Abstract
Purpose
We evaluated the association of microvascular invasion (MVI) and capillary-lymphatic invasion (CLI) with patient outcome following nephrectomy for renal cell carcinoma (RCC).
Material and Methods
We identified 1,433 patients surgically treated for sporadic, unilateral RCC between 2001 and 2008. All specimens were reviewed by a single uropathologist for MVI and CLI. Associations with time to metastases and death from RCC were evaluated using Cox proportional hazards models, controlling for established clinicopathologic prognostic variables.
Results
MVI and CLI were identified in 11% (119/1,103) and 2% (17/1,103) with clear cell, 2% (5/219) and <1% (1/219) with papillary, and 1% (1/86) and 0 with chromophobe RCC, respectively. Median follow-up for patients still alive was 6.4 years (range 0-11). In clear cell RCC, MVI was univariately associated with an increased risk of metastases (HR 3.5,p<0.001) and cancer-specific death (HR 3.0,p<0.001). However, on multivariate analyses, these associations were no longer statistically significant (HR 1.2,p=0.4 and HR 1.3,p=0.1, respectively). CLI remained significantly associated with an increased risk of metastases and death both univariately (HR 15.9,p<0.001 and HR 11.6,p<0.001, respectively) and on multivariate analyses (HR 3.2,p<0.001 and HR 3.1,p<0.001, respectively).
Conclusions
MVI is associated with an increased risk of metastases and cancer death for patients with clear cell RCC, although this does not remain significant after controlling for established prognostic variables. Meanwhile, CLI appears to be independently associated with metastases and cancer death even after controlling for known prognostic risk factors; however, given its rarity, this feature may prove to be of limited clinical significance.
Keywords: Renal cell carcinoma, microvessels
INTRODUCTION
There are an estimated 64,770 new cases and 13,570 deaths from renal cancer in the United States in 2012.1 Given the variable course after surgical management, much effort has been expended to accurately predict disease outcomes based on patient specific factors. Since 2000, several models have been created using clinicopathologic features such as stage, size, grade, tumor necrosis, symptoms, and performance status.2-5 These tools have improved our ability to counsel patients, however further refinement in prognostication is needed.
Microvascular invasion (MVI) and capillary-lymphatic invasion (CLI) represent invasion into the local small vessel architecture and are predictors of adverse outcome in other urologic malignancies, however their predictive value in renal cell carcinoma (RCC) remains unclear.6,7 Numerous studies have evaluated the effect of MVI on metastasis-free survival (MFS) and cancer-specific survival (CSS) with variable results, however most have been constrained by a limited sample size or lack of centralized pathologic review.8-18
In the current study, we evaluated the univariate and multivariate associations of MVI and CLI with MFS and CSS in the entire cohort, as well as in those with and without metastases, in low-stage and low-grade disease, and in patients undergoing systemic therapy.
MATERIALS AND METHODS
