Abstract
The α7-nicotinic acetylcholine receptor (nAChR) has long been a procognitive therapeutic target to treat schizophrenia. Evidence on the role of this receptor in cognition has been lacking, however, in part due to the limited availability of suitable ligands. The behavior of α7-nAChR knockout (KO) mice has been examined previously, but cognitive assessments using tests with cross-species translatability have been limited to date. Here, we assessed the cognitive performance of α7-nAChR KO and wild-type (WT) littermate mice in the attentional set-shifting task of executive functioning, the radial arm maze test of spatial working memory span capacity and the novel object recognition test of short-term memory. The reward motivation of these mutants was assessed using the progressive ratio breakpoint test. In addition, we assessed the exploratory behavior and sensorimotor gating using the behavioral pattern monitor and prepulse inhibition, respectively. α7-nAChR KO mice exhibited normal set-shifting, but impaired procedural learning (rule acquisition) in multiple paradigms. Spatial span capacity, short-term memory, motivation for food, exploration and sensorimotor gating were all comparable to WT littermates. The data presented here support the notion that this receptor is important for such procedural learning, when patterns in the environment become clear and a rule is learned. In combination with the impaired attention observed previously in these mice, this finding suggests that agonist treatments should be examined in clinical studies of attention and procedural learning, perhaps in combination with cognitive behavioral therapy.
Keywords: α7-nicotinic acetylcholine receptor, exploration, motivation, mice, mutant, prepulse inhibition, procedural learning, schizophrenia, set-shifting, short-term memory
Procognitive agents are needed to improve functional outcome in patients with schizophrenia. The lack of an approved drug for treating cognitive dysfunction in schizophrenia prompts basic research on specific targets. Relative to healthy comparison subjects, patients with schizophrenia are more likely to smoke cigarettes, smoke more cigarettes per day and extract more nicotine per cigarette (Kumari & Postma 2005). It is theorized that these patients are self-medicating because nicotine exerts procognitive effects across several cognitive domains (Barr et al. 2008; D’souza & Markou 2011; Levin et al. 1998; Myers et al. 2008; Newhouse et al. 2004; Poltavski & Petros 2006). Unfortunately, nicotine also possesses deleterious effects such as nausea and addiction, limiting its use as a therapeutic drug. Nicotine is the prototypical ligand for the nicotinic acetylcholine receptors (nAChRs), of which the α7 and α4β2 nAChRs are the most populous. Given links between the α7-nAChR and schizophrenia, this receptor has been hailed as one of the most promising targets for treating cognition dysfunction in these patients (Geyer & Tamminga 2004; Tamminga 2006).
Several lines of evidence support the α7-nAChR as a target for treating patients with schizophrenia (Levin et al 2006; Martin et al 2004). Abnormalities in the 15q13-15 region of chromosome 15, which contains the α7-nAChR subunit genotype CHRNA7, are linked to poor sensory gating in schizophrenia patients and may constitute a susceptibility genotype (Freedman et al 1997). Moreover, lower α7-nAChR protein levels are observed in the post-mortem brains of patients with schizophrenia and associated with cognitive dysfunction (Martin-Ruiz et al 2003), despite no alterations in cortical mRNA expression (De Luca et al 2006; Martin-Ruiz et al 2003). Early clinical tests of a partial α7-nAChR agonist (GTS-21, aka DMXBA) were promising with regard to improving cognition in patients with schizophrenia (Olincy et al 2006), but were not replicated in larger studies (Freedman et al 2008).
Determining α7-nAChR pharmacological effects has been problematic due to the lack of available compounds that are both selective and blood–brain barrier (BBB) permeable. The prototypical α7-nAChR antagonist, α-Bungarotoxin (α-BgT) is BBB impermeable, while the antagonist methyllycaconitine (MLA) exhibits only 5–20% permeability (Turek et al 1995). MLA permeability is further lowered by co-administration of nicotine (Lockman et al 2005). The full α7-nAChR agonist AR-R1779 also exhibits poor BBB permeability (Cilia et al 2005), while DMXBA is only a partial agonist (Olincy et al 2006). Although more selective BBB-permeable compounds are being created by companies (Thomsen et al 2009), the availability and thus independent assessment of these drugs remain limited.
A more precise mechanism to understand contributions of α7-nAChRs to behavior is to phenotype α7-nAChR knockout (KO) and heterozygous (HT) littermate mice (Orr-Urtreger et al 1997). Previously, Paylor et al (1998) compared α7-nAChR null mutants to wild-type (WT) littermates and found no abnormalities in a variety of behaviors, including activity levels, water maze spatial learning, prepulse inhibition (PPI) and Pavlovian fear conditioning. Nevertheless, the limited cognitive paradigms used were minimally relevant to the cognitive deficits characteristic of patients with schizophrenia (Young et al 2009b). When more complex food-motivated tasks are used, α7-nAChR null mutant mice exhibit impaired sustained attention as measured by the five-choice serial reaction time task (5-CSRTT) (Hoyle et al 2006; Young et al 2004, 2007a), as well as impaired operant learning (Keller et al 2005; Levin et al 2009; Young et al 2004). Patients with schizophrenia, however, exhibit deficits in other cognitive domains, including executive functioning, working memory (WM) and short-term memory, which can be assessed preclinically in paradigms with putative cross-species translational validity, e.g. the attentional set-shifting task (ASST), radial arm maze (RAM) and novel object recognition task (NORT), respectively (Young et al 2009b).
Like the 5-CSRTT, the ASST is an appetitive-rewarding task and although deficits in such tasks could mean impaired cognition, poor performance of α7-nAChR KOs in these paradigms may reflect reduced motivation (Hoyle et al 2006), given the interaction between nicotine and food motivation (Perkins et al 1991).While α7-nAChR null mutant mice did not differ in their speed to collect food rewards in the 5-CSRTT (Hoyle et al 2006; Young et al 2004, 2007a), a putative measure of motivation (Robbins 2002), there are more sensitive measures of reward motivation such as the progressive ratio breakpoint paradigm (PRBP) (Bensadoun et al 2004; Young & Geyer 2010).
A greater understanding is required on the cognitive domains affected by null mutation of the α7-nAChR using cross-species relevant tasks. Moreover, cross-species paradigms are available to assess exploratory behavior using multivariate analysis (Perry et al 2009; Young et al 2007c) and there are further aspects of PPI to assess (Ouagazzal et al 2006; Young et al 2010c). Finally, the motivation levels of α7-nAChR mutant mice requires clarification to interpret apparent deficits in attention, as do other aspects of cognition that are relevant to schizophrenia (Marder 2006; Nuechterlein et al 2004). Here, we describe the behavioral characterization of α7-nAChR WT, HT and KO mice in the ASST, NORT, RAM, BPM (behavioral pattern monitor), PPI and PRBP.
Methods
Animals
The α7-nAChR mutant and WT mice were derived from HT breeding pairs. The mice were backcrossed for at least 11 generations onto a C57B/6J background. Male WT, HT and KO mice were used in the present studies. Genotype was confirmed by tail tipping mice at around 28 days under isoflurane anesthesia, DNA obtained by proteinase K digestion of tail, with PCR conducted as described (Marks et al 1999; Orr-Urtreger et al 1997) (www.jax.org). The mice had unlimited access to water and food (Harlan, Madison, WI, USA), except during treatment and testing where stated below. Age, sex, sample sizes, weights and test naivety are provided for each experiment below. During training and testing periods that used food rewards, mice were maintained at 85% of free-feeding weight, with water available ad libitum. Mice were housed separately in groups of maximum four per cage in a vivarium on a reversed day–night cycle (lights on at 2000 h, off at 0800 h). Mice were brought to the laboratory 60 min before testing between 0900 h and 1800 h. All behavioral testing procedures were approved by the UCSD Institutional Animal Care and Use Committee. All mice were maintained in an animal facility that meets all federal and state requirements for animal care and was approved by the American Association for Accreditation of Laboratory Animal Care.
Attentional set-shifting task
Fourteen WT (10 ♀ and 4 ♂) and 14 KO mice (11 ♀ and 3 ♂), naive to behavioral testing, were assessed using the ASST when they were approximately 5 months old and weighed between 20 and 40 g. The ASST apparatus and procedure were performed as previously described (Young et al 2010b). Ceramic pots (4.5 × 2.5 cm) were used as digging bowls, given that mice readily dig in bedding placed in such bowls to retrieve a food reward using olfactory cues to solve tasks (Young et al 2007a, b, 2008). These bowls were located on platforms (11 × 5 cm). Specific odors and platform textures were utilized as cues to guide the selection of a bowl. Odors were derived from commercially available powdered spices including nutmeg, ground ginger, coriander, garlic, thyme and cinnamon (Albertsons®, San Diego, CA, USA). Platforms included tile, sandpaper, neoprene, metal wire, wood and a scrubber (Homebase®, San Diego, CA, USA). We have previously demonstrated that mice readily use platforms to identify reward location (Young et al 2010b). The food reward was a single 25 mg food pellet (Noyes Precision, TestDiet, Richmond, IN, USA). The test apparatus was an adapted perspex home cage (30 × 18 × 12 cm). Clear plastic panels were used to separate half of the cage into two equal sections. Two digging bowls were placed in each quarter section with access limited by removable dividers, used to deny the mice access to the other bowl after they had made a selection.
Shaping
Day 1: Mice were brought into the testing room and chamber and trained to dig in unscented bedding for food reward. Day 2: Mice were required to dig in bowls containing each of the odors mounted on each of the platforms that they would encounter in the main task. The criterion for a dig was defined as when the nose or paws of the mouse broke the surface of the digging medium and was used throughout testing.
ASST paradigm
The ASST procedure utilized was consistent with previous studies (Young et al 2010b). Briefly, mice were required to perform a series of discriminations selecting bowls dependent on the stimulus in a particular dimension, either odor or platform. These discrimination stages included a simple discrimination (SD) stage (only one relevant dimension presented), a compound discrimination (CD) stage (introducing the second dimension), CD reversal (CDR) stage (requiring mice to respond to the bowl associated with the previously irrelevant stimulus), an intradimensional (ID) shift (novel stimuli presented), an ID reversal (IDR) stage (reversal of ID rule), an extradimensional (ED) shift (a stimulus from the previously irrelevant dimension would identify the baited bowl) and finally ED reversal (EDR) stage (reversal of the ED rule). In trials 1–4 of SD and CD, mice were permitted to dig in the unbaited bowl without consequence, although an error was recorded, so that the mouse could move to the baited bowl and learn the cue contingency. Subsequent errors in trials resulted in the mouse being denied entry to the other area, an error was recorded and the trial restarted. The mice were required to make six consecutive correct responses at each stage prior to moving to the next. Mice were counterbalanced so that the initial SD would be either odor or platform. Stimuli combinations and locations were selected in a varied order. Trials to criterion, errors and mean correct latencies (cumulative correct latency/total correct responses) were recorded for each stage. Latencies were measured using stopwatches; initiated as the doors were raised and stopped when the mouse dug in one of the choices available. Despite all stages requiring some form of learning, it has been demonstrated that different stages are mediated by different neuroanatomical substrates, in rats andmice, e.g. ED shifts mediated by the medial prefrontal cortex (Birrell & Brown 2000; Bissonette et al 2008), while reversal learning is mediated by the orbitofrontal cortex (Bissonette et al 2008; McAlonan & Brown 2003). Given the different neuroanatomical contributions to stages, performance in the task is often analyzed by conceptual grouping of ED shifting (the ‘set-shifting’ measure), reversal learning and SDs.
