-well culture plates (Nunc, Wiesbaden, Germany) containing 500-µl serum-free RPMI 1640 medium supplemented with penicillin (100 U/ml) and streptomycin (100 µg/ml; PAA Laboratories). After 18 h at 37 °C, culture supernatants were tested for IL-6 by enzyme-linked immunosorbent assay (ELISA) (BD Biosciences, Heidelberg, Germany) as described previously [18, 20, 23, 24].
Analysis of the intestinal microflora
Cultural analysis and biochemical identification of luminal E. coli loads from colon samples were performed as previously described [20, 23, 24].
Statistical analysis
Medians, mean values, standard deviations (SD), standard error of the means (SEM), and levels of significance were determined using appropriate tests as indicated (two-tailed Student’s t-Test, Mann–Whitney U Test). Two-sided probability (P) values ≤0.05 were considered significant. All experiments were repeated at least twice.
Results
Less colonic immunopathology in MMP-2-, but not MMP-9-deficient mice in acute DSS colitis
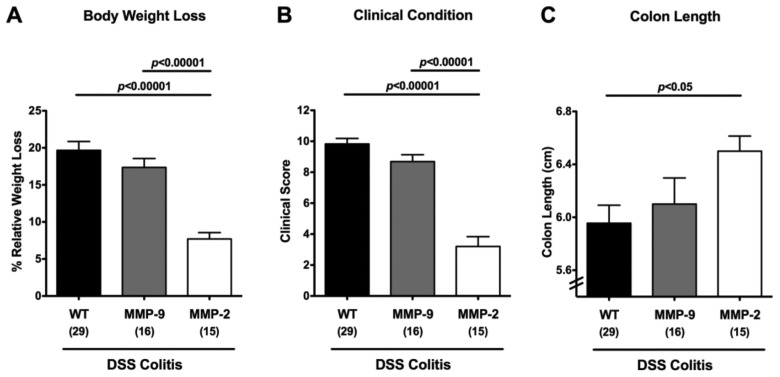
Given that synthetic selective gelatinase blockage was shown effective in preventing acute small intestinal as well as colonic inflammation [17, 18], we were interested which gelatinase in particular (A, B, or both) was predominantly involved in mediating acute DSS colitis. In order to address this question, MMP-2- and MMP-9-deficient mice were subjected to acute colitis induced by DSS treatment for 7 days. At day 8 post colitis induction (p.i.), MMP-9-deficient and wt mice had lost 17.4 ± 1.2% and 19.7 ± 1.2% of their initial body weights, respectively, whereas the body weight loss in MMP-2-deficient mice was significantly less distinct (Fig. 1A). The better clinical outcome of MMP-2-deficient mice when compared to wt and MMP-9-deficient animals was further highlighted by a reduced clinical colitis score evaluating body weight loss, occurrence of blood in and the consistency of stool (see Materials and methods; Fig. 1B). Given that colonic inflammation is accompanied by a significant shortening of the lower intestinal tract, we determined the lengths of the large intestines in mice lacking either gelatinase gene. At day 8 following induction of acute DSS colitis, MMP-2-, but not MMP-9-deficient mice displayed significantly longer colons as compared to wt controls (Fig. 1C) supporting a better clinical outcome. Taken together, whereas DSS treatment resulted in the induction of severe colitis in wt and MMP-9-deficient mice, symptoms were only moderate in MMP-2-deficient mice.
Fig. 1.
Better clinical outcome of acute DSS colitis in MMP-2-, but not MMP-9-deficient mice. Relative body weight loss (A), clinical condition (as indicated by a clinical colitis score, see Materials and methods) (B), and absolute colon lengths (C) of wildtype (WT, black bars), MMP-9–/– (MMP-9; gray bars), or MMP-2–/– (MMP-2; white bars) mice were recorded at day 8 after DSS treatment for 7 days. Numbers of analyzed animals are given in parentheses. Mean values, standard errors of the mean (SEM), and significance levels as indicated were determined by the Student’s t-test. Data are pooled from at least four independent experiments
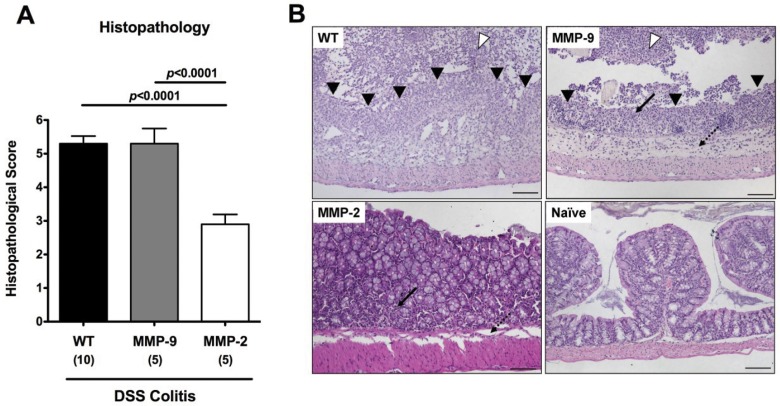
We next assessed the histopathological changes in colon sections. The better clinical outcome in MMP-2-deficient mice was paralleled by less distinct colonic histopathology when compared to MMP-9-deficient and wt mice as indicated by significantly lower histopathological scores (Fig. 2A). In mice lacking MMP-2, only few epithelial lesions and a discrete influx of inflammatory cells into the colonic lamina propria, but not into the submucosal layer were observed (Fig. 2B). However, similar to wt animals, MMP-9-deficient mice developed severe mucosal damage with massive ulcerations affecting the entire colon length extending into the bowel and severe intraluminal bleeding with granulocytes and fibrinous exudate were observed in the colon lumen (Fig. 2B). Taken together, MMP-2-, but not MMP-9-deficient mice exhibited less clinical signs of colitis and reduced histopathological signs of colonic inflammation in acute DSS colitis when compared to wt mice.
Fig. 2.