After Institutional Review Board approval was obtained, we queried the Mayo Clinic Nephrectomy Registry to identify 1,433 patients treated with radical or partial nephrectomy for sporadic, unilateral, RCC between 2001 and 2008 in which information regarding the presence or absence of MVI or CLI was available.
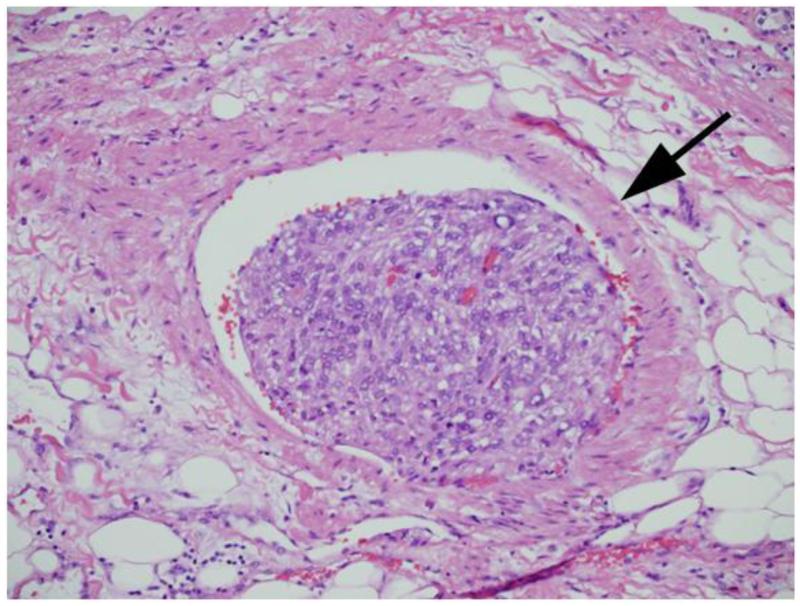
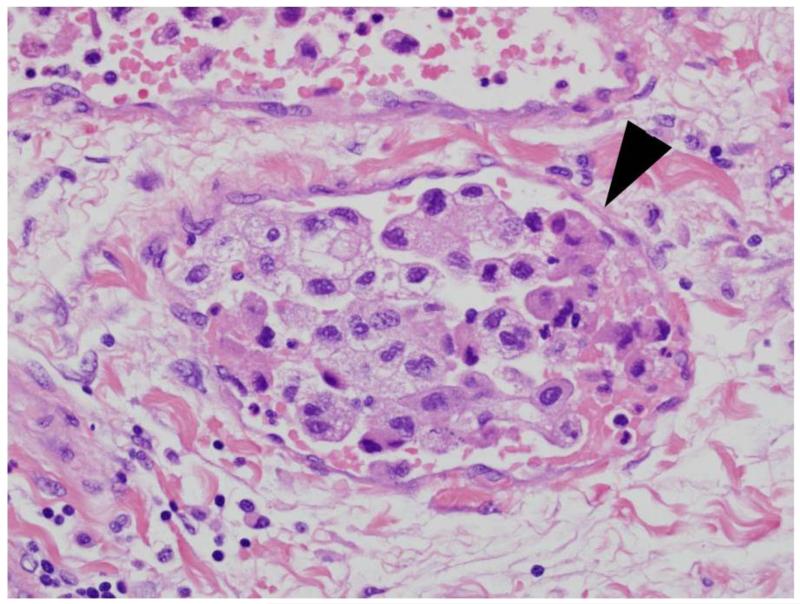
The clinical features studied included age, gender, symptoms at presentation, Eastern Cooperative Oncology Group (ECOG) performance status, and type of surgery. The pathologic features included histologic subtype, tumor size, the 2010 AJCC TNM staging for primary tumor, lymph node, and metastases classifications, nuclear grade, coagulative tumor necrosis, sarcomatoid differentiation, MVI, CLI, and collecting system invasion. MVI was defined as the presence of tumor within microscopic veins or venules with a muscular coat, regardless of gross tumor in the renal vein (Figure 1). The term CLI was specifically used to define the presence of tumor within a microscopic capillary or lymphatic channel, vessels lacking a muscular coat (Figure 2). A single genitourinary pathologist (J.C.C.) reviewed all specimens without knowledge of patient outcome. Pathologic features were assessed using hemotoxylin and eosin staining only, without the use of immunohistochemical stains, to reflect current standard clinical practice.
Figure 1.
Microvascular invasion. Arrow illustrates smooth muscle around vessel.
Figure 2.
Capillary-lymphatic invasion. Arrowhead illustrates endothelial lining of vessel lacking surrounding smooth muscle.
The primary tumor, lymph node, and metastases classifications, tumor size, grade, and tumor necrosis were combined to calculate the Mayo Clinic SSIGN and PROG scores for patients with clear cell RCC (ccRCC).2,19 Briefly, the SSIGN score is calculated using weighted scores for each of the parameters listed above (Table 1), and the sum is used to estimate risk of cancer specific death.2 The PROG score was created using similar methodology in patients with M0 disease and estimates risk of subsequent metastases.19 The impact of MVI and CLI was adjusted for each patient’s SSIGN or PROG score during multivariate analysis.
Table 1.
SSIGN and PROG scoring systems
| SSIGN Score2 |
PROG Score19 |
|
|---|---|---|
| Pathologic Tumor Stage | ||
| pT1a | 0 | 0 |
| pT1b | 0 | 2 |
| pT2 | 1 | 3 |
| pT3 | 2 | 4 |
| pT4 | 0 | 4 |
| Pathologic Node Stage | ||
| pNx/N0 | 0 | 0 |
| pN1/N2 | 2 | 2 |
| Pathologic Metastasis | ||
| Stage | ||
| pM0 | 0 | - |
| pM1 | 4 | - |
| Tumor Size (cm) | ||
| <5 | 0 | - |
| ≥5 | 2 | - |
| <10 | - | 0 |
| ≥10 | - | 1 |
| Nuclear Grade | ||
| 1/2 | 0 | 0 |
| 3 | 1 | 1 |
| 4 | 3 | 3 |
| Histologic Tumor Necrosis | ||
| Absent | 0 | 0 |
| Present | 2 | 1 |
Continuous features were summarized with means, standard deviations (SDs), medians, and ranges; categorical features were summarized with frequency counts and percentages. Comparisons were evaluated using Wilcoxon rank sum, chi-square, and Fisher exact tests. CSS and MFS were estimated using the Kaplan-Meier method. Associations of features with time to death from RCC and time to distant metastases were evaluated using Cox proportional hazards regression models and summarized with hazard ratios (HR) and 95% confidence intervals (CI).