Radial arm maze
Apparatus
Eight WT (♂) and 6 KO (♂) mice naive to behavioral testing were trained in the RAM WM span task when they were approximately 3 months old and weighed between 20 and 30 g. We used an automated 12-arm RAM (Coulbourn Instruments, Whitehall, PA, USA) interfaced with Med Associates controlling software (Med Associates Inc., St. Albans, VT, USA) as described previously (Tarantino et al 2011). Twelve arms radiated from a dodecahedral central hub (43 cm in diameter), with each arm (36 × 10 × 12 cm) separated by 30°. Access from the central hub to each of the 12 arms was controlled using automated guillotine doors. The floor of each arm was a metal grid and the walls and ceiling were made of acrylic glass. Arm activity was monitored using an infrared beam 5.5 cm from the entrance. An infrared beam also monitored entries into the dipper mechanism located at the distal end of each arm. Each dipper provided liquid reinforcement via computer-controlled dipping into a liquid-filled bowl below the dipper. The entire RAM was fixed to a circular acrylic table (124 cm in diameter) raised 53 cm from the floor painted black to avoid a potential confound of depth perception-induced anxiety (Komada et al 2008). Seven extra-maze spatial cues were created and fixed at conspicuous locations around the four walls at varying heights. Dim fluorescent lighting was provided in the room (110 lux in the dodecahedral central hub). A camera was positioned above the maze to track animal behavior. External auditory distractions were minimized by providing a constant background white noise (60 dB(A) scale) throughout training and testing.
Habituation to the testing environment
Acclimation to strawberry milkshake (Nesquik® + non-fat milk) reinforcement occurred prior to training in the home cage tominimize a neophobic response to the reinforcer during training. Mice were initially placed in the RAM with all 12 doors opened and food dippers raised providing a single reward to acclimate the mice to the RAM. Exploration of the RAM was encouraged by randomly placing strawberry milkshake throughout the apparatus. Habituation occurred for five consecutive days with exposure time initially at 10 min (day 1), then reduced to 7 min (days 2–5).
RAM protocol
WM span capacity (WMC) was assessed within a session by baiting ten arms (designated WM arms) only at the start of each session. The two remaining arms (allocated as RM arms) were never baited throughout training or testing. For each session, mice were placed in the hub with all doors closed. The session began with all 12 doors being raised allowing entry into all 12 arms. Entry into an arm resulted in the doors to the remaining 11 arms being closed, barring entry into any other arm. Upon retrieval of the food reward from the magazine (if a WM arm), returning to the hub resulted in all 11 doors being raised after a 1-second delay. The 1-second delay was instituted to interfere with the development of simple turn left/right strategies (Wenk 2004). Entry into any of the 12 arms was again possible. WMC was measured as the number of WM arms entered prior to a repeat entry. Repeat entries into a WM arm (recorded as a WM error) did not require a magazine head entry to initiate the arms to reopen. Similarly, for entries into RM arms (recorded as an RM error), magazine head entries were not required to reopen the remaining doors. The total number of WM errors were totaled and divided by the total number of WM arms entered (%WM errors). The total number of RM errors were totaled and divided by the total number of RM arms entered (%RM errors). These percentages were calculated to provide a measure of WM and RM errors as a function of total arms entered. Total arms entered (Arms) composed of total WM and RM arms entered. The development and use of a simple turn left/right strategy was assessed by totaling all the possible relative transitions from one arm to another. The degree of strategy use was then quantified by calculating the coefficient of variation (StrategyCV) of the total possible relative transitions for each subject. Sessions continued until 7 min had elapsed or all 10 WM arms were entered.
Novel object recognition task
The short-term memory of 11 WT (4 ♀ and 7 ♂) and 9 KO (3 ♀ and 6 ♂) mice was assessed using the NORT as described previously (Ali et al 2011).Mice weighed between 20 and 30 g, were approximately 3 months old and were naive to behavioral testing. Single trial NORT was performed in an open arena (60 × 60 cm) using two object types (Lego pyramid and 50 ml plastic conical tube) affixed to the floor using Velcro tape in opposing corners of the open field 10 cm away from the walls. Each mouse completed one session that comprised of three successive trials. In trial 1, habituation phase, the mouse was placed in the center of the empty open field box and allowed to freely explore. During trial 2, the sample phase, two of the same objects were placed in opposite corners of the box and the mouse was allowed to explore the objects. Half of the mice were randomly assigned to either starting with the Lego pyramid or the conical tube as the familiar object. In trial 3, one of the objects was replaced with a new object to assess novel object exploration. Each trial was 5 min with a 3-min inter-trial interval (ITI). This ITI was chosen to allow for the largest behavioral window to detect group differences. The mouse was removed from the testing box and placed in a holding cage during the ITI. The box and the objects were carefully cleaned with water between each trial and cleaned with 70% alcohol at the end of each testing session. Locomotor activity was assessed using Ethovision tracking software (Noldus Information Technology, Wageningen, Netherlands). Object exploration was defined as when the mouse’s nose was within 2 cm of the object. Performance was calculated as the percentage of novel object interaction (%NI): the length of time exploring the novel object/the time exploring the novel object + the familiar object.
Progressive ratio breakpoint study
The motivation/value of reward for 12 WT (♂), 16 HT (♂) and 13 KO (♂) mice was trained in the PRBP as described previously (Young & Geyer 2010). Mice weighed between 20 and 30 g, were approximately 3 months old and were naive to behavioral testing. Shaping and progressive ratio testing took place in 16 five-hole operant chambers (25 × 25 × 25 cm; Med Associates Inc.). Each chamber consisted of an array of five square apertures (2.5 × 2.5 × 2.5 cm) arranged horizontally on a curved wall 2.5 cm above the grid floor opposite to a reward delivery area (Lafayette Instruments, Lafayette, IN, USA) at floor level with a house light near the ceiling. The chamber was housed in a sound-attenuating box with ventilation provided by a fan that also provided a low level of background noise. Performance was monitored during training and testing using an infrared camera installed in each chamber. Responses with a nose-poke to illuminated LEDs recessed into the apertures were detected by infrared beams mounted vertically located 3 mm from the opening of the aperture (in-house modification). Liquid reinforcement in the form of strawberry milkshake (Nesquik® + non-fat milk, 30 µl, Nestle, Glendale, CA, USA) was utilized and delivered by peristaltic pump (Lafayette Instruments) to a well located in the magazine opposite the five-hole wall. Reward delivery area entries were monitored using an infrared beam mounted horizontally, 5 mm from the floor and recessed 6 mm into the magazine. The control of stimuli and recording of responses were managed by a SmartCtrl Package 8-In/16-Out with additional interfacing by MED-PC for Windows (Med Associates Inc.) using custom in-house programming.
Progressive ratio breakpoint testing
Mice were initially shaped to nose-poke in the central aperture for a single food reward (fixed ration, FR1) as described previously (Young et al 2009a). Each trial was initiated by nose-poking in the reward delivery area, after which the central light was immediately illuminated. The central light remained illuminated until the mouse nose-poked that aperture. A nose-poke in the central aperture resulted in a single food reward being delivered in the reward delivery area. Each session lasted 30 min with no trial limit. Standard acquisition criterion was >70 responses for two consecutive days, although training continued until the number of responses were stable (no effect of day when assessed Tuesday through Friday).
Once nose-poking reliably, mice were challenged in the breakpoint study. The number of nose-pokes required to gain a reward increased according to the following progression: 1, 2, 4, 7, 11, 16, 22, 29, 37, 46, 56 and 67. To maintain responding, the mice could respond three times at each ratio before moving to the next, receiving one reward each time. The session continued for 60 min or until 5 min had passed without a single nose-poke. The breakpoint was defined as the last ratio to be completed before the session end. Mean response latency (MRL) was also calculated. The progressive ratio challenge was conducted on Tuesday with normal shaping on the previous day.
Behavioral pattern monitor
The exploratory behaviors of 12 WT (5 ♀ and 7 ♂) and 14 KO (8 ♀ and 6 ♂) mice were assessed using the BPM. Mice weighed between 23 and 47 g, were approximately 7 months old and a subset of which were assessed in the NORT. Nine mouse BPM chambers were used to assess the spontaneous exploratory behavior as described previously (Halberstadt et al 2009; Risbrough et al 2006). Each chamber is illuminated from a single light source above the arena (30.5 × 61 × 38 cm) area with a Plexiglas hole board floor equipped with three floor holes and eight wall holes (Young et al 2010a). Nose-poking behavior was detected using an infrared photobeam. The location of the mouse was recorded every 0.1 seconds using a grid of 12× 24 infrared photobeams located 1 cm above the floor. The position of the mouse was defined across nine unequal regions (Geyer et al 1986; Risbrough et al 2006; Young et al 2010a). Rearing behavior was recorded using an array of 16 infrared photobeams 2.5 cm above the floor aligned with the long axis of the chamber. At the start of each test session, mice were placed in the bottom left hand corner of the chamber, facing the corner and the test session started immediately.
Three main factors were investigated based on previous analysis of multivariate factor loading (Paulus & Geyer 1993): locomotor activity as measured by transitions (calculated as a movement across a defined region); exploratory behavior as measured by holepoking, varied holepoking (total holepokes minus repetitious poking) and rearing; and locomotor pattern as assessed by the spatial d measure of dimensionality. Spatial d uses analyses based on fractal geometry to quantify the geometrical structure or dimensionality of the locomotor path, where a value of 2 represents highly localized two-dimensional movements and 1 represents one-dimensional straight distance-covering movements (for calculations of the spatial d value, please see Paulus & Geyer 1991).
Prepulse inhibition
The sensorimotor gating of 15 WT (8 ♀ and 7 ♂), 17 HT (8 ♀ and 9 ♂) and 16 KO (9 ♀ and 7 ♂) mice was assessed using the PPI paradigm. Mice weighed between 23 and 52 g, were approximately 10 months old and a subgroup had previously been tested in the NORT and the BPM. Startle chambers (SR-LAB; San Diego Instruments, San Diego, CA, USA) consisted of nonrestrictive Plexiglas cylinders 5 cm in diameter resting on a Plexiglas platform in a ventilated chamber. High-frequency speakers mounted 33 cm above the cylinders produced all acoustic stimuli. Piezoelectric accelerometers mounted under the cylinders transduced movements of the animal, which were digitized and stored by an interface and computer assembly. Beginning at the stimulus onset, 65 consecutive 1 millisecond readings were recorded to obtain the peak amplitude of the animals’ startle response to acoustic (40 milliseconds) startle stimuli. Peak responses to these stimuli are presented in arbitrary units. A dynamic calibration system was used to ensure comparable sensitivities across chambers. Sound levels were measured as described elsewhere (Mansbach & Geyer 1988) using the A weighting scale in units of dB(A) SPL. The light was delivered via a bare 15 W incandescent bulb located on the ceiling of the testing chamber. A 65 dB background noise was presented continuously throughout the session.
PPI challenge 1
The experimental session consisted of a 5-min acclimatization period to a 65-dB background noise, followed by the PPI test. Because no effect of genotype on PPI using variable prepulses was observed previously (Paylor et al 1998), we used a mixed modality PPI test session which we have previously demonstrated to be sensitive to aging effects (Young et al 2010c). During the session, four trial types that varied in modality (auditory, visual) were presented 12 times in a pseudorandom order. Trial types were 120 dB pulse (pulse-alone), 73 dB prepulse preceding a 120 dB pulse (prepulse + pulse), 100 milliseconds light prepulse preceding a 120 dB pulse (light + pulse). No stimulus (nostim) trials occurred between each trial with an average ITI of 15 seconds. In addition, six of the pulse-alone trials, which were not included in the calculation of PPI values, were presented at the beginning of the test session to achieve a relatively stable level of startle reactivity for the reminder of the session (based on the observation that the most rapid habituation of the startle reflex occurs within the first few presentations of the startling stimulus (Geyer et al 1986).
PPI challenge 2
The next PPI test session consisted of three testing blocks. Block 1 (pulse intensities) assessed acoustic startle responding across stimulus intensities with four presentations of 80, 90, 100, 110 and 120 dB pulse-alone trials. Block 2 (prepulse intensities) consisted of twelve 120 dB startle pulse-alone intensities interspersed with 10 each of 3 different prepulse trials: 69, 73 and 81 dB prepulses preceding a 120 dB pulse. Prepulses preceded the pulse by 100 milliseconds [i.e. interstimulus interval (ISI), onset to onset]. Block 3 (ISI variation) varied the ISI. The block consisted of 7 startle pulses at 120 dB and 4 each of 73 dB prepulses preceding a 120 dB pulse by 20, 50, 100, 200, 500 and 1000 milliseconds (onset to onset).