Less colonic histopathology in MMP-2-, but not MMP-9-deficient mice with acute DSS colitis. (A) Histopathology scores of the colon were determined in wildtype (WT, black bars), MMP-9–/– (MMP-9; gray bars), or MMP-2–/– (MMP-2; white bars) mice at day 8 after DSS treatment for 7 days. Numbers of analyzed animals are given in parentheses. Mean values, standard deviation (SD), and significance levels as indicated were determined by the Student’s t-test. Data shown are representative for four independent experiments. (B) Paraffin sections of colon samples were obtained from wildtype (WT; upper left), MMP-9–/– (MMP-9; upper right), and MMP-2–/– (MMP-2; lower left) mice at day 8 after DSS treatment for 7 days as well as from naïve controls (Naïve; lower right) and HE-stained as described (see Materials and methods). Open arrow heads indicate severe bleeding into the colon lumen with granulocytes and fibrinous exudate, and solid arrow heads indicate mucosal ulcerations. Solid and dotted arrows point towards mucosal and submucosal leukocyte infiltrates, respectively, the latter missing in MMP-2-deficient animals. Representative photomicrographs (magnification ×100) from three independent experiments are shown
Less pronounced immune cell responses in colonic mucosa of MMP-2-, but not MMP-9-deficient mice in acute DSS colitis
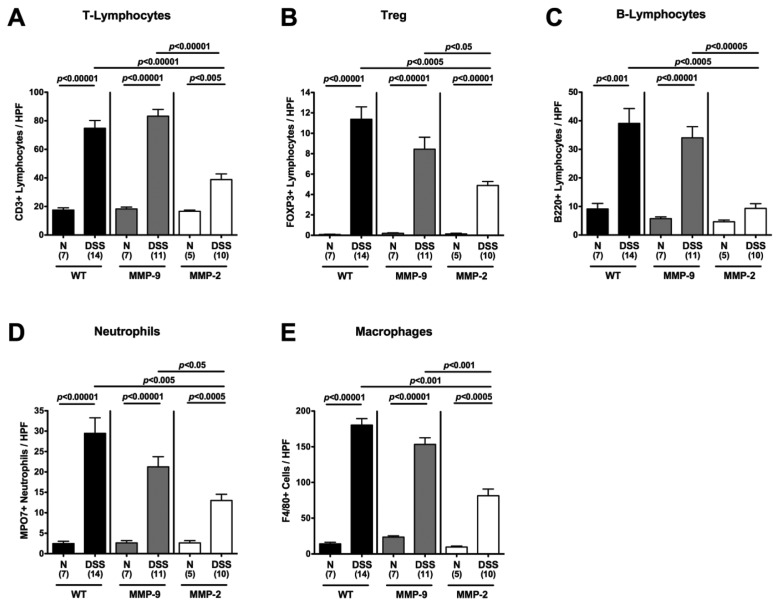
Given that ulcerative colitis in humans is accompanied by the recruitment of pro-inflammatory immune cell populations to sites of inflammation in the large intestine [25, 26], we next quantified the amount of CD3+ (T-lymphocytes), FOXP3+ (regulatory T-cells, Tregs), B220+ (B-lymphocytes), MPO7+ (neutrophilic granulocytes), and F4/80+ (macrophages) cells within the colon mucosa and submucosa in situ. The amount of all immune cells tested within the colon was substantially increased in DSS-treated MMP-9-deficient and wt mice as compared to the respective naïve controls (Fig. 3). This influx of immune cells, however, was not as distinct in MMP-2-deficient mice. Thus, amelioration of colitis in gelatinase A-deficient mice might be linked to the significantly reduced recruitment of immune cell into the colonic mucosa and submucosa (Fig. 3).
Fig. 3.
Quantification of defined cell populations in the colon of MMP-2- and MMP-9-deficient mice in situ following acute DSS colitis. The average number of cells positive for CD3 (T-Lymphocytes; A), FOXP3 (regulatory T cells, Treg; B), B220 (B-Lymphocytes; C), MPO7 (Neutrophils; D), and F4/80 (Macrophages; E) from at least six high power fields (HPF, ×400 magnification) per animal were determined microscopically in immunostained colon sections of wildtype (WT; black bars), MMP-9–/– (MMP-9; gray bars), or MMP-2–/– (MMP-2; white bars) mice without (naïve, N) or with acute colitis (DSS) isolated at day 8 after the start of DSS treatment. Numbers of analyzed animals are given in parentheses. Mean values, standard errors of the mean (SEM), and significance levels as indicated were determined by the Student’s t-test. Data are pooled from three independent experiments
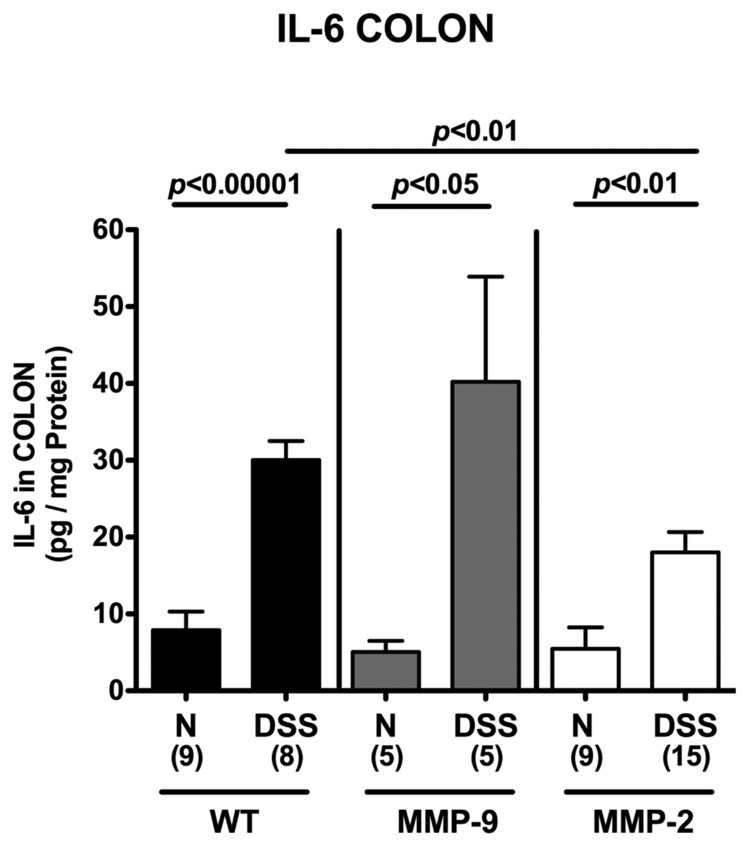
Less pro-inflammatory cytokine expression in colons of MMP-2-, but not MMP-9-deficient mice in acute DSS colitis
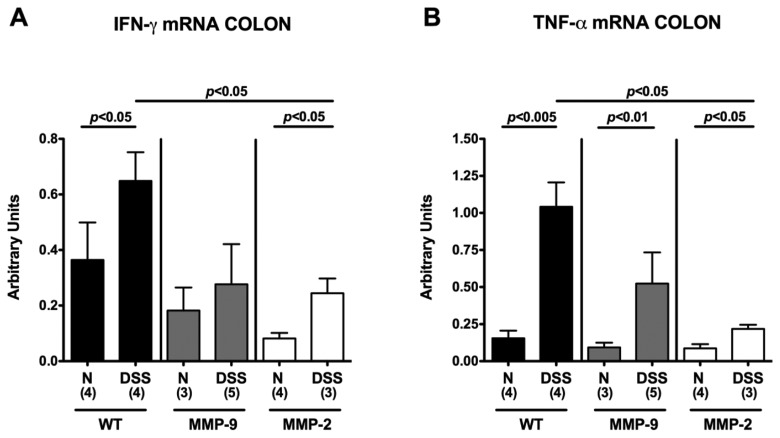