Statistical analyses were performed using the SAS software package (SAS Institute, Cary, NC). All tests were two-sided with p-values <0.05 considered statistically significant.
RESULTS
There were 1,103 (77%) patients with non-cystic ccRCC, 219 (15%) with papillary RCC, 86 (6%) with chromophobe RCC, 5 (<1%) with collecting duct RCC, and 20 (1%) with RCC, not otherwise specified (NOS). MVI was identified in 129 (9%) of these tumors and CLI in 20 (1%) tumors. The frequency of MVI and CLI within each RCC histologic subtype is summarized in Table 2. MVI was significantly more common in ccRCC (p<0.001) compared with papillary and chromophobe RCC. CLI was uniformly rare across the three most common histologic subtypes. Associations of MVI and CLI with clinicopathologic features and patient outcome were further evaluated in ccRCC patients only since they were rarely observed in the other subtypes (Table 3).
Table 2.
Comparisons of MVI and CLI invasion by histologic subtype for 1,433 patients with RCC
| Histologic Subtype, N (%) | |||||
|---|---|---|---|---|---|
| Clear Cell N=1,103 |
Papillary N=219 |
Chromophobe N=86 |
Collecting Duct N=5 |
RCC, NOS N=20 |
|
| Microvascular invasion | 119 (11) | 5 (2) | 1 (1) | 1 (20) | 3 (15) |
| Capillary-lymphatic invasion | 17 (2) | 1 (<1) | 0 | 1 (20) | 1 (5) |
Table 3.
Comparisons of clinical and pathologic features by microvascular and capillary lymphatic invasion for 1,103 patients with clear cell RCC
| Microvascular Invasion | Capillary-lymphatic Invasion | |||||
|---|---|---|---|---|---|---|
| No N=984 |
Yes N=119 |
No N=1,086 |
Yes N=17 |
|||
| Feature | Mean (Median; Range) | P-value | Mean (Median; Range) | P-value | ||
| Age at surgery (years) | 61.9 (62; 19–93) | 65.2 (66; 36–90) | 0.005 | 62.2 (63; 19–93) | 66.2 (70; 39–88) | 0.14 |
| Tumor size (cm; N=1,102) | 6.2 (5.0; 0.5–22.0) | 10.0 (9.7; 2.6–29.0) | <0.001 | 6.5 (5.5; 0.5–29.0) | 10.7 (10.0; 4.0–24.0) | <0.001 |
| SSIGN score (N=1,102) | 3.6 (3; 0–15) | 7.5 (7; 1–15) | <0.001 | 4.0 (3; 0–15) | 10.0 (9; 5–15) | <0.001 |
| Gender | N (%) | N (%) | ||||
| Female | 353 (36) | 40 (34) | 0.63 | 391 (36) | 2 (12) | 0.04 |
| Male | 631 (64) | 79 (66) | 695 (64) | 15 (88) | ||
| Symptoms (N=1,101) | 462 (47) | 85 (71) | <0.001 | 533 (49) | 14 (82) | 0.007 |
| Constitutional symptoms (N=1,101) | 177 (18) | 42 (35) | <0.001 | 212 (20) | 7 (41) | 0.06 |
| ECOG performance status (N=1,102) | ||||||
| 0 | 825 (84) | 86 (73) | 0.003 | 898 (83) | 13 (76) | 0.52 |
| ≥1 | 159 (16) | 32 (27) | 187 (17) | 4 (24) | ||
| Type of surgery | ||||||
| Open radical | 445 (45) | 104 (87) | <0.001 | 533 (49) | 16 (94) | 0.002 |
| Open partial | 352 (36) | 4 (3) | 356 (33) | 0 | ||
| Laparoscopic radical | 131 (13) | 11 (9) | 141 (13) | 1 (6) | ||