Statistical analyses
For every study, an analysis of variance (ANOVA) was used to examine performance by genotype and sex as between-subject factors. For ASST, performance was analyzed in terms of trials to criterion using a repeated-measure ANOVA with stage (SD, CD, CDR, ID, IDR, EDS and EDR) as a within-subject factor and starting dimension as a between-subject factor. Selected stages were also analyzed separately to examine specific cognitive constructs: discrimination learning was assessed by comparing SD, CD and ID; reversal learning was evaluated by comparing CDR, IDR and EDR; attentional set formation was investigated by comparing ID and ED. The RAM was analyzed during acquisition and stability using day as a within-subject factor. Acquisition of the PRB study was measured in terms of days to criterion, while PRB was assessed in a single day. NORT performance was compared with chance (50%) using a one sample t test. For the BPM, time was analyzed as a within-subject factor. For PPI, varying prepulses, modalities and ISIs were analyzed as within-subject factors. Tukey post hoc analyses were performed on any significant main effect or interaction. Alpha level was set to 0.05. Data were analyzed using SPSS for Windows 14 (Chicago, IL, USA) and Biomedical Data Programs statistical software (Statistical Solutions Inc., Saugus, MA, USA).
Results
Attentional set-shifting task
Overview of task performance
The performance of α7-nAChR WT and KO mice was compared in the ASST. Performance was initially assessed for conceptual validity – that is, an attentional set was formed by the mice as measured by a significant difference in the total trials to criterion between ID and ED, irrespective of starting dimension. A main effect of stage was observed on trials to criterion (F6,120 = 10.3, P < 0.0001; Fig. 1) and mean correct latency (F6,96 = 4.1, P < 0.05). No stage by starting dimension interaction was observed (F < 1.1, ns). Moreover, no sex by stage interaction was observed (F < 1, ns).
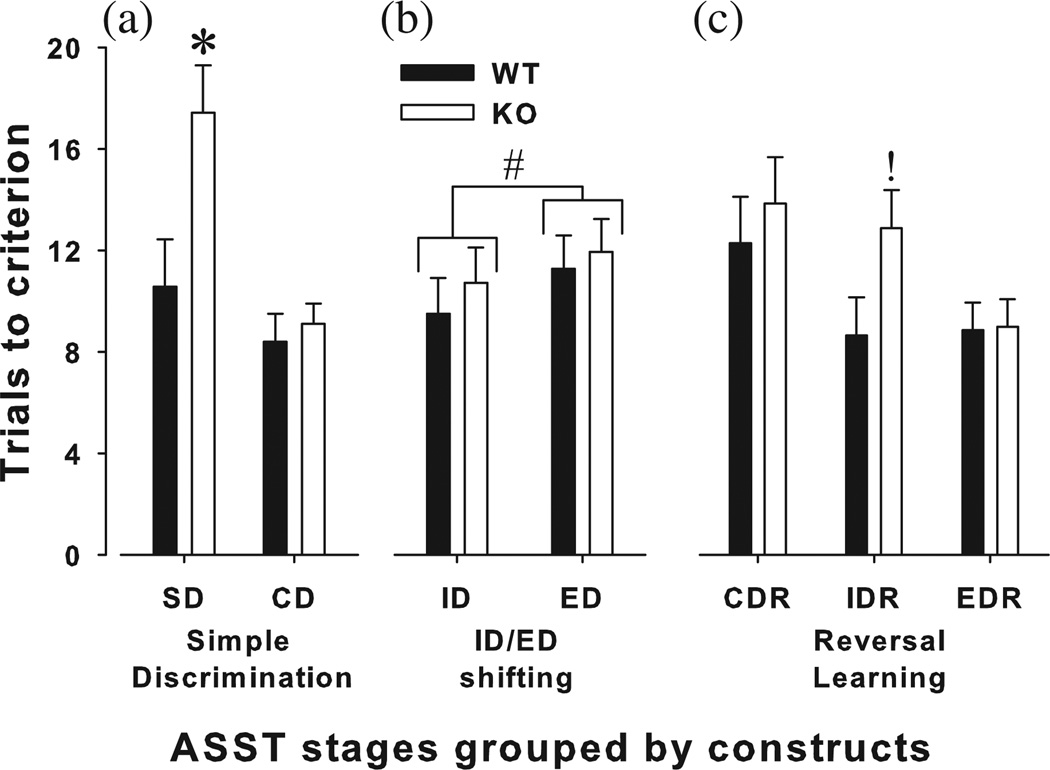
Figure 1. Performance of α7-nAChR mutant mice in the ASST.
The performance of α7-nAChR KO mice in the ASST was compared with their WT littermates. KO mice exhibited impaired acquisition of the rule to associate a specific stimulus with reward location, taking longer to acquire the SD but not CD stage as compared to WT mice (a). Set-shifting was normal in KO mice, with both KO and WT mice demonstrating the formation of an attentional set given that it took both groups more trials to complete the ED stage compared with the ID stage (b). KO mice exhibited (1) normal reversal learning at the first reversal stage (CDR stage), (2) took more trials to complete the second reversal stage (IDR stage) due to WT mice taking fewer trials compared to CDR while KO did not and (3) exhibited comparable reversal learning to WT mice at the third stage (EDR) because KO mice improved by this stage when compared to the IDR stage (c). Data are presented as mean + SEM, grouped by construct as opposed to the order of testing (SD, CD, CDR, ID, IDR, ED, EDR), *P < 0.05 when compared to WT mice, P < 0.1 when compared to WT mice, #P < 0.05 when ID is compared to ED shifting.
To provide evidence that an attentional set had been formed and thus the mice performed set-shifting behavior, the performance of ID vs. ED stages was compared. A main effect of stage was observed in trials to criterion (F1,20 = 4.7, P < 0.05; Fig. 1b) with no interaction with genotype (F < 1, ns) or starting dimension (F < 1, ns). A main effect of ID/ED stage was also observed in mean correct latencies (F1,20 = 5.8, P < 0.05) with no interaction with genotype (F < 1.8, ns) or starting dimension (F < 1.1, ns). Thus, more trials to criterion were required and longer mean correct latencies were observed in the ED stage relative to the ID stage irrespective of genotype. No main effect of genotype was observed for trials to criterion or MCL in the ED stage (F < 1, ns; data not shown).
Genotypic effects on specific stages were investigated based on the underlying cognitive constructs described previously (Birrell & Brown 2000; Young et al 2009).
Discrimination learning
The ability of α7-nAChR WT and KO mice to perform discriminations within a dimension (set of stimuli) as measured by SD, CD and ID was compared. A significant effect of discrimination stage on trials to criterion was observed (F2,40 = 24.3, P < 0.0001), as was a genotype × stage interaction (F2,40 = 3.3, P < 0.05). Post hoc analysis revealed that KO mice took more trials to reach criterion relative to WT mice in the SD stage (F1,26 = 6.8, P < 0.05; Fig. 1a), but not in the CD or ID stage (F < 1, ns).
Reversal learning
The reversal learning of the mutant mice was assessed in the CDR, IDR and EDR stages. A main effect of reversal stage on trials to criterion was found (F2,48 = 5.4, P < 0.05), with no interaction with genotype (F2,48 = 1.2, P = 0.310). Consistent with previous reports, we observed that the number of trials taken to obtain criterion at these stages reduced with increasing reversal stages. Thus, fewer trials were required for the EDR when compared to the CDR. Upon closer examination it was observed that this learning to reverse was unequal between genotypes with a trend toward a genotype effect on IDR (F1,27 = 3.3, P = 0.076). When comparing CDR to IDR, WT mice took fewer trials to complete the latter (F1,10 = 18.2, P < 0.005) unlike KO mice (F < 1, ns). In the last two reversals (IDR and EDR), however, KO mice exhibited fewer trials to criterion in the latter stage (F1,10 = 6.7, P < 0.05; Fig. 1c), an effect not observed in WT mice (F < 1, ns).
Extradimensional shifting
To assess executive functioning specifically, the ED shifting of α7-nAChR WT and KO mice was compared using a t test. No effect of genotype on trials to criterion or mean correct latency was observed (t< 1, ns).
Radial arm maze
Acquisition of RAM performance was assessed over 12 days. A significant main effect of days was observed (F11,132 = 3.0, P < 0.005; Fig. 2), while a trend toward a genotype × day interaction was observed (F11,132 = 1.9, P = 0.052). Post hoc analyses revealed that while the performance of WT and KO mice did not differ on days 1 and 2 (P > 0.2), WT mice exhibited significantly greater performance on days 3, 4, 5 and 6 (P < 0.05). Post hoc analyses across days are depicted in Fig. 2a. Performance did not differ between the two groups thereafter (days 7–12; P > 0.2). In terms of strategy development, a trend toward reduced strategy use over continuing sessions was observed (F11,132 = 1.8, P = 0.06; Fig. 2b), with no interaction with genotype (F < 1, ns).
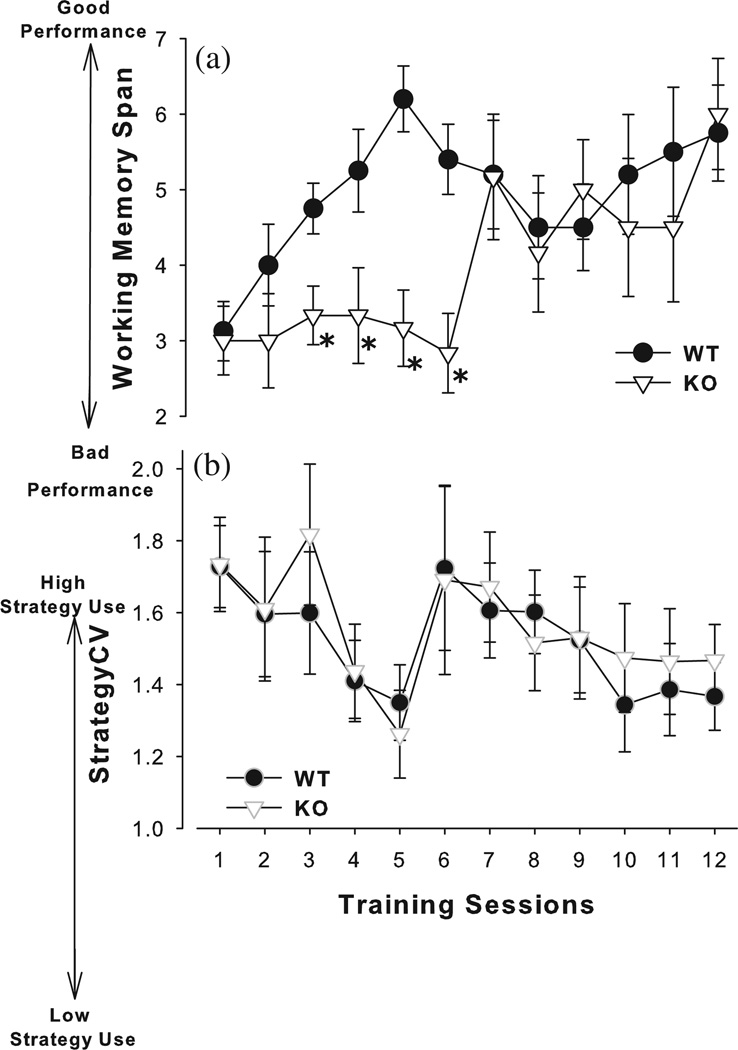
Figure 2. Acquisition of α7-nAChR mutant mice in the RAM.
α7-nAChR KO and WT littermate mice were trained to perform a win-shift search strategy using the RAM. Doors to the arms closed between arm choices for 1 second to limit simple turnleft/right strategies. KO mice took more sessions to acquire the win-shift rule compared to WT mice, as measured by the number of arm entries prior to repeat [WM span (a)]. The strategy used by these mice also changed with increasing sessions, indicative of a simple strategy being used less as time went on, as measured by strategyCV (b). Data are presented as mean ± SEM, *P < 0.05 when compared to WT mice on that session day.