To further dissect the mechanisms underlying the role of MMP-2 in acute DSS colitis, we determined gene expression and secretion of pro-inflammatory cytokines in ex vivo isolated colonic biopsies. In all colitic mice irrespective of their genotype, IFN-γ and TNF-α mRNA levels (Fig. 4) as well as IL-6 protein concentrations (Fig. 5) were increased. However, concentrations of these pro-inflammatory mediators were significantly lower in MMP-2-deficient mice when compared to wt animals. Thus, amelioration of colitis in mice lacking the gelatinase A gene was paralleled by lower pro-inflammatory cytokine expression in the inflamed colon.
Fig. 4.
Less colonic IFN- and TNF-α mRNA expression in MMP-2-, but not MMP-9-deficient mice with acute DSS colitis. Expression levels of IFN-γ (A) and TNF-α (B) mRNA were determined by RT-PCR (see Materials and methods) in colon biopsies derived from wildtype (WT; black bars), MMP-9–/– (MMP-9; gray bars), or MMP-2–/– (MMP-2; white bars) mice without (naïve, N) or with acute colitis (DSS) at the end of the experiment. Data are expressed as fold changes relative to HPRT mRNA expression (arbitrary units). Numbers of analyzed animals are given in parentheses. Mean values, standard deviations (SD), and significance levels as indicated were determined by the Student’s t-test. Data shown are representative for three independent experiments
Fig. 5.
Less colonic IL-6 secretion in MMP-2-, but not MMP-9-deficient mice with acute DSS colitis. Supernatants of colon tissue isolated at the end of the experiments from wildtype (WT; black bars), MMP-9–/– (MMP-9; gray bars), or MMP-2–/– (MMP-2; white bars) mice without (naïve, N) or with acute colitis (DSS), and cultured for 24 h, were characterized by ELISA for the presence of IL-6. Numbers of analyzed animals are given in parentheses. Mean values, standard errors of the mean (SEM), and significance levels as indicated were determined by the Student’s t-test. Data are representative for three independent experiments
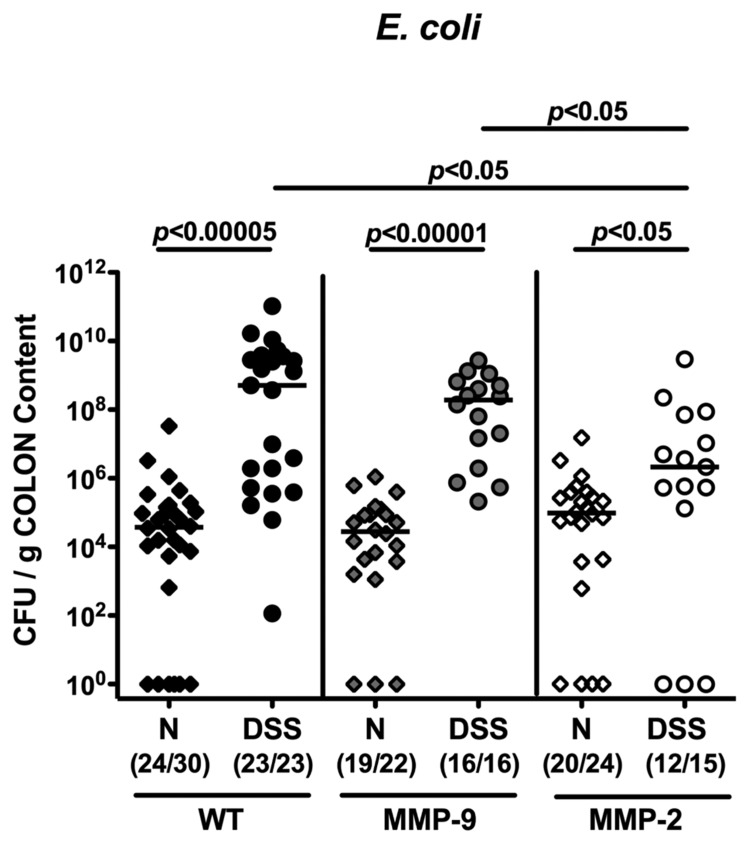
Lower E. coli loads in colons of MMP-2-, but not MMP-9-deficient mice in acute DSS colitis
We could recently demonstrate that acute small intestinal [17, 23, 24] and large intestinal inflammation [18, 20, 21] are accompanied by a significant overgrowth of the gut lumen with commensal enterobacteria such as E. coli. In addition, we clearly showed that amelioration of acute DSS colitis following selective gelatinase blockage by the synthetic compound RO28-2653 (lacking any anti-bacterial properties) was paralleled by a substantial decrease of E. coli loads within the colon lumen [18], suggesting an effect of the colonic E. coli load on the outcome of colitis. Therefore, we determined the amount of E. coli present in the colon lumen of mice lacking gelatinases A or B following colitis induction. At the end of the experiment, luminal E. coli loads increased by approximately 4 orders of magnitude in MMP-9-deficient and wt control mice with severe colitis, but to a significantly lower extent in MMP-2-deficient mice (1.5 orders of magnitude versus naïve controls (Fig. 6)). Mice lacking gelatinase A displayed significantly lower E. coli numbers (2.5 orders of magnitude) in the colon, when compared to MMP-9-deficient and wt mice (Fig. 6). Interestingly, whereas in colonic samples of some naïve but none of the colitic wt and MMP-9-deficient mice E. coli were absent, even in 20% (3 out of 15) of the DSS-treated MMP-2-deficient mice no E. coli were detectable.