| Laparoscopic partial | 56 (6) | 0 | 56 (5) | 0 | ||
| 2010 primary tumor classification (N=1,102) | ||||||
| pT1a | 359 (37) | 2 (2) | <0.001 | 361 (33) | 0 | <0.001 |
| pT1b | 220 (22) | 8 (7) | 228 (21) | 0 | ||
| pT2a | 85 (9) | 5 (4) | 89 (8) | 1 (6) | ||
| pT2b | 30 (3) | 4 (3) | 34 (3) | 0 | ||
| pT3a | 213 (22) | 65 (55) | 267 (25) | 11 (65) | ||
| pT3b | 49 (5) | 23 (19) | 69 (6) | 3 (18) | ||
| pT3c | 10 (1) | 6 (5) | 16 (1) | 0 | ||
| pT4 | 17 (2) | 6 (5) | 21 (2) | 2 (12) | ||
| 2010 regional lymph node involvement | ||||||
| pNx | 696 (71) | 47 (40) | <0.001 | 740 (68) | 3 (18) | <0.001 |
| pN0 | 226 (23) | 47 (40) | 268 (25) | 5 (29) | ||
| pN1 | 62 (6) | 25 (21) | 78 (7) | 9 (53) | ||
| Distant metastases | ||||||
| M0 | 876 (89) | 95 (80) | 0.004 | 959 (88) | 12 (71) | 0.04 |
| M1 | 108 (11) | 24 (20) | 127 (12) | 5 (29) | ||
| Nuclear grade | ||||||
| 1 | 75 (8) | 0 | <0.001 | 75 (7) | 0 | <0.001 |
| 2 | 383 (39) | 11 (9) | 394 (36) | 0 | ||
| 3 | 417 (42) | 71 (60) | 481 (44) | 7 (41) | ||
| 4 | 109 (11) | 37 (31) | 136 (13) | 10 (59) | ||
| Coagulative tumor necrosis | 274 (28) | 74 (62) | <0.001 | 331 (30) | 17 (100) | <0.001 |
| Sarcomatoid differentiation | 35 (4) | 10 (8) | 0.02 | 43 (4) | 2 (12) | 0.15 |
| Capillary-lymphatic invasion | 6 (1) | 11 (9) | <0.001 | - | - | - |
| Microvascular invasion | - | - | - | 108 (10) | 11 (65) | <0.001 |
| Collecting system invasion | 36 (4) | 19 (16) | <0.001 | 52 (5) | 3 (18) | 0.049 |
Among the 971 M0 patients, 223 subsequently developed metastases at a mean of 1.7 years following surgery (median 1.0; range 0–10). The mean PROG score was 3.2 (SD 2.9; median 3; range 0–11). At last follow-up of all ccRCC patients, 414 had died, including 256 who died from RCC at a mean of 2.3 years following surgery (median 1.7; range 0–10); 20 patients died from unknown causes and were excluded from analyses of CSS. The mean follow-up for the 689 patients who were alive at last visit was 6.5 years (median 6.4; range 0–11). At 2 years, 7.8% (16/205) of censored patients were lost to follow-up.
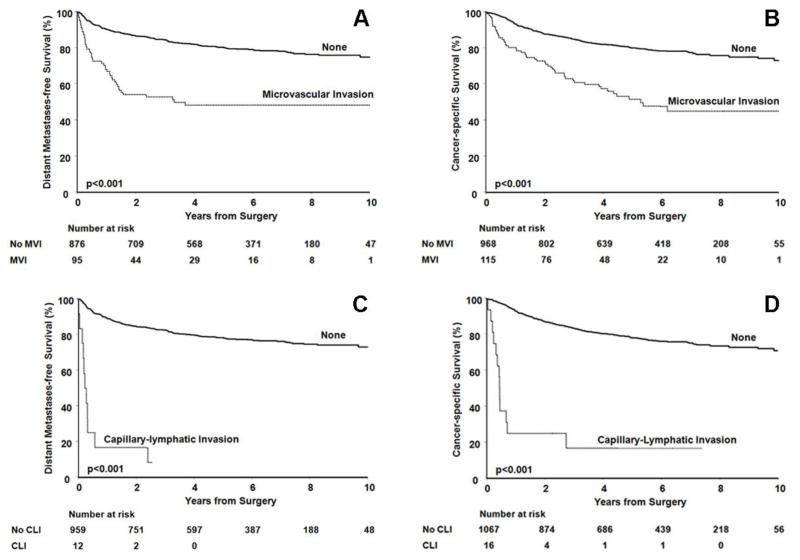
Associations of MVI and CLI metastases and cancer specific death are summarized in Table 4. Distant metastases occurred in 47% (45/95) with MVI and 20% (178/876) without MVI. Univariately, MVI was associated with a 3.5-fold increased risk of metastasis (p<0.001). However, after adjusting for the PROG score, the increased risk of metastasis was no longer statistically significant (HR 1.2; p=0.43). The estimated 5-year MFS (95%CI) following surgery was 48% (39–60%) for patients whose tumors demonstrated MVI compared with 80% (78–83%) for patients whose tumors did not (Figure 3a).