Performance was assessed over 3 days once stable (Table 1). Performance did not vary over the 3 days in terms of WM span, RM errors or WM omissions (F < 1.1, ns), nor was there an effect of genotype (F < 1, ns) or a genotype × day interaction for any of these measures (F < 1.5, ns).
Table 1.
Stable RAM performance in α7-nAChR WT and KO mice
| Measure | WT | KO |
|---|---|---|
| WM span | 5.575 ± 0.46 | 5.72 ± 0.55 |
| RM errors | 2.54 ± 0.46 | 2.22 ± 0.53 |
| WM omissions | 1.83 ± 0.33 | 1.89 ± 0.38 |
| StrategyCV | 1.39 ± 0.12 | 1.45 ± 0.13 |
Novel object recognition memory
The mutants did not differ in any aspect of NORT performance. No main effect of genotype, sex or a sex × genotype interaction (F < 0.3, ns) was observed for %NI (WT = 71.6% ± 5.7, KO ♀ = 71.1% ± 6.6). Each group interacted with the novel object at rates higher than chance (P < 0.05).
Progressive ratio breakpoint study
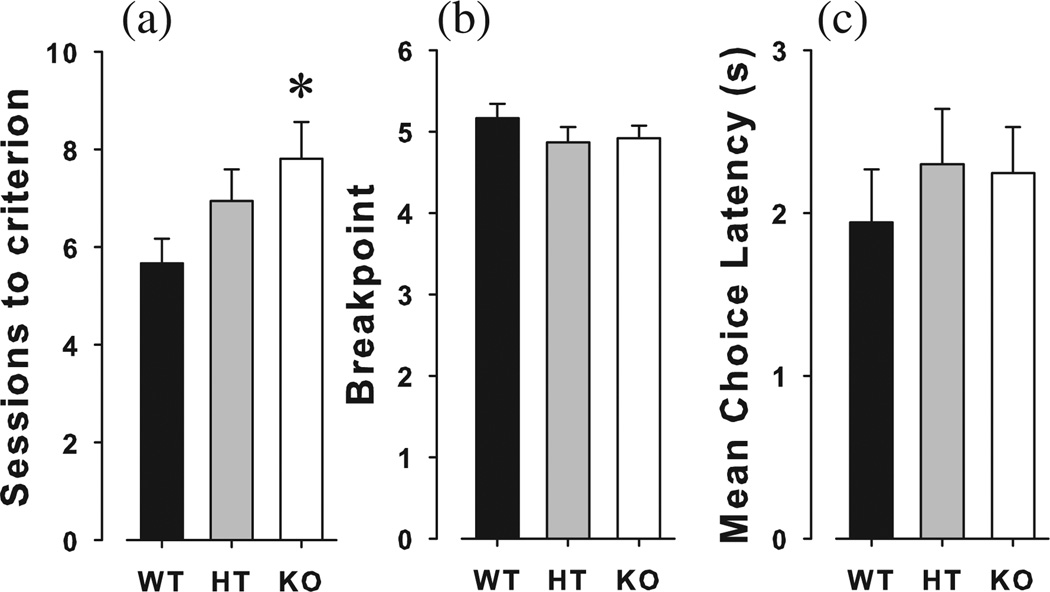
Mice were trained to nose-poke in a single lit hole on an FR1 schedule. When compared directly, α7-nAChR KO required significantly more training sessions to attain criterion (>70 responses on two consecutive days) than WT mice (t = 2.3, P < 0.05), while HT mice did not differ from either group (P > 0.1). Once trained to a stable performance, however, the number of responses made during the FR1 schedule did not differ between the three genotypes (F < 1, ns; Fig. 3a). When challenged with the PRB reinforcement schedule, there was no difference between genotypes on the breakpoint, total trials completed or MRL (F < 1.2, ns; Fig. 3b–d).
Figure 3. Acquisition and performance of α7-nAChR mutant mice in the progressive ratio breakpoint study.
α7-nAChR KO, HT and WT littermate mice were trained to nose-poke in a single lit hole. KO mice took significantly more sessions than WT mice to attain criterion of >70 responses for two consecutive days with HT mice exhibiting a numerically intermediate learning speed (a). Once trained, however, the number of nose-pokes did not differ between the groups on a fixed ratio 1 schedule. When challenged with a progressive ratio breakpoint schedule, the KO, HT and WT littermates were willing to nose-poke the same number of times for one reward (b), and did not differ in response latency (c). Data are presented as mean + SEM, *P < 0.05 when compared to WT littermate mice.
Behavioral pattern monitor
The exploratory profile of α7-nAChR null mutants was compared to WT littermate mice using the cross-species exploratory BPM paradigm over 60 min binned into 20 min intervals. No effect of genotype was observed on activity (transitions and center entries; Table 2), specific exploration (total holepoking, varied holepoking, rearing and center duration) or the predictability of locomotor pattern (temporal and spatial CV) (all F < 1, ns). Moreover, no genotype × time bin interaction was observed for any of these measures (F < 1.3, ns), except varied holepoking. A genotype × time bin interaction was observed for varied holepoking (F2,48 = 4.0, P < 0.05) with post hoc analyses revealing that KO mice produced more holepokes in the first 20 min compared to WT mice (P < 0.05), but this effect was not observed for subsequent time bins (P > 0.1). The linearity of activity (spatial d) was similarly unaffected (F1,24 = 1.8, P = 0.18), with a trend toward a genotype × time bin interaction observed (F2,48 = 2.8, P < 0.08). Both groups reduced the linearity of their locomotor pattern over time (increased spatial d; F2,48 = 28.7, P < 0.001), with no effect of genotype observed at any time bin (P > 0.1).
Table 2.
Measures of exploration of α 7- nAChRWT and KO mice as assessed in the BPM
| Factor | Measure | Time Bin (min) |
Mean |
|---|---|---|---|
| Activity | Transitions | 0–20 | WT = 506.3 ± 9.1 |
| KO = 486.4 ± 9.3 | |||
| 20–40 | WT = 366.9 ± 7.5 | ||
| KO = 374.1 ± 10 | |||
| 40–60 | WT = 307.3 ± 8.1 | ||
| KO = 277,5 ± 6.5 | |||
| Center entries | 0–20 | WT = 59.17 ± 1.7 | |
| KO = 55.57 ± 1.6 | |||
| 20–40 | WT = 38.83 ± 1.2 | ||
| KO = 44.14 ± 1.6 | |||
| 40–60 | WT = 32.75 ± 1.4 | ||
| KO = 34.00 ± 1.2 | |||
| Specific exploration | Total holepoking | 0–20 | WT = 65.33 ± 1.2 |
| KO = 65.86 ± 1.8 | |||
| 20–40 | WT = 67.00 ± 1.7 | ||
| KO = 69.50 ± 1.7 | |||
| 40–60 | WT = 74.42 ± 2.7 | ||
| KO = 59.93 ± 1.6 | |||
| Varied holekeeping | 0–20 | WT = 7.8 ± 0.6 | |
| KO = 11.8 ± 0.9* | |||
| 20–40 | WT = 9.4 ± 0.8 | ||
| KO = 11.1 ± 1.1 | |||
| 40–60 | WT = 11.1 ± 1.1 | ||
| KO = 9.1 ± 0.8 | |||
| Rearing | 0–20 | WT = 87.92 ± 2.9 | |
| KO = 84.42 ± 1.7 | |||
| 20–40 | WT = 97.75 ± 3.8 | ||
| KO = 81.50 ± 2.1 | |||
| 40–60 | WT = 79.17 ± 3.0 | ||
| KO = 71.07 ± 2.1 | |||
| Locomotor patterns | Temporal CV | 0–20 | WT = 1.166 ± 0.012 |
| KO = 1.245 ± 0.023 | |||
| 20–40 | WT = 1.377 ± 0.017 | ||
| KO = 1.372 ± 0.024 | |||
| 40–60 | WT = 1.496 ± 0.035 | ||
| KO = 1.687 ± 0.044 | |||
| Spatial CV | 0–20 | WT =1.024 ± 0.007 | |
| KO = 1.017 ± 0.007 | |||
| 20–40 | WT = 1.106 ± 0.010 | ||
| KO = 1.087 ± 0.008 | |||
| 40–60 | WT = 1.094 ± 0.010 | ||
| KO = 1.089 ± 0.011 |
P < 0.05 when compared to WT mice.
Prepulse inhibition
PPI challenge 1
The sensorimotor gating of the mutant mice as measured by PPI was intact, irrespective of the stimulus modality used (Table 3). When using light as the prepulse stimulus, no effect of genotype, sex or a sex × genotype interaction was observed (F < 1.2, ns). For prepulse + pulse trials, while there was no effect of genotype (F < 1.4), a main effect of sex (F1,42 = 12.6, P < 0.005) and a trend toward a sex × genotype interaction (F2,42 = 3.1, P = 0.0554) were observed. Tukey’s post hoc analysis revealed that male KO and WT mice exhibited higher PPI than female KO and WT mice (P < 0.05), with no sex effect in HT mice (P > 0.1). A main effect of genotype was observed for P120 (F2,42 = 7.7, P < 0.005), but no effects of sex or genotype × sex interaction were observed (F < 1, ns). Tukey’s post hoc analyses revealed that KO mice exhibited higher startle values than WT or HT mice (P < 0.05).
Table 3.
Multivariate assessment of sensorimotor gating of α7-nAChR WT and KO mice using PPI
| Study | Measure | Genotype | Mean startle |
|---|---|---|---|
| PPI challenge 1: multiple modalities | 120 dB startle pulse alone | WT | ♀ = 90.4 ± 10.7 |
| ♂ = 92.9 ± 16.3 | |||
| HT | ♀ = 78.7 ± 20.7 | ||
| ♂ = 78.4 ± 11.9 | |||
| KO | ♀ = 125.6 ± 19.2* | ||
| ♂ = 160.7 ± 23.5* | |||
| 73 dB (65 dB background) preceding 120 dB startle | WT | ♀ = 45.0 ± 3.3 | |
| ♂ = 67.6 ± 6.2# | |||
| HT | ♀ = 49.0 ± 3.0 | ||
| ♂ = 49.3 ± 6.2 | |||
| KO | ♀ = 45.6 ± 5.2 | ||
| ♂ = 68.0 ± 4.6# | |||
| Light preceding 120 dB pulse | WT | ♀ = 33.0 ± 7.7 | |
| ♂ = 12.0 ± 10.9 | |||
| HT | ♀ = 10.8 ± 10.0 | ||
| ♂ = 14.6 ± 8.0 | |||
| KO | ♀ = 27.5 ± 3.0 | ||
| ♂ = 26.2 ± 5.8 |
P < 0.05 when compared to WT mice;
P < 0.05 when compared to male HT mice.