Fig. 6.
Less overgrowth of the colon lumen with commensal E. coli in MMP-2-, but not MMP-9-deficient mice with acute DSS colitis. Quantitative analysis of E. coli loads of the colon lumen was performed by culture (see Materials and methods). Individual bacterial counts (CFU, colony forming units) were determined in wildtype (WT; black symbols), MMP-9–/– (MMP-9; gray symbols), or MMP-2–/– (MMP-2; white symbols) mice without (naïve, N; diamonds) or with acute colitis (DSS; circles) at the end of the experiment. Numbers of mice harboring E. coli out of the total number of analyzed animals are given in parentheses. Medians and levels of significance determined by Mann–Whitney U test are indicated. Data are pooled from at least three independent experiments
Taken together, gelatinase A deficiency ameliorates inflammation in acute DSS colitis as indicated by a better clinical, macroscopic as well as histopathological outcome, paralleled by less influx of pro-inflammatory immune cells and less pro-inflammatory cytokine expression in the colon as well as less distinct overgrowth of the colonic lumen by potentially pro-inflammatory enterobacteria from the commensal colon microbiota.
Discussion
Matrix metalloproteinases play essential roles during inflammatory episodes in human IBD. The gelatinases A (MMP-2) and B (MMP-9) have been shown to be highly upregulated in small as well as large intestinal inflammation in mice and men [11–16]. We have recently reported that selective blockage of gelatinases by the synthetic compound RO28-2653 was effectively ameliorating acute ileitis [17] and colitis [18]. In addition, MMP-2-, but not MMP-9-deficient animals were protected from acute murine ileitis [17], indicating that in this hyper-acute model, MMP-2 is essential for the establishment of immune cell recruitment and inflammatory responses in the very early stages of inflammation. In the present study, we could extend these results by demonstrating that MMP-2-, but not MMP-9-deficient mice were protected from acute DSS colitis. Like in the small intestinal inflammation model, MMP-2-deficient mice presented with a better clinical and macroscopic outcome as well as significantly less histopathology of the colon mucosa. Given that MMPs are involved in the recruitment of immune cells into the gut [27, 28] and shed biologically active IL-1, IL-6, and TNF-α molecules from the surfaces of macrophage, and thus cytokines which are able to induce MMP expression from immune, epithelial, and parenchymal cells [28, 29], the disruption of this vicious cycle all in one by blocking one specific MMP without compromising beneficial MMP properties is of therapeutic interest. In our study, MMP-2, but not MMP-9 deficiency resulted in less influx of macrophages into the colon as indicated by significantly lower F4/80+ cell numbers in the colonic lamina propria and submucosa at day 8 which was paralleled by reduced cytokine expression in colon biopsies. Furthermore, we [18] and others [30, 31] could show in acute experimental colitis that following MMP blockage and, as depicted in the presented study, in MMP-2-, but not MMP-9-deficient mice, the influx of neutrophilic granulocytes into the colon is diminished: An effect that, in turn, results in reduced oxidative stress for the colonic epithelium. In addition, these effects were paralleled by less infiltration of the colonic lamina propria and submucosa by T-lymphocytes including Tregs as well as B-cells.
In experimental acute ileitis [17, 23, 24, 32, 33] and colitis [18, 20, 21, 32], inflammation was accompanied by a marked overgrowth of the intestinal lumen with commensal enterobacteria such as E. coli which possess potentially pro-inflammatory properties. Overgrowing E. coli easily translocate through a disrupted epithelial cell barrier into the intestinal lamina propria and subsequently come in contact with immune cells thereby exacerbating the inflammation by TLR4-dependent signaling [23, 24, 33]. In our study, following colitis induction by DSS, luminal E. coli loads increased to a much lesser extent in the colonic lumen of MMP-2-deficient mice as compared to MMP-9-deficient mice and wt controls.
The pro-inflammatory function of MMP-2 in acute DSS colitis, however, is in sharp contrast to earlier studies describing the role of gelatinases A and B large intestinal inflammation in mice. MMP-9-deficient mice were shown to be protected from acute colitis induced either by DSS [34, 35] or by Salmonella Typhimurium [34]. Studies with bone marrow chimera revealed that MMP-2 and MMP-9 derived from epithelial but not immune cells, mediated colonic inflammation [34, 36]. Furthermore, Garg and coworkers could show that besides MMP-2 knockout [36], MMP-2/MMP-9 double deficient animals [37] also suffered from acute colitis following DSS treatment or Salmonella Typhimurium infection. Taken together, these studies revealed that epithelial-derived MMP-9 is an important mediator in colitis at early stages of disease development whereas MMP-2 exerts protective function preserving intestinal epithelial barrier integrity [36, 37]. The colitis-mediating properties of MMP-9, however, were shown to override MMP-2’s protective function during colitis [37].