Table 4.
Associations with time to distant metastases and clear cell RCC specific mortality
| Univariate | Multivariate† | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | |
| Time to Distant Metastasis (N = 971) | ||||||
| Microvascular invasion | 3.5 | (2.5-4.8) | <0.001 | 1.2 | (0.82-1.6) | 0.43 |
| Capillary-lymphatic invasion | 15.9 | (8.5-29.4) | <0.001 | 3.2 | (1.7-6.0) | <0.001 |
| Time to Cancer Specific Mortality (N =1,083) | ||||||
| Microvascular invasion | 3.0 | (2.2-4.0) | <0.001 | 1.3 | (0.95-1.8) | 0.10 |
| Capillary-lymphatic invasion | 11.6 | (6.6-20.3) | <0.001 | 3.1 | (1.7-5.4) | <0.001 |
Controlled for PROG or SSIGN score which include: primary tumor, regional lymph node, and distant metastases classifications, tumor size, nuclear grade, and coagulative tumor necrosis
Figure 3.
A) Metastasis-free survival and B) Cancer-specific survival in clear cell RCC patients, with and without MVI. C) Metastasis-free survival and D) Cancer-specific survival in clear cell RCC patients, with and without CLI
Univariately, MVI was associated with a 3-fold increased risk of death from RCC (p<0.001). The estimated 5-year CSS (95%CI) following surgery was 52% (42–63%) for patients whose tumors demonstrated MVI compared with 80% (77–83%) for patients whose tumors did not (Figure 3b). However, after adjusting for the SSIGN score, the increased risk of death associated with MVI was no longer statistically significant (HR 1.3; p=0.10). We had adequate (80%) statistical power to identify a HR of 1.85 and 1.80 for the univariate association of MVI with progression to distant metastases and cancer specific mortality, respectively.
CLI was significantly associated with an increased risk of metastasis both univariately (HR 15.9; p<0.001) and after adjusting for the PROG score (HR 3.2; p<0.001). The estimated 5-year MFS (95%CI) following surgery was 78% (76-81%) for patients whose tumors did not demonstrate CLI (Figure 3c). At 2 years the estimated MFS for this group was 17% (5–59%).
M1 rates were similar in patients with MVI and CLI (20% (24/119) vs. 29% (5/17), respectively, p=0.4), while positive lymph nodes were less frequently identified in patients with MVI (35% (25/72) vs. 64% (9/14), respectively, p=0.04).
CLI was significantly associated with an increased risk of death from RCC both univariately (HR 11.6; p<0.001) and after adjusting for the SSIGN score (HR 3.1; p<0.001). The estimated 5-year CSS (95%CI) following surgery was 17% (5–54%) for patients whose tumors demonstrated CLI compared with 78% (76–81%) for patients whose tumors did not (Figure 3d).
When the entire cohort was analyzed based on M0 or M1 status, consistent results were found. In M0 patients, risk of death associated with MVI was significant on univariate (HR 3.4, p<0.001) but not multivariate analysis (HR 1.2, p=0.49) and risk of death associated with CLI was significant on univariate (HR 16.0, p<0.001) and multivariate analysis (HR 2.7, p=0.008). In M1 patients, risk of death associated with MVI was not significant on univariate (HR 1.4, p=0.18) or multivariate analysis (HR 1.3, p=0.35) and risk of death associated with CLI was significant on univariate (HR 3.3, p=0.01) and multivariate analysis (HR 2.8, p=0.026).
Associations of MVI with metastasis and death from RCC in patients with low-stage (pT1 and pT2) and low-grade (nuclear grade 1 and 2) M0 disease are summarized in Table 5. Among the 971 M0 patients, 687 (71%) were low-stage, 462 (48%) were low-grade, and 428 (44%) were low-stage and low-grade. MVI was seen in 18 (2.6%) of low-stage, 11 (2.4%) of low-grade, and 6 (1.4%) of combined low-stage and grade patients. In these patients an association of MVI with time to metastasis was seen in the low-grade patients on univariate, but not multivariate analysis. CLI was seen in 1 patient with low-stage disease and in 0 with low-grade disease, precluding analysis.
Table 5.