PPI challenge 2
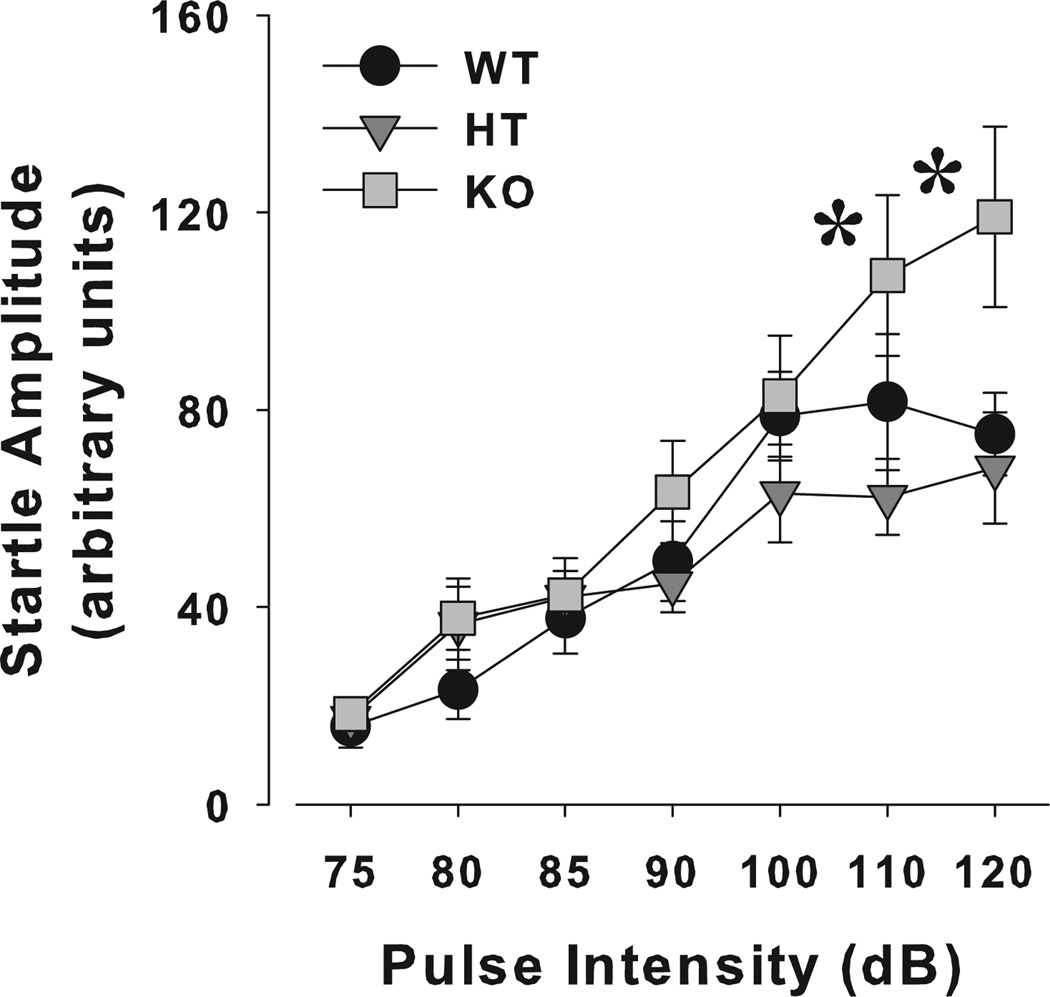
The sensorimotor gating of the mice was challenged across multiple parameters in separate blocks within the same session. These parameters included varying (1) the prepulse intensities (67, 69, 73 and 81 dB), (2) the ISIs (25, 50, 100, 200, 500 and 1000 milliseconds between a 73 dB prepulse and 120 dB pulse) and (3) the pulse intensities. No effect of sex or genotype, nor a sex × genotype interaction was observed for varying prepulse intensities (F < 1.2, ns; Table 4). A main effect of prepulse intensity was observed (F3,126 = 126.3, P < 0.0001) with a trend toward a sex × prepulse intensity interaction (F3,126 = 2.2, P < 0.1). No interaction of genotype, or sex × genotype with prepulse intensity was observed (F < 1.3, ns). No effects of sex (F1,42 = 2.2, P = 0.112), genotype or a sex × genotype interaction (F < 1, ns) were observed on PPI across ISIs (Table 4). A main effect of ISI was observed (F5,210 = 19.8, P < 0.0001), but no ISI interaction with sex, genotype or sex × genotype was observed (F < 1, ns). A main effect of pulse intensity was observed (F6,252 = 38.7, P < 0.0001), with trends toward an effect of genotype (F2,42 = 3.1, P < 0.06) and sex (F1,42 = 3.8, P < 0.06), with males exhibiting higher startle values than females. A pulse intensity × genotype interaction was observed (F12,252 = 2.5, P < 0.005; Fig. 4), but no sex × pulse intensity or sex × genotype × pulse intensity (F < 1, ns). Post hoc analyses revealed that while startle reactivity increased with increasing pulse intensity for all three genotypes, KO mice exhibited higher startle reactivity when compared with WT and HT mice (P < 0.05) at pulse intensities of 110 and 120 dB but not at smaller intensities (P > 0.1).
Table 4.
Effects of varying the (a) prepulse dB level and (b) interstimulus interval duration on PPI in female and male α 7-nAChR mutant mice
| Study | Measure | dB | Genotype | Mean startle |
|---|---|---|---|---|
| (a) PPI challenge 2 part A: varying prepulse intensities on PPI | Prepulse intensities (65 dB background) | 67 dB | WT | ♀ = 8.3 ± 7.3 |
| ♂ = 12.7 ± 9.1 | ||||
| HT | ♀ = 22.2 ± 5.0 | |||
| ♂ = 5.9 ± 8.8 | ||||
| KO | ♀ = 19.3 ± 6.3 | |||
| ♂ = 18.1 ± 5.4 | ||||
| 69 dB | WT | ♀ = 20.4 ± 5.3 | ||
| ♂ = 30.6 ±8.8 | ||||
| HT | ♀ = 31.5 ±9.1 | |||
| ♂ = 27.3 ±9.3 | ||||
| KO | ♀ = 30.0 ±9.1 | |||
| ♂ = 25.9 ± 9.3 | ||||
| 73 dB | WT | ♀ = 43.7 ± 9.1 | ||
| ♂ = 53.0 ± 9.3 | ||||
| HT | ♀ = 49.6 ± 9.1 | |||
| ♂ = 55.9 ± 9.3 | ||||
| KO | ♀ = 46.9 ± 9.1 | |||
| ♂ = 48.3 ± 9.3 | ||||
| 81 dB | WT | ♀ = 61.1 ± 9.1 | ||
| ♂ = 72.6 ± 9.3 | ||||
| HT | ♀ = 59.2 ± 9.1 | |||
| ♂ = 59.6 ± 9.3 | ||||
| KO | ♀ = 68.3 ± 9.1 | |||
| ♂ = 67.8 ± 9.3 | ||||
| (b) PPI challenge 2 part A: varying ISI duration PPI | ISI | Isi1000 | WT | ♀ = 3.4 ± 10.6 |
| ♂ = 22.5 ± 7.3 | ||||
| HT | ♀ = 10.4 ± 15.4 | |||
| ♂ = 20.9 ± 9.4 | ||||
| KO | ♀ = 19.9 ± 9.2 | |||
| ♂ = 16.5 ± 14.8 | ||||
| Isi500 | WT | ♀ = 22.8 ± 11.8 | ||
| ♂ = 39.1 ± 12.3 | ||||
| HT | ♀ = 33.9 ± 11.7 | |||
| ♂ = 41.6 ± 7.9 | ||||
| KO | ♀ = 3.4 ± 13.1 | |||
| ♂ = 25.1 ± 7.9 | ||||
| Isi200 | WT | ♀ = 27.8 ± 16.9 | ||
| ♂ = 55.7 ± 7.5 | ||||
| HT | ♀ = 40.8 ± 8.7 | |||
| ♂ = 46.7 ± 8.0 | ||||
| KO | ♀ = 39.8 ± 8.1 | |||
| ♂ = 43.0 ± 6.3 | ||||
| Isi100 | W | ♀ = 51.4 ± 7.9 | ||
| ♂ = 57.3 ± 9.3 | ||||
| HT | ♀ = 45.7 ± 10.6 | |||
| ♂ = 51.7 ± 7.1 | ||||
| KO | ♀ = 40.6 ± 8.1 | |||
| ♂ = 41.8 ± 4.2 | ||||
| Isi50 | WT | ♀ = 29.7 ± 9.9 | ||
| ♂ = 51.7 ± 7.2 | ||||
| HT | ♀ = 46.8 ± 5.9 | |||
| ♂ = 48.0 ± 10.6 | ||||
| KO | ♀ = 32.5 ± 15.2 | |||
| ♂ = 41.8 ± 4.2 | ||||
| Isi25 | WT | ♀ = 12.2 ± 23.4 | ||
| ♂ = 25.1 ± 18.1 | ||||
| HT | ♀ = 3.6 ± 17.8 | |||
| ♂ = 30.6 ± 11.9 | ||||
| KO | ♀ = 12.0 ± 12.0 | |||
| ♂ = 15.0 ± 6.5 |
Figure 4. Startle reactivity of α7-nAChR mutant mice across pulse intensities.
The startle reactivity of α7-nAChR KO, HT and WT littermate mice to various pulse intensities was assessed. Startle reactivity increased with increasing pulse intensity. The startle amplitude of HT and WT mice reached plateau at 110 and 120 dB, while KO mice continued to increase in amplitude. Data are presented as mean + SEM, *P < 0.05 when compared to WT and HT mice.
Discussion
We assessed the effects of null mutation of the α7-nAChR on mice in a variety of cognitive and behavioral paradigms. Null mutation of the α7-nAChR impaired procedural learning irrespective of the rule being acquired or the stimuli employed. The α7-nAChR KO mice took longer to learn to associate obtaining a food reward with: (1) digging in a bowl of specific odor or platform, (2) nose-poking in a lit hole and (3) entering previously unvisited arms. While reduced motivation for food rewards could impact learning for a reward, these mice exhibited normal breakpoint in a progressive ratio study, indicative of normal motivation for a single food reward in an operant task. Despite impaired learning of the initial rule and previous reports of impaired sustained attention in these mice, null mutation of the α7-nAChR did not affect attentional set-shifting, reversal learning, WM span capacity or short-term memory. Exploratory behavior and sensorimotor gating were unaffected, although KO mice exhibited increased startle reactivity when compared to WT mice. The data presented here support a role for the α7-nAChR in procedural learning.
Null mutation of the α7-nAChR appears to affect specific cognitive domains. Sustained attention is impaired (Hoyle et al 2006; Young et al 2004, 2007a) as well as learning as measured by learning to lever press (Keller et al 2005), to nose-poke in a lit hole (Young et al 2004) or to enter previously unvisited arms (Levin et al 2009) for food rewards. Interestingly, however, α7-nAChR KO mice learned (i.e. performance improved with training) and remembered (during probe challenges) the location of the escape platform as readily as WT mice when aversively motivated in the water maze (Paylor et al 1998). The differentiation of performance in these cognitive domains could reflect reduced motivation for food rewards. In the 5-CSRTT, α7-nAChR mutant mice exhibit genotype dose-dependent increases in omissions (Hoyle et al 2006; Young et al 2004, 2007a), which could reflect reduced motivation for reward in the task (Hoyle et al 2006). In the present studies, we specifically assessed the motivation of α7-nAChR KO mice for a food reward using the PRBP (Bensadoun et al 2004; Young & Geyer 2010). While the KO mice took more sessions to attain criterion on an FR1 schedule (nose-poke in a single lit hole), once trained to a stable level of performance, there was no genotype effect on the number of nose-pokes performed. When challenged, WT, HT and KO mice all attained the same breakpoint, nose-poking 22 times for a single reward. Previously, we reported that dopamine D1 receptor WT and HT mice also made approximately 22 responses for a single reward while D1 KO mice did not (Young & Geyer 2010). Thus, the breakpoint of these mice appears consistent both within and between mutant lines. These data support the interpretation that: (1) increased omissions of α7-nAChR KO mice are attributable to impaired attention and (2) differences in performance in various paradigms reflect differentially affected cognitive domains in these mice.