It is tempting to speculate that these contradicting findings might be caused by a cumulative effect of differences in factors of the experimental setups such as DSS concentrations, duration of colitis, as well as age and sex of the used animals. For instance, the sex of the host is known to be correlated to differences in the immune responses of males and females to inflammatory stimuli [38–40]. According to our experiences (Prof. Britta Siegmund, Charité Berlin; personal communication) male mice (as used in [35]) might be more resistant to colitis induction by DSS as compared to female animals (in our study) whereas mice younger than 8 weeks of age (as used in [36]) are more susceptible to acute DSS colitis as compared to 3-month-old animals (in the presented study). Furthermore, the important role of the host microbiota composition in initiating, mediating, and perpetuating acute and chronic intestinal inflammation in mice and men is of current debate and highlighted by a plethora of recent studies [41–47]. For this reason, marked differences in the composition of the commensal intestinal microbiota might represent a general problem when comparing the data from different research that are based on in vivo studies in mice. It is well known that the colonization status of mice tremendously varies between animal facilities, units within the same facility, between rooms within the same unit and, in addition, between cages within the same room [48, 49]. Moreover, factors such age, sex, genetic background, infection status, and diet can all synergistically modulate the intestinal microbiota composition [46]. These differences in hygiene and microbial colonization status might be subtle at the first glance, but have a huge biologic impact, especially in inflammation and infection experiments [46, 47]. For instance, members of the gut microbiota such as Bacteroides spp. [50, 51] and Enterococcus faecealis strains [47, 52, 53] are able to express molecules exerting MMP activity which in turn might affect epithelial barrier function and thus represent potential triggers toward higher susceptibility of the host to inflammatory stimuli [46]. Indeed, mice purchased from different commercial vendors exhibited differences in susceptibility or resistance in inflammation and infection models due to their distinct colonization status [46, 48]. Futhermore, susceptible mice can be diverted into a resistant condition by transplanting gut flora from a resistant to a susceptible host (and vice versa), irrespective of the underlying genotype (Finlay et al., manuscript in press, PLoSONE; personal communication).
Taken together, we show for the first time that MMP-2, but not MMP-9 is essentially involved in acute DSS colitis. These results opposite to what has been published so far might be due to substantial differences in age, sex, underlying background of the respective genotype, and fundamental differences in the colonic microflora composition that is dependent on the source and the animal facility. Consequently, a detailed survey of the gut microbiota should be generally implemented in immunological/inflammatory studies in the future in order to be able to compare results from different facilities.
Acknowledgments
This work was supported by grants from the German Research Foundation (DFG) to SB and UBG (GO363/12-1, CampyGerm; SFB633, TP A7), AK and CL (SFB633, TP Z1), BS and AB(SFB633, TP A12), MMH (SFB633, TP B6), and from the German Federal Ministry of Education and Research (BMBF) to SB (“Lab in a hanky” projects TP1.1 and TP 8.2). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. We gratefully acknowledge providing breeding pairs of MMP-2- and MMP-9-deficient mice by Prof. Claude Libert (University of Ghent, Belgium) and Prof. Leif R. Lund (University of Copenhagen, Denmark), respectively.
We thank Michaela Wattrodt, Ursula Rüschendorf, Gernot Reifenberger, Uwe Lohmann, and the staff of the animal research facility for excellent technical assistance, animal breeding, and genotyping of mice. We are grateful to Simone Spieckermann for immunohistochemistry staining of colon sections. We further acknowledge critical discussions with Prof. Dr. Dr. h.c. Helmut Hahn, Prof. Dr. Rajan Somasundaram, and Dr. Martin Rühl.
Glossary
Abbreviations
- DSS:
dextrane sulfate sodium
- MMP:
matrixmetalloproteinase
- IBD:
inflammatory bowel disease
- ELISA:
enzyme-linked immunosorbent assay
- HE:
hematoxylin eosin
- PBS:
phosphate-buffered saline
- TIMPs:
tissue inhibitors of matrixmetalloproteinases
- Tregs:
regulatory T cells
- TNF:
tumor necrosis factor
- IFN:
interferon
- PCR:
polymerase chain reaction
- spp:
species
- HPRT:
hypoxanthine-guanine-phosphoribosyltransferase
- wt:
wildtype
- p.i.:
post induction
Contributor Information
M. M. Heimesaat, 1Department of Microbiology and Hygiene, Charité – University Medicine Berlin, Berlin, Germany.
I. R. Dunay, 2Department of Microbiology and Hygiene, University of Magdeburg, Magdeburg, Germany.
D. Fuchs, 1Department of Microbiology and Hygiene, Charité – University Medicine Berlin, Berlin, Germany.
D. Trautmann, 1Department of Microbiology and Hygiene, Charité – University Medicine Berlin, Berlin, Germany.
A. Fischer, 1Department of Microbiology and Hygiene, Charité – University Medicine Berlin, Berlin, Germany.
A. A. Kühl, 3Department of Pathology/Research Center ImmunoSciences (RCIS), Charité – University Medicine Berlin, Berlin, Germany.
C. Loddenkemper, 3Department of Pathology/Research Center ImmunoSciences (RCIS), Charité – University Medicine Berlin, Berlin, Germany.