Associations of MVI with time to distant metastases and cancer specific death for M0 low-stage and grade clear cell RCC
| Univariate | Multivariate† | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | |
| Time to Distant Metastasis | ||||||
| pT1/pT2 | 1.9 | (0.6 - 6.1) | 0.27 | 0.8 | (0.2 - 2.5) | 0.67 |
| Grade 1-2 | 4.6 | (1.1 - 19.3) | 0.04 | 1.7 | (0.4 - 7.4) | 0.49 |
| pT1/pT2 and Grade 1-2 | 5.3 | (0.7 - 39.4) | 0.11 | 3.3 | (0.43 - 25.1) | 0.25 |
| Time to Cancer specific mortality | ||||||
| pT1/pT2 | 2.4 | (0.6 – 10.0) | 0.23 | 0.5 | (0.1 – 2.3) | 0.35 |
| Grade 1-2 | 7.5 | (0.9 – 60.9) | 0.06 | 2.5 | (0.3 – 20.6) | 0.41 |
| pT1/pT2 and Grade 1-2 | * | * | * | * | * | * |
Controlled for PROG or SSIGN score which include: primary tumor, regional lymph node, and distant metastases classifications, tumor size, nuclear grade, and coagulative tumor necrosis
Only 5 deaths were seen in the pT1/T2 and Grade 1-2 group precluding statistical analysis
Next, we evaluated the outcomes after systemic therapy in patients with and without MVI. Analysis of CLI was not possible given only 3 and 0 patients received immunotherapy and targeted therapy, respectively. Of the 132 M1 ccRCC patients, 38 (29%) were treated with immunotherapy, 39 (30%) were treated with targeted agents, and 13 (10%) were treated with both. In immunotherapy patients, median survival was 0.7 and 1.7 years for those with and without MVI, respectively (HR 2.5, p=0.04). In patients receiving targeted therapy, no association with survival was seen on univariate analysis in those with and without MVI, with median survival of 2.1 and 3.4 years, respectively (HR 1.8, p=0.18).
DISCUSSION
The current study represents the largest analysis of patients with MVI and CLI in RCC at a single institution as well as the largest with central pathologic review to date. We found that although MVI was significantly associated with MFS and CSS, this association was not maintained when accounting for established clinicopathologic prognostic variables. CLI, while rare, was associated with MFS and CSS in both univariate and multivariate analyses.
Since 1997 when van Poppel et al observed that MVI was the most important predictor of progression for RCC patients, this feature has been revisited numerous times with groups showing both a positive and negative association on multivariate analysis.11 Recently, Katz et al and Kroeger et al reported their single institution and multicenter findings, respectively.8,16 Along with the current study, these represent the three largest evaluations of MVI in the literature to date.
Our findings mirror those reported by Katz et al where MVI in 91 patients with non-metastatic disease showed a significant association with MFS and CSS on univariate, but not multivariate analysis.16 Similarly, Kroeger et al analyzed 412 patients with MVI in ccRCC and found a significant association on univariate analysis with CSS, but no association on multivariate analysis with MFS or CSS.8 Taken together, these findings indicate that MVI is a component of the neoplastic changes that affect disease outcome, but do not add prognostic ability beyond other commonly assessed features.
Although MVI was not an independent prognostic factor for the entire ccRCC cohort, we evaluated it further to see if it adds information in low-stage or low-grade tumors. Kroeger et al noted that in tumors <4cm MVI was one of the most important predictors of CSS on univariate analysis.8 Others have documented similar statistically significant findings in pT1-2 patients on multivariate analysis of MVI and MFS and CSS.12,15 Interestingly, Katz et al did not see an association of MVI with MFS or CSS in patients with T1 or T2 disease.16 In the current study, no association with recurrence was seen in patients with pT1-2 disease, however there was an association in patients with low-grade disease on univariate but not multivariate analysis. In the subset of patients with pT1-2 and low-grade disease, no association of MVI with recurrence was seen.
We found that M1 rates were similar in patients with MVI and CLI, while positive lymph nodes were higher in patients with CLI. While this was statistically significant, we hesitate to draw firm conclusions based on limitations in statistical power. If this finding is confirmed in subsequent evaluation, it may point to differences in underlying tumor biology. Lymph node invasion is a known risk factor for subsequent metastatic disease, as well as an independent predictor of cancer specific death in the setting of metastatic disease.19,20 Thus lymph node invasion in the setting of CLI may be an early event in the pathway of a more aggressive subset of systemic disease. This may also help explain the association with cancer specific death seen with CLI but not MVI in M1 patients. It must be emphasized that evaluating these hypotheses was beyond the scope of the current study.
CLI is a pathologic characteristic that is distinct, yet infrequently evaluated, when compared with MVI. Indeed, the evaluation of small vessel invasion is commonly made without distinction between blood vessels and lymphatic channels.16,17 To our knowledge, the current study represents the largest evaluation of CLI in RCC patients. While CLI maintained an independent association with risk of metastasis and cancer specific death on multivariate analysis, its presence in only 2% (17/1103) of clear cell and 0.9% (3/330) of non-clear cell cases likely indicates limited clinical utility.