Evidence for normal motivation for food rewards assists in the interpretation of the present data. Spatial location learning and long-term memory appear normal in α7-nAChR KO mice (Paylor et al 1998). In the present studies, attentional set-shifting and reversal learning was also unimpaired in α7-nAChR KO mice as measured by the ASST. Surprisingly, however, α7-nAChR KO mice took longer to acquire SD learning (stage 1) of the ASST, despite normal learning in nearly every other ASST stage. Ordinarily, if these mice exhibited a generalized learning deficit, one might expect that performance would be impaired at every stage of the ASST (Birrell & Brown 2000). The KO mice, however, exhibited impaired learning at the SD stage and a trend toward impaired learning at the IDR stage. The SD stage requires not only learning the appropriate stimulus that predicts the reward, but also the premise that a stimulus could predict a reward. While CDR was the first reversal stage, IDR represents the first stage to which the knowledge can be applied that rule reversals occur. Subjecting rodents or primates to repeated reversal challenges, i.e. serial reversal learning, commonly results in the rodents appearing to learn to rule reverse faster (Castane et al 2010; Dickson et al 2010; Leary 1962; Rygula et al 2010; Seu & Jentsch 2009), due to increasing awareness that such events occur. Here, improved reversal learning was evident in the WT mice by the second reversal stage but only by the third reversal stage in KO mice. Improved reversal learning has also been observed within previous ASST studies in rats (Izquierdo et al 2010; Tait et al 2009) andmice (Young et al 2010b). Therefore, the SD ASST data provide support for the notion that α7-nAChR KO mice exhibit impaired procedural learning (rule acquisition). While only a trend toward a genotype effect was observed in the IDR stage, the faster learning exhibited by the WT from the first to second reversal – not seen in the KO mice – supports this hypothesis of impaired procedural learning in the KO mice. By the third stage of reversal (EDR), the KO mice took fewer trials to criterion resulting in similar performance to WT mice. Given the unimpaired performance of α7-nAChR KO mice in CD, CDR, ID, ED and EDR stages, the data support the hypothesis that once acquired, these mice can apply a rule and are motivated to obtain food rewards.
The present RAM study provided further evidence of procedural learning deficits in α7-nAChR KO mice. The KO mice took longer to learn or apply the rule of not reentering arms. This observation is consistent with earlier reports, although previous reports interpreted these findings of the mice exhibiting poorer choice accuracy and not necessarily impaired acquisition (Levin et al 2009). Fully trained stable performance was not presented in the previous studies, however, and therefore it is unclear whether performance would have improved to WT levels with more training sessions, as the present findings suggest. When stable, RAM performance did not differ between the two genotypes. These data support the hypothesis presented here that these mice exhibit impaired procedural learning, but normal choice accuracy once the task is acquired. This RAM procedure was developed to assess spatial WM span capacity, controlling for simple turn left strategies by briefly closing doors for 1 second between choices (Wenk 2004). The StrategyCV measure was developed to quantify the degree to which repeated strategy choices are made. Consistent with previous reports (Tarantino et al 2011), as performance in this RAM procedure improved, the use of a simple strategy decreased. No difference in strategy use was observed between α7-nAChR WT and KO mice. Thus, impaired procedural learning of the KO mice was unlikely to be due to utilizing a different strategy.
When using non-spatial cues requiring digging, α7-nAChR KO exhibited impaired learning of the odor span task (Young et al 2007a). These mice also exhibited poorer performance on this non-spatial WM span task, suggesting that spatial and non-spatial WM span capacity are dissociable in mice as in humans (Buchanan et al 2005; Gruzelier et al 1988; Milner 1971). Another possibility is that because the testing environment for the odor span task was a large open space, the α7-nAChR KO were more distracted, thus taking longer than the 10 min allotted to complete the task (Young et al 2007a). Thus, inattention may mediate the odor span and procedural learning deficits of α7-nAChR KO mice (Young et al 2007a).
In humans, attentional aspects of cognitive abilities play a role in learning and WM (Vanderploeg et al 1994). Specifically, attention can impact procedural/perceptual learning (Feldman 2004; Tsushima & Watanabe 2009). Procedural/perceptual learning, observing patterns in the environment to understand an action, utilizes distinct hippocampofronto-parietal networks (Feldman 2004; Wimber et al 2010). Once the pattern is observed and the rule learned, it can be applied to other aspects of behavior. This rule realization has been referred to as the ‘eureka’ effect (Giovannelli et al 2010). It could be hypothesized that these studies provide some support that removal of the α7-nAChR delays this ‘eureka effect’ in mice, while leaving rule application, reversal learning and other aspects of behavior intact. The precise mechanism of action by which the α7-nAChR contributes toward procedural learning remains unclear, however. Cholinergic projections from the basal forebrain innervate a number of structures including the hippocampus and frontal cortices (Miwa et al 2011) where α7-nAChRs are located (Whiteaker et al 1999). It has been hypothesized that α7-nAChR located on inhibitory neurons in the hippocampus participate in controlling sensory responses indirectly resulting in pyramidal neurons decreasing their response to repeated stimuli (Miwa et al 2011; Poorthuis et al 2009). Given that the hippocampus contributes toward relational learning between sensory stimuli (DeVito et al 2010a; Eichenbaum & Fortin 2009), the loss of α7-nAChRs may limit the ability of the hippocampus to integrate incoming sensory stimuli, inhibiting sensory input to the cortex. While speculative, the primary location of causal procedural deficits in these mice could be investigated using the transitive inference task. This task requires animals to make relational inferences between stimuli that had not previously been associated, based on the knowledge previously gained about the stimuli (DeVito et al 2010a, b). Because hippocampal and prefrontal lesions differentially affect performance in this task, one could predict that if hippocampal α7-nAChR mediate this procedural learning and integration of stimuli, their deficits in the task would resemble hippocampal lesioned mice as opposed to prefrontal lesioned mice (DeVito et al 2010a, b). Future studies are warranted to test this hypothesis.
The studies presented here extend the behavioral phenotype of α7-nAChR mutant mice. While normal PPI has been observed previously in male and female α7-nAChRmice at younger ages (3–4 months; Paylor et al 1998) the present study extends these findings using a multivariate/multimodal approach (Barr et al 2006; Csomor et al 2008; Young et al 2010c). Thus, it is clear α7-nAChR KO exhibit normal auditory, tactile and light-induced PPI. Moreover, these mice exhibit normal PPI across multiple inter-stimulus intervals. Increased startle amplitude was observed in KO mice compared to WT and HT mice although only at higher pulse intensities (110 and 120 dB). The present studies also extend the exploratory behavioral profile of these α7-nAChR mutants at an older age (10 months) using the cross-species test of exploration, the BPM (Henry et al 2010; Perry et al 2009; Young et al 2007c). Null mutation of the α7-nAChR mice had no effect on multivariate measures of activity, specific exploratory responses or locomotor path patterns, consistent with activity measures of younger (3–4 months) mice (Paylor et al 2008). Finally, the lack of difference between α7-nAChR KO mice and WT mice in the NORT suggests that these mice do not exhibit impaired short-term memory (Ali et al 2011; Young et al 2009b). No sex by genotype effects were observed in any of the current studies. The potential for such interactions cannot be readily discounted, however, given the limited sample sizes utilized in the current studies. Moreover, the female estrus cycle was not taken into account in these studies, which may have increased the variance observed. Thus, while certain aspects of behavior are affected by null mutation of the α7-nAChR, numerous behaviors remain unchanged, but to fully investigate sex × genotype interactions, greater sample sizes will be required with estrus cycles being examined concurrently.
Previous studies have assessed the efficacy of the partial α7-nAChR agonist GTS-21 for improving cognitive dysfunction in schizophrenia. Although early studies were promising (Olincy et al 2006), larger multisite studies failed to support the efficacy of this drug (Freedman et al 2008). GTS-21 is one of a long list of pharmacological treatments that have so far failed to gain approval for improving cognition in schizophrenia. There is evidence that cognitive behavioral (remediation) therapy, where patients learn to perform cognitive tasks (Twamley et al 2008), may be a viable treatment option (Adcock et al 2009; Genevsky et al 2010; Rathod et al 2010; Tarrier 2010) and such therapy can even produce fronto-parietal structural changes (Eack et al 2010; Haut et al 2010; Premkumar et al 2010). It is a common complaint that while patients take longer to acquire the rule governing the task, they can perform adequately once acquired (Twamley et al 2008). In the future, cognitive behavioral therapy may and perhaps should be commonly used to assist in the treatment of cognitive dysfunction in schizophrenia (Swerdlow 2010). Add-on pharmacotherapy may assist cognitive behavioral therapy and, based on the data presented here, perhaps future studies could examine the effects of an α7-nAChR agonist on assisting learning during cognitive behavioral therapy in patients with schizophrenia.
The present studies provide a more in-depth behavioral and cognitive characterization of α7-nAChRmutantmice than previously described. These studies, using tasks with varying degrees of cross-species translational validity (Young et al 2009b), support the notion that the α7-nAChR contributes toward normal procedural learning because of impaired learning in these mice observed across several testing paradigms. While the internal representation of learning is difficult to assess in animals given the requirement of behavioral output to interpret the animal’s knowledge, we feel these data may reflect at least in part a delayed ‘eureka’ of α7-nAChR KO mice. These deficits are unlikely to be attributable to reduced motivation for reward because these mice exhibited normal breakpoint behavior, albeit in an operant chamber. The poor attention of these mice may contribute to this delayed procedural learning and requires further investigation. Future studies evaluating α7-nAChR agonists for the treatment of schizophrenia should examine the effects of specific α7-nAChR ligands on procedural learning both in preclinical and in clinical settings.
Acknowledgments
We thank Dr Dean Acheson for his advice and support. This study was supported by NIH grants R21-MH085221 and R01-MH071916, and the Veteran’s Administration VISN 22 Mental Illness Research, Education and Clinical Center. The production of these mice was supported by a grant from NIDAP30 DA015663. We thank Drs Michael Marks and Athina Markou for providing breeding stock of the α7-nAChR mutant mice. During the past 3 years, M.A.G. has received compensation from Acadia, Addex, Amylin, Cerca Insights, Johnson & Johnson, Medivation, Merck, Omeros, Sepracor, Takeda, Teva and Wyeth-Ayerst and holds an equity interest in San Diego Instruments (San Diego, CA, USA). J.W.Y. has received compensation from Wyeth Nutrition. J.M.M., I.S.T. and S.C. have nothing to disclose.