B. Siegmund, 4Department of Internal Medicine, Charité – University Medicine Berlin, Berlin, Germany.
A. Batra, 4Department of Internal Medicine, Charité – University Medicine Berlin, Berlin, Germany.
S. Bereswill, 1Department of Microbiology and Hygiene, Charité – University Medicine Berlin, Berlin, Germany.
O. Liesenfeld, 1Department of Microbiology and Hygiene, Charité – University Medicine Berlin, Berlin, Germany.
References
- 1.Goetzl EJ, Banda MJ, Leppert D. Matrix metalloproteinases in immunity. J Immunol. 1996 Jan 1;156(1):1–4. [PubMed] [Google Scholar]
- 2.Brinckerhoff CE, Matrisian LM. Matrix metalloproteinases: a tail of a frog that became a prince. Nat Rev Mol Cell Biol. 2002 Mar;3(3):207–214. doi: 10.1038/nrm763. [DOI] [PubMed] [Google Scholar]
- 3.Birkedal-Hansen H, Moore WG, Bodden MK, Windsor LJ, Birkedal-Hansen B, DeCarlo A, Engler JA. Matrix metalloproteinases: a review. Crit Rev Oral Biol Med. 1993;4(2):197–250. doi: 10.1177/10454411930040020401. [DOI] [PubMed] [Google Scholar]
- 4.Nelson AR, Fingleton B, Rothenberg ML, Matrisian LM. Matrix metalloproteinases: biologic activity and clinical implications. J Clin Oncol. 2000 Mar;18(5):1135–1149. doi: 10.1200/JCO.2000.18.5.1135. [DOI] [PubMed] [Google Scholar]
- 5.Crawford HC, Matrisian LM. Mechanisms controlling the transcription of matrix metalloproteinase genes in normal and neoplastic cells. Enzyme Protein. 1996;49(1-3):20–37. doi: 10.1159/000468614. [DOI] [PubMed] [Google Scholar]
- 6.Sarén P, Welgus HG, Kovanen PT. TNF-alpha and IL-1beta selectively induce expression of 92-kDa gelatinase by human macrophages. J Immunol. 1996 Nov 1;157(9):4159–4165. [PubMed] [Google Scholar]
- 7.Cawston TE, Billington C. Metalloproteinases in the rheumatic diseases. J Pathol. 1996 Oct;180(2):115–117. doi: 10.1002/(SICI)1096-9896(199610)180:2<115::AID-PATH674>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 8.Itoh T, Matsuda H, Tanioka M, Kuwabara K, Itohara S, Suzuki R. The role of matrix metalloproteinase-2 and matrix metalloproteinase-9 in antibody-induced arthritis. J Immunol. 2002 Sep 1;169(5):2643–2647. doi: 10.4049/jimmunol.169.5.2643. [DOI] [PubMed] [Google Scholar]
- 9.Uzui H, Harpf A, Liu M, Doherty TM, Shukla A, Chai NN, Tripathi PV, Jovinge S, Wilkin DJ, Asotra K, Shah PK, Rajavashisth TB. Increased expression of membrane type 3-matrix metalloproteinase in human atherosclerotic plaque: role of activated macrophages and inflammatory cytokines. Circulation. 2002 Dec 10;106(24):3024–3030. doi: 10.1161/01.cir.0000041433.94868.12. [DOI] [PubMed] [Google Scholar]
- 10.Salmela MT, MacDonald TT, Black D, Irvine B, Zhuma T, Saarialho-Kere U, Pender SL. Upregulation of matrix metalloproteinases in a model of T cell mediated tissue injury in the gut: analysis by gene array and in situ hybridisation. Gut. 2002 Oct;51(4):540–547. doi: 10.1136/gut.51.4.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bailey CJ, Hembry RM, Alexander A, Irving MH, Grant ME, Shuttleworth CA. Distribution of the matrix metalloproteinases stromelysin, gelatinases A and B, and collagenase in Crohn's disease and normal intestine. J Clin Pathol. 1994 Feb;47(2):113–116. doi: 10.1136/jcp.47.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baugh MD, Perry MJ, Hollander AP, Davies DR, Cross SS, Lobo AJ, Taylor CJ, Evans GS. Matrix metalloproteinase levels are elevated in inflammatory bowel disease. Gastroenterology. 1999 Oct;117(4):814–822. doi: 10.1016/s0016-5085(99)70339-2. [DOI] [PubMed] [Google Scholar]
- 13.Heuschkel RB, MacDonald TT, Monteleone G, Bajaj-Elliott M, Smith JA, Pender SL. Imbalance of stromelysin-1 and TIMP-1 in the mucosal lesions of children with inflammatory bowel disease. Gut. 2000 Jul;47(1):57–62. doi: 10.1136/gut.47.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.von Lampe B, Barthel B, Coupland SE, Riecken EO, Rosewicz S. Differential expression of matrix metalloproteinases and their tissue inhibitors in colon mucosa of patients with inflammatory bowel disease. Gut. 2000 Jul;47(1):63–73. doi: 10.1136/gut.47.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Louis E, Ribbens C, Godon A, Franchimont D, De Groote D, Hardy N, Boniver J, Belaiche J, Malaise M. Increased production of matrix metalloproteinase-3 and tissue inhibitor of metalloproteinase-1 by inflamed mucosa in inflammatory bowel disease. Clin Exp Immunol. 2000 May;120(2):241–246. doi: 10.1046/j.1365-2249.2000.01227.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stallmach A, Chan CC, Ecker KW, Feifel G, Herbst H, Schuppan D, Zeitz M. Comparable expression of matrix metalloproteinases 1 and 2 in pouchitis and ulcerative colitis. Gut. 2000 Sep;47(3):415–422. doi: 10.1136/gut.47.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muñoz M, Heimesaat MM, Danker K, Struck D, Lohmann U, Plickert R, Bereswill S, Fischer A, Dunay IR, Wolk K, Loddenkemper C, Krell HW, Libert C, Lund LR, Frey O, Hölscher C, Iwakura Y, Ghilardi N, Ouyang W, Kamradt T, Sabat R, Liesenfeld O. Interleukin (IL)-23 mediates Toxoplasma gondii-induced immunopathology in the gut via matrixmetalloproteinase-2 and IL-22 but independent of IL-17. J Exp Med. 2009 Dec 21;206(13):3047–3059. doi: 10.1084/jem.20090900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heimesaat MM, Dunay IR, Fuchs D, Trautmann D, Fischer A, Kühl AA, Loddenkemper C, Batra A, Siegmund B, Krell H-W, Bereswill S, Liesenfeld O. Selective gelatinase blockage ameliorates acute DSS colitis. EuJMI. 2011;1:228–236. doi: 10.1556/EuJMI.1.2011.3.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Siegmund B, Rieder F, Albrich S, Wolf K, Bidlingmaier C, Firestein GS, Boyle D, Lehr HA, Loher F, Hartmann G, Endres S, Eigler A. Adenosine kinase inhibitor GP515 improves experimental colitis in mice. J Pharmacol Exp Ther. 2001 Jan;296(1):99–105. [PubMed] [Google Scholar]