Based on previous observations, the rates of MVI vary considerably ranging from 5-45% in specimens evaluated with hematoxylin and eosin staining.8,10-16 This variability is likely attributable to variation in patient inclusion criteria such as stage and grade, as well as use of central pathologic review. It has been suggested that a more accurate identification of MVI and CLI may be obtained with the use of specialized stains such as CD31, CD34, or D2-40 given the MVI and CLI rates when these are used tend to be higher (9-39%). However, even with increased detection, the independent predictive capability of MVI and CLI after multivariate analysis is mixed.9,15,17,18 In the current study, MVI and CLI were assessed using hemotoxylin and eosin staining only, to reflect standard clinical practice.
Our evaluation of the interaction of MVI and adjuvant therapy provided hypothesis generating results. In patients receiving systemic therapy for metastatic disease, survival was prolonged in patients without MVI, although this was only marginally significant (p=0.04) in the immunotherapy group, and non-significant in the targeted therapy group. Given the post-hoc nature of our analysis and limited sample sizes in these subsets, further study and validation are needed. A formal evaluation of this interaction in future adjuvant therapy studies is warranted.
While the current study adds to the volume of literature regarding MVI and CLI, several limitations must be acknowledged. The study is limited by its retrospective design, however, comprehensive pathologic re-review was conducted over several months with the specific intent of identifying MVI and CLI if present. Only hematoxylin and eosin staining was used to assess MVI and CLI which may limit its identification as discussed. The median follow for patients still alive is 77 months. It is possible with longer follow up, differences in recurrence rates and survival would be seen for MVI on multivariate analysis. Additionally, detection of CLI in only 17 clear cell patients limits the ability to make definitive conclusions regarding this feature and prevented further subset analysis.
CONCLUSION
MVI in patients with ccRCC is associated with an increased risk of metastases and cancer death, although this feature does not retain its prognostic significance after controlling for other clinicopathologic variables. CLI appears to be independently associated with metastases and cancer death even after controlling for known prognostic risk factors, however given its rarity; it may prove to be of limited clinical significance. Further study is require to determine if pathologically ascertained MVI or CLI in apparent localized RCC should merit more intense therapy or surveillance.
Acknowledgments
Funding Source:
Department of Urology, Mayo Clinic
This project was supported by NIH/NCRR CTSA Grant Number UL1 RR024150. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Key of Abbreviations
- CI
Confidence interval
- CLI
Capillary-lymphatic invasion
- CSS
Cancer-specific survival
- ECOG
Eastern Cooperative Oncology Group
- HR
Hazard ratio
- MFS
Metastasis-free survival
- MVI
Microvascular invasion
- NOS
Not otherwise specified
- ccRCC
Clear cell renal cell carcinoma
- RCC
Renal cell carcinoma
Footnotes
Financial Disclosure: None
REFERENCES
- 1.American Cance Society . Cancer Facts and Figures 2012. American Cancer Society; Atlanta: 2012. [Google Scholar]
- 2.Frank I, Blute M, Cheville J, et al. An outcome prediction model for patients with clear cell renal cell carcinoma treated with radical nephrectomy based on tumor stage, size, grade and necrosis: the SSIGN score. J Urol. 2002;168(6):2395–2400. doi: 10.1016/S0022-5347(05)64153-5. [DOI] [PubMed] [Google Scholar]
- 3.Kattan MW, Reuter V, Motzer RJ, et al. A postoperative prognostic nomogram for renal cell carcinoma. J Urol. 2001;166(1):63–7. [PubMed] [Google Scholar]
- 4.Zisman A, Pantuck AJ, Dorey F, et al. Improved prognostication of renal cell carcinoma using an integrated staging system. J Clin Oncol. 2001;19(6):1649–57. doi: 10.1200/JCO.2001.19.6.1649. [DOI] [PubMed] [Google Scholar]