References
- Adcock RA, Dale C, Fisher M, Aldebot S, Genevsky A, Simpson GV, Nagarajan S, Vinogradov S. When top-down meets bottom-up: auditory training enhances verbal memory in schizophrenia. Schizophr Bull. 2009;35:1132–1141. doi: 10.1093/schbul/sbp068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali SS, Young JW, Wallace CK, Gresack J, Jeste DV, Geyer MA, Dugan LL, Risbrough VB. Initial evidence linking synaptic superoxide production with poor short-term memory in aged mice. Brain Res. 2011;1368:65–70. doi: 10.1016/j.brainres.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr AM, Powell SB, Markou A, Geyer MA. Iloperidone reduces sensorimotor gating deficits in pharmacological models, but not a developmental model, of disrupted prepulse inhibition in rats. Neuropharmacology. 2006;51:457–465. doi: 10.1016/j.neuropharm.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Barr RS, Culhane MA, Jubelt LE, Mufti RS, Dyer MA, Weiss AP, Deckersbach T, Kelly JF, Freudenreich O, Goff DC, Evins AE. The effects of transdermal nicotine on cognition in nonsmokers with schizophrenia and nonpsychiatric controls. Neuropsychopharmacology. 2008;33:480–490. doi: 10.1038/sj.npp.1301423. [DOI] [PubMed] [Google Scholar]
- Bensadoun JC, Brooks SP, Dunnett SB. Free operant and discrete trial performance of mice in the nine-hole box apparatus: validation using amphetamine and scopolamine. Psychopharmacology (Berl) 2004;174:396–405. doi: 10.1007/s00213-003-1751-0. [DOI] [PubMed] [Google Scholar]
- Birrell JM, Brown VJ. Medial frontal cortex mediates perceptual attentional set shifting in the rat. J Neurosci. 2000;20:4320–4324. doi: 10.1523/JNEUROSCI.20-11-04320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissonette GB, Martins GJ, Franz TM, Harper ES, Schoenbaum G, Powell EM. Double dissociation of the effects of medial and orbital prefrontal cortical lesions on attentional and affective shifts in mice. J Neurosci. 2008;28:11124–11130. doi: 10.1523/JNEUROSCI.2820-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan RW, Davis M, Goff D, Green MF, Keefe RS, Leon AC, Nuechterlein KH, Laughren T, Levin R, Stover E, Fenton W, Marder SR. A summary of the FDA-NIMH-MATRICS workshop on clinical trial design for neurocognitive drugs for schizophrenia. Schizophr Bull. 2005;31:5–19. doi: 10.1093/schbul/sbi020. [DOI] [PubMed] [Google Scholar]
- Castane A, Theobald DE, Robbins TW. Selective lesions of the dorsomedial striatum impair serial spatial reversal learning in rats. Behav Brain Res. 2010;210:74–83. doi: 10.1016/j.bbr.2010.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cilia J, Cluderay JE, Robbins MJ, Reavill C, Southam E, Kew JN, Jones DN. Reversal of isolation-rearing-induced PPI deficits by an alpha7 nicotinic receptor agonist. Psychopharmacology (Berl) 2005;182:214–219. doi: 10.1007/s00213-005-0069-5. [DOI] [PubMed] [Google Scholar]
- Csomor PA, Yee BK, Vollenweider FX, Feldon J, Nicolet T, Quednow BB. On the influence of baseline startle reactivity on the indexation of prepulse inhibition. Behav Neurosci. 2008;122:885–900. doi: 10.1037/0735-7044.122.4.885. [DOI] [PubMed] [Google Scholar]
- D’Souza MS, Markou A. Schizophrenia and tobacco smoking comorbidity: nAChR agonists in the treatment of schizophrenia-associated cognitive deficits. Neuropharmacology. 2011 doi: 10.1016/j.neuropharm.2011.01.044. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luca V, Likhodi O, Van Tol HH, Kennedy JL, Wong AH. Regulation of alpha7-nicotinic receptor subunit and alpha7-like gene expression in the prefrontal cortex of patients with bipolar disorder and schizophrenia. Acta Psychiatr Scand. 2006;114:211–215. doi: 10.1111/j.1600-0447.2006.00785.x. [DOI] [PubMed] [Google Scholar]
- DeVito LM, Kanter BR, Eichenbaum H. The hippocampus contributes to memory expression during transitive inference in mice. Hippocampus. 2010a;20:208–217. doi: 10.1002/hipo.20610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVito LM, Lykken C, Kanter BR, Eichenbaum H. Prefrontal cortex: role in acquisition of overlapping associations and transitive inference. Learn Mem. 2010b;17:161–167. doi: 10.1101/lm.1685710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson PE, Rogers TD, Del Mar N, Martin LA, Heck D, Blaha CD, Goldowitz D, Mittleman G. Behavioral flexibility in a mouse model of developmental cerebellar Purkinje cell loss. Neurobiol Learn Mem. 2010;94:220–228. doi: 10.1016/j.nlm.2010.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eack SM, Hogarty GE, Cho RY, Prasad KM, Greenwald DP, Hogarty SS, Keshavan MS. Neuroprotective effects of cognitive enhancement therapy against gray matter loss in early schizophrenia: results from a 2-year randomized controlled trial. Arch Gen Psychiatry. 2010;67:674–682. doi: 10.1001/archgenpsychiatry.2010.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H, Fortin NJ. The neurobiology of memory based predictions. Philos Trans R Soc Lond B Biol Sci. 2009;364:1183–1191. doi: 10.1098/rstb.2008.0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman J. How surprising is a simple pattern? Quantifying “Eureka!”. Cognition. 2004;93:199–224. doi: 10.1016/j.cognition.2003.09.013. [DOI] [PubMed] [Google Scholar]
- Freedman R, Coon H, Myles-Worsley M, et al. Linkage of a neurophysiological deficit in schizophrenia to a chromosome 15 locus. Proc Natl Acad Sci U S A. 1997;94:587–592. doi: 10.1073/pnas.94.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman R, Olincy A, Buchanan RW, Harris JG, Gold JM, Johnson L, Allensworth D, Guzman-Bonilla A, Clement B, Ball MP, Kutnick J, Pender V, Martin LF, Stevens KE, Wagner BD, Zerbe GO, Soti F, Kem WR. Initial phase 2 trial of a nicotinic agonist in schizophrenia. Am J Psychiatry. 2008;165:1040–1047. doi: 10.1176/appi.ajp.2008.07071135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genevsky A, Garrett CT, Alexander PP, Vinogradov S. Cognitive training in schizophrenia: a neuroscience-based approach. Dialogues Clin Neurosci. 2010;12:416–421. doi: 10.31887/DCNS.2010.12.3/agenevsky. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer MA, Tamminga CA. Measurement and treatment research to improve cognition in schizophrenia: Neuropharmacological aspects. Psychopharmacology. 2004;174:1–2. [Google Scholar]
- Geyer MA, Russo PV, Masten VL. Multivariate assessment of locomotor behavior: pharmacological and behavioral analyses. Pharmacol Biochem Behav. 1986;25:277–288. doi: 10.1016/0091-3057(86)90266-2. [DOI] [PubMed] [Google Scholar]
- Giovannelli F, Silingardi D, Borgheresi A, Feurra M, Amati G, Pizzorusso T, Viggiano MP, Zaccara G, Berardi N, Cincotta M. Involvement of the parietal cortex in perceptual learning (Eureka effect): an interference approach using rTMS. Neuropsychologia. 2010;48:1807–1812. doi: 10.1016/j.neuropsychologia.2010.02.031. [DOI] [PubMed] [Google Scholar]
- Gruzelier J, Seymour K, Wilson L, Jolley A, Hirsch S. Impairments on neuropsychologic tests of temporohippocampal and frontohippocampal functions and word fluency in remitting schizophrenia and affective disorders. Arch Gen Psychiatry. 1988;45:623–629. doi: 10.1001/archpsyc.1988.01800310027003. [DOI] [PubMed] [Google Scholar]
- Halberstadt AL, van der Heijden I, Ruderman MA, Risbrough VB, Gingrich JA, Geyer MA, Powell SB. 5-HT(2A) and 5-HT(2C) receptors exert opposing effects on locomotor activity in mice. Neuropsychopharmacology. 2009;34:1958–1967. doi: 10.1038/npp.2009.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haut KM, Lim KO, MacDonald A., III Prefrontal cortical changes following cognitive training in patients with chronic schizophrenia: effects of practice, generalization, and specificity. Neuropsychopharmacology. 2010;35:1850–1859. doi: 10.1038/npp.2010.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry BL, Minassian A, Young JW, Paulus MP, Geyer MA, Perry W. Cross-species assessments of motor and exploratory behavior related to bipolar disorder. Neurosci Biobehav Rev. 2010;34:1296–1306. doi: 10.1016/j.neubiorev.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyle E, Genn RF, Fernandes C, Stolerman IP. Impaired performance of alpha7 nicotinic receptor knockout mice in the five-choice serial reaction time task. Psychopharmacology (Berl) 2006;189:211–223. doi: 10.1007/s00213-006-0549-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo A, Belcher AM, Scott L, Cazares VA, Chen J, O’Dell SJ, Malvaez M, Wu T, Marshall JF. Reversal-specific learning impairments after a binge regimen of methamphetamine in rats: possible involvement of striatal dopamine. Neuropsychopharmacology. 2010;35:505–514. doi: 10.1038/npp.2009.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller JJ, Keller AB, Bowers BJ, Wehner JM. Performance of alpha7 nicotinic receptor null mutants is impaired in appetitive learning measured in a signaled nose poke task. Behav Brain Res. 2005;162:143–152. doi: 10.1016/j.bbr.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Komada M, Takao K, Miyakawa T. Elevated plus maze for mice. J Vis Exp. 2008 doi: 10.3791/1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari V, Postma P. Nicotine use in schizophrenia: the self medication hypotheses. Neurosci Biobehav Rev. 2005;29:1021–1034. doi: 10.1016/j.neubiorev.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Leary RW. “Spontaneous reversal” in the serial-discrimination reversal learning of monkeys. Can J Psychol. 1962;16:228–233. doi: 10.1037/h0083248. [DOI] [PubMed] [Google Scholar]
- Levin ED, Conners CK, Silva D, Hinton SC, Meck WH, March J, Rose JE. Transdermal nicotine effects on attention. Psychopharmacology (Berl) 1998;140:135–141. doi: 10.1007/s002130050750. [DOI] [PubMed] [Google Scholar]
- Levin ED, McClernon FJ, Rezvani AH. Nicotinic effects on cognitive function: behavioral characterization, pharmacological specification, and anatomic localization. Psychopharmacology (Berl) 2006;184:523–539. doi: 10.1007/s00213-005-0164-7. [DOI] [PubMed] [Google Scholar]
- Levin ED, Petro A, Rezvani AH, Pollard N, Christopher NC, Strauss M, Avery J, Nicholson J, Rose JE. Nicotinic alpha7- or beta2-containing receptor knockout: effects on radialarm maze learning and long-term nicotine consumption in mice. Behav Brain Res. 2009;196:207–213. doi: 10.1016/j.bbr.2008.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockman PR, Van der Schyf CJ, Abbruscato TJ, Allen DD. Chronic nicotine exposure alters blood–brain barrier permeability and diminishes brain uptake of methyllycaconitine. J Neurochem. 2005;94:37–44. doi: 10.1111/j.1471-4159.2005.03162.x. [DOI] [PubMed] [Google Scholar]
- Mansbach RS, Geyer MA. Blockade of potentiated startle responding in rats by 5-hydroxytryptamine1A receptor ligands. Eur J Pharmacol. 1988;156:375–383. doi: 10.1016/0014-2999(88)90283-x. [DOI] [PubMed] [Google Scholar]
- Marder SR. The NIMH-MATRICS project for developing cognition-enhancing agents for schizophrenia. Dialogues Clin Neurosci. 2006;8:109–113. doi: 10.31887/DCNS.2006.8.1/smarder. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks MJ, Whiteaker P, Calcaterra J, Stitzel JA, Bullock AE, Grady SR, Picciotto MR, Changeux JP, Collins AC. Two pharmacologically distinct components of nicotinic receptor-mediated rubidium efflux in mouse brain require the beta2 subunit. J Pharmacol Exp Ther. 1999;289:1090–1103. [PubMed] [Google Scholar]
- Martin LF, Kem WR, Freedman R. Alpha–7 nicotinic receptor agonists: potential new candidates for the treatment of schizophrenia. Psychopharmacology (Berl) 2004;174:54–64. doi: 10.1007/s00213-003-1750-1. [DOI] [PubMed] [Google Scholar]
- Martin-Ruiz CM, Haroutunian VH, Long P, Young AH, Davis KL, Perry EK, Court JA. Dementia rating and nicotinic receptor expression in the prefrontal cortex in schizophrenia. Biol Psychiatry. 2003;54:1222–1233. doi: 10.1016/s0006-3223(03)00348-2. [DOI] [PubMed] [Google Scholar]
- McAlonan K, Brown VJ. Orbital prefrontal cortex mediates reversal learning and not attentional set shifting in the rat. Behav Brain Res. 2003;146:97–103. doi: 10.1016/j.bbr.2003.09.019. [DOI] [PubMed] [Google Scholar]