- 20.Heimesaat MM, Fischer A, Siegmund B, Kupz A, Niebergall J, Fuchs D, Jahn HK, Freudenberg M, Loddenkemper C, Batra A, Lehr HA, Liesenfeld O, Blaut M, Göbel UB, Schumann RR, Bereswill S. Shift towards pro-inflammatory intestinal bacteria aggravates acute murine colitis via Toll-like receptors 2 and 4. PLoS One. 2007 Jul 25;2(7):e662. doi: 10.1371/journal.pone.0000662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heimesaat MM, Nogai A, Bereswill S, Plickert R, Fischer A, Loddenkemper C, Steinhoff U, Tchaptchet S, Thiel E, Freudenberg MA, Göbel UB, Uharek L. MyD88/TLR9 mediated immunopathology and gut microbiota dynamics in a novel murine model of intestinal graft-versus-host disease. Gut. 2010 Aug;59(8):1079–1087. doi: 10.1136/gut.2009.197434. [DOI] [PubMed] [Google Scholar]
- 22.Bereswill S, Fischer A, Plickert R, Haag LM, Otto B, Kühl AA, Dasti JI, Zautner AE, Muñoz M, Loddenkemper C, Gross U, Göbel UB, Heimesaat MM. Novel murine infection models provide deep insights into the "ménage à trois" of Campylobacter jejuni, microbiota and host innate immunity. PLoS One. 2011;6(6):e20953. doi: 10.1371/journal.pone.0020953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heimesaat MM, Bereswill S, Fischer A, Fuchs D, Struck D, Niebergall J, Jahn HK, Dunay IR, Moter A, Gescher DM, Schumann RR, Göbel UB, Liesenfeld O. Gram-negative bacteria aggravate murine small intestinal Th1-type immunopathology following oral infection with Toxoplasma gondii. J Immunol. 2006 Dec 15;177(12):8785–8795. doi: 10.4049/jimmunol.177.12.8785. [DOI] [PubMed] [Google Scholar]
- 24.Heimesaat MM, Fischer A, Jahn HK, Niebergall J, Freudenberg M, Blaut M, Liesenfeld O, Schumann RR, Göbel UB, Bereswill S. Exacerbation of murine ileitis by Toll-like receptor 4 mediated sensing of lipopolysaccharide from commensal Escherichia coli. Gut. 2007 Jul;56(7):941–948. doi: 10.1136/gut.2006.104497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Podolsky DK. Inflammatory bowel disease. N Engl J Med. 2002 Aug 8;347(6):417–429. doi: 10.1056/NEJMra020831. [DOI] [PubMed] [Google Scholar]
- 26.Basset C, Holton J. Inflammatory bowel disease: is the intestine a Trojan horse? Sci Prog. 2002;85(Pt 1):33–56. doi: 10.3184/003685002783238861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martinesi M, Treves C, Bonanomi AG, Milla M, Bagnoli S, Zuegel U, Steinmeyer A, Stio M. Down-regulation of adhesion molecules and matrix metalloproteinases by ZK 156979 in inflammatory bowel diseases. Clin Immunol. 2010 Jul;136(1):51–60. doi: 10.1016/j.clim.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 28.Naito Y, Takagi T, Kuroda M, Katada K, Ichikawa H, Kokura S, Yoshida N, Okanoue T, Yoshikawa T. An orally active matrix metalloproteinase inhibitor, ONO-4817, reduces dextran sulfate sodium-induced colitis in mice. Inflamm Res. 2004 Sep;53(9):462–498. doi: 10.1007/s00011-004-1281-1. [DOI] [PubMed] [Google Scholar]
- 29.Wang M, Qin X, Mudgett JS, Ferguson TA, Senior RM, Welgus HG. Matrix metalloproteinase deficiencies affect contact hypersensitivity: stromelysin-1 deficiency prevents the response and gelatinase B deficiency prolongs the response. Proc Natl Acad Sci U S A. 1999 Jun 8;96(12):6885–6889. doi: 10.1073/pnas.96.12.6885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Medina C, Videla S, Radomski A, Radomski MW, Antolín M, Guarner F, Vilaseca J, Salas A, Malagelada JR. Increased activity and expression of matrix metalloproteinase-9 in a rat model of distal colitis. Am J Physiol Gastrointest Liver Physiol. 2003 Jan;284(1):G116–G122. doi: 10.1152/ajpheart.00036.2002. [DOI] [PubMed] [Google Scholar]
- 31.Huang TY, Chu HC, Lin YL, Lin CK, Hsieh TY, Chang WK, Chao YC, Liao CL. Minocycline attenuates experimental colitis in mice by blocking expression of inducible nitric oxide synthase and matrix metalloproteinases. Toxicol Appl Pharmacol. 2009 May 15;237(1):69–82. doi: 10.1016/j.taap.2009.02.026. [DOI] [PubMed] [Google Scholar]
- 32.Erridge C, Duncan SH, Bereswill S, Heimesaat MM. The induction of colitis and ileitis in mice is associated with marked increases in intestinal concentrations of stimulants of TLRs 2, 4, and 5. PLoS One. 2010 Feb 9;5(2):e9125. doi: 10.1371/journal.pone.0009125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bereswill S, Muñoz M, Fischer A, Plickert R, Haag LM, Otto B, Kühl AA, Loddenkemper C, Göbel UB, Heimesaat MM. Anti-inflammatory effects of resveratrol, curcumin and simvastatin in acute small intestinal inflammation. PLoS One. 2010 Dec 3;5(12):e15099. doi: 10.1371/journal.pone.0015099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Castaneda FE, Walia B, Vijay-Kumar M, Patel NR, Roser S, Kolachala VL, Rojas M, Wang L, Oprea G, Garg P, Gewirtz AT, Roman J, Merlin D, Sitaraman SV. Targeted deletion of metalloproteinase 9 attenuates experimental colitis in mice: central role of epithelial-derived MMP. Gastroenterology. 2005 Dec;129(6):1991–2008. doi: 10.1053/j.gastro.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 35.Santana A, Medina C, Paz-Cabrera MC, Díaz-Gonzalez F, Farré E, Salas A, Radomski MW, Quintero E. Attenuation of dextran sodium sulphate induced colitis in matrix metalloproteinase-9 deficient mice. World J Gastroenterol. 2006 Oct 28;12(40):6464–6472. doi: 10.3748/wjg.v12.i40.6464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Garg P, Rojas M, Ravi A, Bockbrader K, Epstein S, Vijay-Kumar M, Gewirtz AT, Merlin D, Sitaraman SV. Selective ablation of matrix metalloproteinase-2 exacerbates experimental colitis: contrasting role of gelatinases in the pathogenesis of colitis. J Immunol. 2006 Sep 15;177(6):4103–4112. doi: 10.4049/jimmunol.177.6.4103. [DOI] [PubMed] [Google Scholar]