- 5.Karakiewicz PI, Suardi N, Capitanio U, et al. Conditional survival predictions after nephrectomy for renal cell carcinoma. J Urol. 2009;182(6):2607–12. doi: 10.1016/j.juro.2009.08.084. [DOI] [PubMed] [Google Scholar]
- 6.Ahmadi H, Mitra AP, Abdelsayed GA, et al. Principal component analysis based pre-cystectomy model to predict pathological stage in patients with clinical organ-confined bladder cancer. BJU Int. 2012 doi: 10.1111/j.1464-410X.2012.11502.x. [DOI] [PubMed] [Google Scholar]
- 7.Tandstad T, Dahl O, Cohn-Cedermark G, et al. Risk-adapted treatment in clinical stage I nonseminomatous germ cell testicular cancer: the SWENOTECA management program. J Clin Oncol. 2009;27(13):2122–8. doi: 10.1200/JCO.2008.18.8953. [DOI] [PubMed] [Google Scholar]
- 8.Kroeger N, Rampersaud EN, Patard JJ, et al. Prognostic value of microvascular invasion in predicting the cancer specific survival and risk of metastatic disease in renal cell carcinoma: a multicenter investigation. J Urol. 2012;187(2):418–23. doi: 10.1016/j.juro.2011.10.024. [DOI] [PubMed] [Google Scholar]
- 9.Yildiz E, Ayan S, Goze F, et al. Relation of microvessel density with microvascular invasion, metastasis and prognosis in renal cell carcinoma. BJU Int. 2008;101(6):758–64. doi: 10.1111/j.1464-410X.2007.07318.x. [DOI] [PubMed] [Google Scholar]
- 10.Sorbellini M, Kattan MW, Snyder ME, et al. A postoperative prognostic nomogram predicting recurrence for patients with conventional clear cell renal cell carcinoma. J Urol. 2005;173(1):48–51. doi: 10.1097/01.ju.0000148261.19532.2c. [DOI] [PubMed] [Google Scholar]
- 11.Van Poppel H, Vandendriessche H, Boel K, et al. Microscopic vascular invasion is the most relevant prognosticator after radical nephrectomy for clinically nonmetastatic renal cell carcinoma. J Urol. 1997;158(1):45–9. doi: 10.1097/00005392-199707000-00013. [DOI] [PubMed] [Google Scholar]
- 12.Ishimura T, Sakai I, Hara I, et al. Microscopic venous invasion in renal cell carcinoma as a predictor of recurrence after radical surgery. Int J Urol. 2004;11(5):264–8. doi: 10.1111/j.1442-2042.2004.00802.x. [DOI] [PubMed] [Google Scholar]
- 13.Lang H, Lindner V, Letourneux H, et al. Prognostic value of microscopic venous invasion in renal cell carcinoma: long-term follow-up. Eur Urol. 2004;46(3):331–335. doi: 10.1016/j.eururo.2004.03.020. [DOI] [PubMed] [Google Scholar]
- 14.Dall’Oglio MF, Antunes AA, Sarkis AS, et al. Microvascular tumour invasion in renal cell carcinoma: the most important prognostic factor. BJU Int. 2007;100(3):552–5. doi: 10.1111/j.1464-410X.2007.07015.x. [DOI] [PubMed] [Google Scholar]
- 15.Madbouly K, Al-Qahtani SM, Ghazwani Y, et al. Microvascular tumor invasion: prognostic significance in low-stage renal cell carcinoma. Urology. 2007;69(4):670–4. doi: 10.1016/j.urology.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 16.Katz M, Serrano M, Humphrey P, et al. The role of lymphovascular space invasion in renal cell carcinoma as a prognostic marker of survival after curative resection. Urol Oncol. 2009;29(6):738–744. doi: 10.1016/j.urolonc.2009.07.034. [DOI] [PubMed] [Google Scholar]
- 17.Horiguchi A, Ito K, Sumitomo M, et al. Intratumoral lymphatics and lymphatic invasion are associated with tumor aggressiveness and poor prognosis in renal cell carcinoma. Urology. 2008;71(5):928–32. doi: 10.1016/j.urology.2007.11.076. [DOI] [PubMed] [Google Scholar]
- 18.Zubac DP, Bostad L, Kihl B, et al. Organ-confined clear cell renal cell carcinoma: the prognostic impact of microvascular invasion, nuclear grade and tumour size. APMIS. 2008;116(12):1027–33. doi: 10.1111/j.1600-0463.2008.01071.x. [DOI] [PubMed] [Google Scholar]
- 19.Leibovich BC, Blute ML, Cheville JC, et al. Prediction of progression after radical nephrectomy for patients with clear cell renal cell carcinoma: a stratification tool for prospective clinical trials. Cancer. 2003;97(7):1663–71. doi: 10.1002/cncr.11234. [DOI] [PubMed] [Google Scholar]
- 20.Lughezzani G, Capitanio U, Jeldres C, et al. Prognostic significance of lymph node invasion in patients with metastatic renal cell carcinoma: a populationbased perspective. Cancer. 2009;115(24):5680–7. doi: 10.1002/cncr.24682. [DOI] [PubMed] [Google Scholar]