- Milner B. Interhemispheric differences in the localization of psychological processes in man. Br Med Bull. 1971;27:272–277. doi: 10.1093/oxfordjournals.bmb.a070866. [DOI] [PubMed] [Google Scholar]
- Miwa JM, Freedman R, Lester HA. Neural systems governed by nicotinic acetylcholine receptors: emerging hypotheses. Neuron. 2011;70:20–33. doi: 10.1016/j.neuron.2011.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers CS, Taylor RC, Moolchan ET, Heishman SJ. Dose-related enhancement of mood and cognition in smokers administered nicotine nasal spray. Neuropsychopharmacology. 2008;33:588–598. doi: 10.1038/sj.npp.1301425. [DOI] [PubMed] [Google Scholar]
- Newhouse P, Singh A, Potter A. Nicotine and nicotinic receptor involvement in neuropsychiatric disorders. Curr Top Med Chem. 2004;4:267–282. doi: 10.2174/1568026043451401. [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH, Barch DM, Gold JM, Goldberg TE, Green MF, Heaton RK. Identification of separable cognitive factors in schizophrenia. Schizophr Res. 2004;72:29–39. doi: 10.1016/j.schres.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Olincy A, Harris JG, Johnson LL, Pender V, Kongs S, Allensworth D, Ellis J, Zerbe GO, Leonard S, Stevens KE, Stevens JO, Martin L, Adler LE, Soti F, Kem WR, Freedman R. Proof-of-concept trial of an alpha7 nicotinic agonist in schizophrenia. Arch Gen Psychiatry. 2006;63:630–638. doi: 10.1001/archpsyc.63.6.630. [DOI] [PubMed] [Google Scholar]
- Orr-Urtreger A, Goldner FM, Saeki M, Lorenzo I, Goldberg L, De Biasi M, Dani JA, Patrick JW, Beaud Al, et al. Mice deficient in the alpha7 neuronal nicotinic acetylcholine receptor lack alpha-bungarotoxin binding sites and hippocampal fast nicotinic currents. J Neurosci. 1997;17:9165–9171. doi: 10.1523/JNEUROSCI.17-23-09165.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouagazzal AM, Reiss D, Romand R. Effects of age-related hearing loss on startle reflex and prepulse inhibition in mice on pure and mixed C57BL and 129 genetic background. Behav Brain Res. 2006;172:307–315. doi: 10.1016/j.bbr.2006.05.018. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Geyer MA. A temporal and spatial scaling hypothesis for the behavioral effects of psychostimulants. Psychopharmacology (Berl) 1991;104:6–16. doi: 10.1007/BF02244547. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Geyer MA. Three independent factors characterize spontaneous rat motor activity. Behav Brain Res. 1993;53:11–20. doi: 10.1016/s0166-4328(05)80262-1. [DOI] [PubMed] [Google Scholar]
- Paylor R, Nguyen M, Crawley JN, Patrick J, Beaudet A, Orr-Urtreger A. Alpha7 nicotinic receptor subunits are not necessary for hippocampal-dependent learning or sensorimotor gating: a behavioral characterization of Acra7-deficient mice. Learn Mem. 1998;5:302–316. [PMC free article] [PubMed] [Google Scholar]
- Perkins KA, Epstein LH, Stiller RL, Fernstrom MH, Sexton JE, Jacob RG, Solberg R. Acute effects of nicotine on hunger and caloric intake in smokers and nonsmokers. Psychopharmacology (Berl) 1991;103:103–109. doi: 10.1007/BF02244083. [DOI] [PubMed] [Google Scholar]
- Perry W, Minassian A, Paulus MP, Young JW, Kincaid MJ, Ferguson EJ, Henry BL, Zhuang X, Masten VL, Sharp RF, Geyer MA. A reverse-translational study of dysfunctional exploration in psychiatric disorders: from mice to men. Arch Gen Psychiatry. 2009;66:1072–1080. doi: 10.1001/archgenpsychiatry.2009.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poltavski DV, Petros T. Effects of transdermal nicotine on attention in adult non-smokers with and without attentional deficits. Physiol Behav. 2006;87:614–624. doi: 10.1016/j.physbeh.2005.12.011. [DOI] [PubMed] [Google Scholar]
- Poorthuis RB, Goriounova NA, Couey JJ, Mansvelder HD. Nicotinic actions on neuronal networks for cognition: general principles and long-term consequences. Biochem Pharmacol. 2009;78:668–676. doi: 10.1016/j.bcp.2009.04.031. [DOI] [PubMed] [Google Scholar]
- Premkumar P, Parbhakar VA, Fannon D, Lythgoe D, Williams SC, Kuipers E, Kumari V. N-acetyl aspartate concentration in the anterior cingulate cortex in patients with schizophrenia: a study of clinical and neuropsychological correlates and preliminary exploration of cognitive behaviour therapy effects. Psychiatry Res. 2010;182:251–260. doi: 10.1016/j.pscychresns.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathod S, Phiri P, Kingdon D. Cognitive behavioral therapy for schizophrenia. Psychiatr Clin North Am. 2010;33:527–536. doi: 10.1016/j.psc.2010.04.009. [DOI] [PubMed] [Google Scholar]
- Risbrough VB, Masten VL, Caldwell S, Paulus MP, Low MJ, Geyer MA. Differential contributions of dopamine D(1), D(2), and D(3) receptors to MDMA-induced effects on locomotor behavior patterns in mice. Neuropsychopharmacology. 2006;31:2349–2358. doi: 10.1038/sj.npp.1301161. [DOI] [PubMed] [Google Scholar]
- Robbins TW. The 5-choice serial reaction time task: behavioural pharmacology and functional neurochemistry. Psychopharmacology (Berl) 2002;163:362–380. doi: 10.1007/s00213-002-1154-7. [DOI] [PubMed] [Google Scholar]
- Rygula R, Walker SC, Clarke HF, Robbins TW, Roberts AC. Differential contributions of the primate ventrolateral prefrontal and orbitofrontal cortex to serial reversal learning. J Neurosci. 2010;30:14552–14559. doi: 10.1523/JNEUROSCI.2631-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seu E, Jentsch DJ. Effect of acute and repeated treatment with desipramine or methylphenidate on serial reversal learning in rats. Neuropharmacology. 2009;57:665–672. doi: 10.1016/j.neuropharm.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow NR. Integrative circuit models and their implications for the pathophysiologies and treatments of the schizophrenias. In: Swerdlow NR, editor. Behavioral Neurobiology of schizophrenia and its treatment. Berlin: Springer-Verlag; 2010. [DOI] [PubMed] [Google Scholar]
- Tait DS, Marston HM, Shahid M, Brown VJ. Asenapine restores cognitive flexibility in rats with medial prefrontal cortex lesions. Psychopharmacology (Berl) 2009;202:295–306. doi: 10.1007/s00213-008-1364-8. [DOI] [PubMed] [Google Scholar]
- Tamminga CA. The neurobiology of cognition in schizophrenia. J Clin Psychiatry. 2006;67(Suppl. 9):9–13. discussion 36–42. [PubMed] [Google Scholar]
- Tarantino IS, Sharp RF, Geyer MA, Meves JM, Young JW. Working memory span capacity improved by a D2 but not D1 receptor family agonist. Behav Brain Res. 2011;219:181–188. doi: 10.1016/j.bbr.2010.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarrier N. Cognitive behavior therapy for schizophrenia and psychosis: current status and future directions. Clin Schizophr Relat Psychoses. 2010;4:176–184. doi: 10.3371/CSRP.4.3.4. [DOI] [PubMed] [Google Scholar]
- Thomsen MS, Christensen DZ, Hansen HH, Redrobe JP, Mikkelsen JD. alpha(7) Nicotinic acetylcholine receptor activation prevents behavioral and molecular changes induced by repeated phencyclidine treatment. Neuropharmacology. 2009;56:1001–1009. doi: 10.1016/j.neuropharm.2009.02.003. [DOI] [PubMed] [Google Scholar]
- Tsushima Y, Watanabe T. Roles of attention in perceptual learning from perspectives of psychophysics and animal learning. Learn Behav. 2009;37:126–132. doi: 10.3758/LB.37.2.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turek JW, Kang CH, Campbell JE, Arneric SP, Sullivan JP. A sensitive technique for the detection of the alpha 7 neuronal nicotinic acetylcholine receptor antagonist, methyllycaconitine, in rat plasma and brain. J Neurosci Methods. 1995;61:113–118. doi: 10.1016/0165-0270(95)00032-p. [DOI] [PubMed] [Google Scholar]
- Twamley EW, Savla GN, Zurhellen CH, Heaton RK, Jeste DV. Development and pilot testing of a novel compensatory cognitive training intervention for people with psychosis. Am J Psychiatr Rehabil. 2008;11:144–163. doi: 10.1080/15487760801963678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderploeg RD, Schinka JA, Retzlaff P. Relationships between measures of auditory verbal learning and executive functioning. J Clin Exp Neuropsychol. 1994;16:243–252. doi: 10.1080/01688639408402635. [DOI] [PubMed] [Google Scholar]
- Wenk GL. Assessment of spatialmemory using the radial arm maze and Morris water maze. Curr Protoc Neurosci. 2004;Chapter 8(Unit 8.5A) doi: 10.1002/0471142301.ns0805as26. [DOI] [PubMed] [Google Scholar]
- Whiteaker P, Davies AR, Marks MJ, Blagbrough IS, Potter BV, Wolstenholme AJ, Collins AC, Wonnacott S. An autoradiographic study of the distribution of binding sites for the novel alpha7-selective nicotinic radioligand [3H]-methyllycaconitine in the mouse brain. Eur J Neurosci. 1999;11:2689–2696. doi: 10.1046/j.1460-9568.1999.00685.x. [DOI] [PubMed] [Google Scholar]
- Wimber M, Heinze HJ, Richardson-Klavehn A. Distinct frontoparietal networks set the stage for later perceptual identification priming and episodic recognition memory. J Neurosci. 2010;30:13272–13280. doi: 10.1523/JNEUROSCI.0588-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Geyer MA. Action of modafinil-increased motivation via the dopamine transporter inhibition and D1 receptors? Biol Psychiatry. 2010;67:784–787. doi: 10.1016/j.biopsych.2009.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Finlayson K, Spratt C, Marston HM, Crawford N, Kelly JS, Sharkey J. Nicotine improves sustained attention in mice: evidence for involvement of the alpha7 nicotinic acetylcholine receptor. Neuropsychopharmacology. 2004;29:891–900. doi: 10.1038/sj.npp.1300393. [DOI] [PubMed] [Google Scholar]
- Young JW, Crawford N, Kelly JS, Kerr LE, Marston HM, Spratt C, Finlayson K, Sharkey J. Impaired attention is central to the cognitive deficits observed in alpha 7 deficient mice. Eur Neuropsychopharmacol. 2007a;17:145–155. doi: 10.1016/j.euroneuro.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Young JW, Kerr LE, Kelly JS, Marston HM, Spratt C, Finlayson K, Sharkey J. The odour span task: a novel paradigm for assessing working memory in mice. Neuropharmacology. 2007b;52:634–645. doi: 10.1016/j.neuropharm.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Young JW, Minassian A, Paulus MP, Geyer MA, Perry W. A reverse-translational approach to bipolar disorder: rodent and human studies in the behavioral pattern monitor. Neurosci Biobehav Rev. 2007c;31:882–896. doi: 10.1016/j.neubiorev.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Sharkey J, Finlayson K. Progressive impairment in olfactory working memory in a mouse model of mild cognitive impairment. Neurobiol Aging. 2008;30:1430–1443. doi: 10.1016/j.neurobiolaging.2007.11.018. [DOI] [PubMed] [Google Scholar]
- Young JW, Light GA, Marston HM, Sharp R, Geyer MA. The 5-choice continuous performance test: evidence for a translational test of vigilance for mice. PLoS ONE. 2009a;4:e4227. doi: 10.1371/journal.pone.0004227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Powell SB, Risbrough V, Marston HM, Geyer MA. Using the MATRICS to guide development of a preclinical cognitive test battery for research in schizophrenia. Pharmacol Ther. 2009b;122:150–202. doi: 10.1016/j.pharmthera.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Goey AK, Minassian A, Perry W, Paulus MP, Geyer MA. The mania-like exploratory profile in genetic dopamine transporter mouse models is diminished in a familiar environment and reinstated by subthreshold psychostimulant administration. Pharmacol Biochem Behav. 2010a;96:7–15. doi: 10.1016/j.pbb.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Powell SB, Geyer MA, Jeste DV, Risbrough VB. The mouse attentional set-shifting task: a method for assaying successful cognitive aging? Cogn Affect Behav Neurosci. 2010b;10:243–251. doi: 10.3758/CABN.10.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Wallace CK, Geyer MA, Risbrough VB. Age-associated improvements in cross-modal prepulse inhibition in mice. Behav Neurosci. 2010c;124:133–140. doi: 10.1037/a0018462. [DOI] [PMC free article] [PubMed] [Google Scholar]