- 37.Garg P, Vijay-Kumar M, Wang L, Gewirtz AT, Merlin D, Sitaraman SV. Matrix metalloproteinase-9-mediated tissue injury overrides the protective effect of matrix metalloproteinase-2 during colitis. Am J Physiol Gastrointest Liver Physiol. 2009 Feb;296(2):G175–G184. doi: 10.1152/ajpgi.90454.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Terres G, Morrison SL, Habicht GS. A quantitative difference in the immune response between male and female mice. Proc Soc Exp Biol Med. 1968 Mar;127(3):664–667. doi: 10.3181/00379727-127-32768. [DOI] [PubMed] [Google Scholar]
- 39.Morell V. Zeroing in on how hormones affect the immune system. Science. 1995 Aug 11;269(5225):773–775. doi: 10.1126/science.7638587. [DOI] [PubMed] [Google Scholar]
- 40.Kovacs A, Ben-Jacob N, Tayem H, Halperin E, Iraqi FA, Gophna U. Genotype is a stronger determinant than sex of the mouse gut microbiota. Microb Ecol. 2011 Feb;61(2):423–428. doi: 10.1007/s00248-010-9787-2. [DOI] [PubMed] [Google Scholar]
- 41.Bloom SM, Bijanki VN, Nava GM, Sun L, Malvin NP, Donermeyer DL, Dunne WM, Jr., Allen PM, Stappenbeck TS. Commensal Bacteroides species induce colitis in host-genotype-specific fashion in a mouse model of inflammatory bowel disease. Cell Host Microbe. 2011 May 19;9(5):390–403. doi: 10.1016/j.chom.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Balish E, Warner T. Enterococcus faecalis induces inflammatory bowel disease in interleukin-10 knockout mice. Am J Pathol. 2002 Jun;160(6):2253–2257. doi: 10.1016/S0002-9440(10)61172-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sydora BC, Macfarlane SM, Walker JW, Dmytrash AL, Churchill TA, Doyle J, Fedorak RN. Epithelial barrier disruption allows nondisease-causing bacteria to initiate and sustain IBD in the IL-10 gene-deficient mouse. Inflamm Bowel Dis. 2007 Aug;13(8):947–954. doi: 10.1002/ibd.20155. [DOI] [PubMed] [Google Scholar]
- 44.Sartor RB. Genetics and environmental interactions shape the intestinal microbiome to promote inflammatory bowel disease versus mucosal homeostasis. Gastroenterology. 2010 Dec;139(6):1816–1819. doi: 10.1053/j.gastro.2010.10.036. [DOI] [PubMed] [Google Scholar]
- 45.Sartor RB. Microbial influences in inflammatory bowel diseases. Gastroenterology. 2008 Feb;134(2):577–594. doi: 10.1053/j.gastro.2007.11.059. [DOI] [PubMed] [Google Scholar]
- 46.Nell S, Suerbaum S, Josenhans C. The impact of the microbiota on the pathogenesis of IBD: lessons from mouse infection models. Nat Rev Microbiol. 2010 Aug;8(8):564–577. doi: 10.1038/nrmicro2403. [DOI] [PubMed] [Google Scholar]
- 47.Steck N, Mueller K, Schemann M, Haller D. Bacterial proteases in IBD and IBS. Gut. 2011 doi: 10.1136/gutjnl-2011-300775. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 48.Deloris Alexander A, Orcutt RP, Henry JC, Baker J, Jr., Bissahoyo AC, Threadgill DW. Quantitative PCR assays for mouse enteric flora reveal strain-dependent differences in composition that are influenced by the microenvironment. Mamm Genome. 2006 Nov;17(11):1093–1104. doi: 10.1007/s00335-006-0063-1. [DOI] [PubMed] [Google Scholar]
- 49.Ge Z, Feng Y, Taylor NS, Ohtani M, Polz MF, Schauer DB, Fox JG. Colonization dynamics of altered Schaedler flora is influenced by gender, aging, and Helicobacter hepaticus infection in the intestines of Swiss Webster mice. Appl Environ Microbiol. 2006 Jul;72(7):5100–5103. doi: 10.1128/AEM.01934-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Medina C, Santana A, Llopis M, Paz-Cabrera MC, Antolín M, Mourelle M, Guarner F, Vilaseca J, Gonzalez C, Salas A, Quintero E, Malagelada JR. Induction of colonic transmural inflammation by Bacteroides fragilis: implication of matrix metalloproteinases. Inflamm Bowel Dis. 2005 Feb;11(2):99–105. doi: 10.1097/00054725-200502000-00002. [DOI] [PubMed] [Google Scholar]
- 51.Wu S, Rhee KJ, Zhang M, Franco A, Sears CL. Bacteroides fragilis toxin stimulates intestinal epithelial cell shedding and gamma-secretase-dependent E-cadherin cleavage. J Cell Sci. 2007 Jun 1;120(Pt 11):1944–1952. doi: 10.1242/jcs.03455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zeng J, Teng F, Murray BE. Gelatinase is important for translocation of Enterococcus faecalis across polarized human enterocyte-like T84 cells. Infect Immun. 2005 Mar;73(3):1606–1612. doi: 10.1128/IAI.73.3.1606-1612.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Steck N, Hoffmann M, Sava IG, Kim SC, Hahne H, Tonkonogy SL, Mair K, Krueger D, Pruteanu M, Shanahan F, Vogelmann R, Schemann M, Kuster B, Sartor RB, Haller D. Enterococcus faecalis metalloprotease compromises epithelial barrier and contributes to intestinal inflammation. Gastroenterology. 2011 Sep;141(3):959–971. doi: 10.1053/j.gastro.2011.05.035. [DOI] [PubMed] [Google Scholar]