Abstract
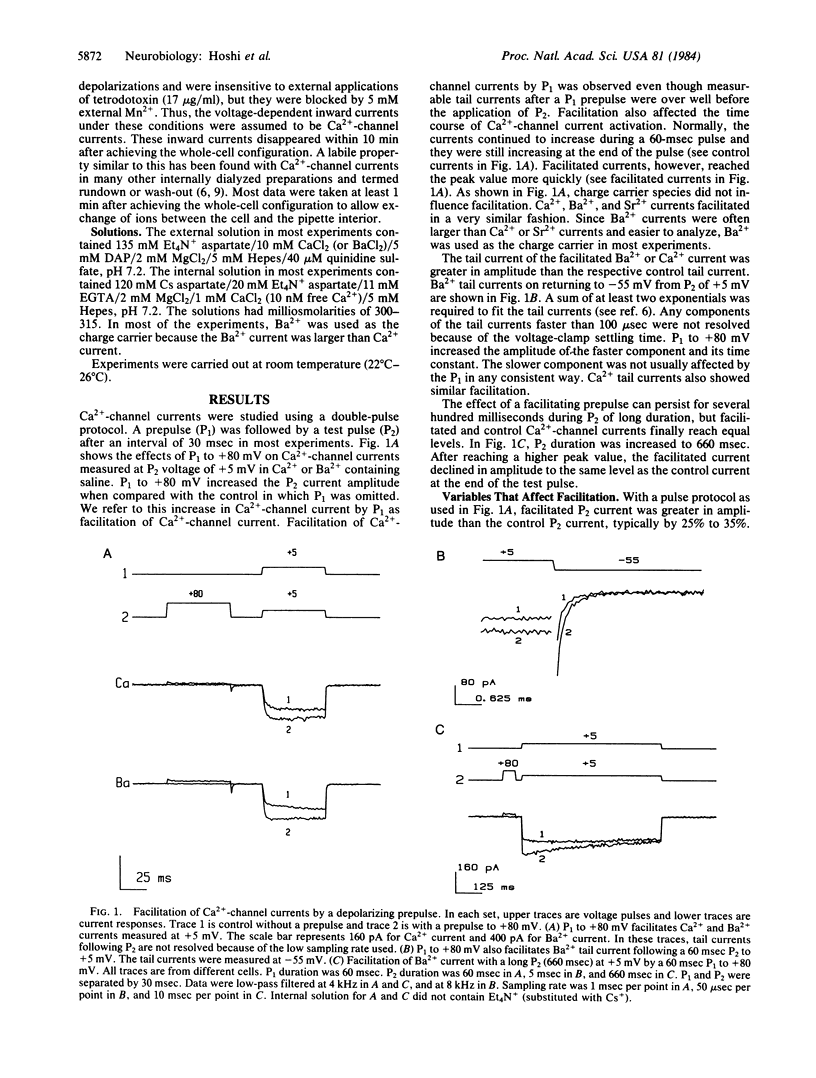
Ca2+-channel currents in primary cultures of bovine adrenal chromaffin cells were studied using the whole-cell patch-clamp method. Parameters of a double-pulse protocol were systematically varied to characterize facilitation by a prepulse (P1) of Ca2+-channel current during a test pulse (P2). The pulses were usually separated by 30 msec, an interval sufficient for decay of any measurable P1 tail currents. The Ca2+-channel current amplitude during P2 increased when P1 voltage was more positive than 0 mV. The effect became progressively greater with more positive P1 voltage. With a 60-msec P1 to +80 mV, the current amplitude typically increased by 25%-35% during a 60-msec P2. Comparison of facilitated and control inward Ca2+-channel current I(V) curves showed that facilitation was also strongly dependent on P2 test voltage. Facilitation of Ca2+-channel currents is a voltage-dependent phenomenon and is not dependent on Ca2+ entry. When short repetitive voltage-clamp pulses were applied, the Ca2+-channel current amplitude increased with each pulse. This suggests that Ca2+-channel facilitation could enhance release of catecholamines from chromaffin cells during a train of action potentials.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Byerly L., Hagiwara S. Calcium currents in internally perfused nerve cell bodies of Limnea stagnalis. J Physiol. 1982 Jan;322:503–528. doi: 10.1113/jphysiol.1982.sp014052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlton M. P., Smith S. J., Zucker R. S. Role of presynaptic calcium ions and channels in synaptic facilitation and depression at the squid giant synapse. J Physiol. 1982 Feb;323:173–193. doi: 10.1113/jphysiol.1982.sp014067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge F. A., Jr, Rahamimoff R. Co-operative action a calcium ions in transmitter release at the neuromuscular junction. J Physiol. 1967 Nov;193(2):419–432. doi: 10.1113/jphysiol.1967.sp008367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas W. W., Kanno T. The effect of amethocaine on acetylcholine-induced depolarization and catecholamine secretion in the adrenal chromaffin cell. Br J Pharmacol Chemother. 1967 Aug;30(3):612–619. doi: 10.1111/j.1476-5381.1967.tb02167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert R., Ewald D. Inactivation of calcium conductance characterized by tail current measurements in neurones of Aplysia californica. J Physiol. 1983 Dec;345:549–565. doi: 10.1113/jphysiol.1983.sp014996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert R., Tillotson D., Ridgway E. B. Voltage-dependent facilitation of Ca2+ entry in voltage-clamped, aequorin-injected molluscan neurons. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1748–1752. doi: 10.1073/pnas.74.4.1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenwick E. M., Marty A., Neher E. A patch-clamp study of bovine chromaffin cells and of their sensitivity to acetylcholine. J Physiol. 1982 Oct;331:577–597. doi: 10.1113/jphysiol.1982.sp014393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenwick E. M., Marty A., Neher E. Sodium and calcium channels in bovine chromaffin cells. J Physiol. 1982 Oct;331:599–635. doi: 10.1113/jphysiol.1982.sp014394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Byerly L. Calcium channel. Annu Rev Neurosci. 1981;4:69–125. doi: 10.1146/annurev.ne.04.030181.000441. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Heyer C. B., Lux H. D. Properties of a facilitating calcium current in pace-maker neurones of the snail, Helix pomatia. J Physiol. 1976 Nov;262(2):319–348. doi: 10.1113/jphysiol.1976.sp011598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. The role of calcium in neuromuscular facilitation. J Physiol. 1968 Mar;195(2):481–492. doi: 10.1113/jphysiol.1968.sp008469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick D. L., Ledbetter F. H., Carson K. A., Kirshner A. G., Slepetis R., Kirshner N. Stability of bovine adrenal medulla cells in culture. J Neurochem. 1980 Sep;35(3):679–692. doi: 10.1111/j.1471-4159.1980.tb03707.x. [DOI] [PubMed] [Google Scholar]
- Lux H. D., Eckert R. Inferred slow inward current in snail neurones. Nature. 1974 Aug 16;250(467):574–576. doi: 10.1038/250574a0. [DOI] [PubMed] [Google Scholar]
- Lux H. D., Heyer C. B. An aequorin study of a facilitating calcium current in bursting pacemaker neurons of Helix. Neuroscience. 1977;2(4):585–592. doi: 10.1016/0306-4522(77)90054-9. [DOI] [PubMed] [Google Scholar]
- Rahamimoff R. A dual effect of calcium ions on neuromuscular facilitation. J Physiol. 1968 Mar;195(2):471–480. doi: 10.1113/jphysiol.1968.sp008468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichardt L. F., Kelly R. B. A molecular description of nerve terminal function. Annu Rev Biochem. 1983;52:871–926. doi: 10.1146/annurev.bi.52.070183.004255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien R. W. Calcium channels in excitable cell membranes. Annu Rev Physiol. 1983;45:341–358. doi: 10.1146/annurev.ph.45.030183.002013. [DOI] [PubMed] [Google Scholar]
- Wilson S. P., Viveros O. H. Primary culture of adrenal medullary chromaffin cells in a chemically defined medium. Exp Cell Res. 1981 May;133(1):159–169. doi: 10.1016/0014-4827(81)90366-9. [DOI] [PubMed] [Google Scholar]


