Abstract
Setting
Inpatient hospitals in South Africa and Uganda
Objective
To evaluate the cost-effectiveness of a lateral flow urine lipoarabinomannan (LAM) test when added to existing strategies for tuberculosis (TB) diagnosis in HIV-infected adults (CD4+ T-cell counts<100 cells/μL) with symptoms of active TB.
Design
Decision-analytic cost-utility model with the primary outcome being the incremental cost-effectiveness ratio (ICER), expressed in 2010 US dollars per disability-adjusted life year (DALY) averted, from the perspective of a public-sector TB control program.
Results and Conclusion
For every 1000 patients tested, adding lateral-flow urine LAM generated 80 incremental appropriate TB treatments and averted 224 DALYs. Estimated cost-utility was $353 per DALY averted (95% uncertainty range: $192–$1161) in South Africa and $86 per DALY averted (95% uncertainty range: $49–$239) in Uganda, reflecting the lower treatment costs in Uganda. Cost-utility was most sensitive to assay specificity, cost of TB treatment, life expectancy after TB cure, and cohort TB prevalence but did not rise above $1500 per DALY averted in South Africa under any one-way sensitivity analysis. The probability of acceptability was >99.8% at a per-DALY willingness-to-pay threshold equal to the per-capita gross domestic product in South Africa ($7275) and Uganda ($509).
INTRODUCTION
Tuberculosis (TB) is the leading cause of infectious death among people living with HIV/AIDS (PLWHA),1,2 accounting for 26% of AIDS-related deaths worldwide.3 Standard diagnostics for TB (e.g., chest X-ray, sputum smear microscopy) perform poorly in PLWHA, and many patients die prior to diagnosis.4–6 Better TB diagnostic tests may substantially reduce mortality in PLWHA,7 but they may also increase costs.8–10 A newly developed lateral flow immunochromatographic assay (Determine TB-LAM Alere, Waltham, MA, USA) detects lipoarabinomannan (LAM)—an immunogenic glycolipid in the cell wall of Mycobacterium tuberculosis11,12—in urine, diagnosing TB with excellent specificity and higher sensitivity than sputum smear among highly immunocompromised adults.13 This lateral-flow assay is the first true point-of-care diagnostic test for TB,14,15 and although inexpensive, its specificity is imperfect and it is optimized for use only in individuals with high underlying mortality rates. Thus, its cost-effectiveness remains uncertain. We performed a cost-utility analysis of lateral-flow LAM detection using data from a cohort of severely immunocompromised HIV-infected individuals with symptoms of TB in a South African hospital.
METHODS
Study Data
We conducted a diagnostic accuracy study in South Africa and Uganda to determine the sensitivity and specificity of Determine TB-LAM.16 We evaluated consenting HIV-infected adults (≥ 18 years) with clinical suspicion of TB based on any one or more of cough, fever, night sweats and weight loss. Individuals on TB treatment for more than 2 days during the prior 2 months were excluded. At enrollment, each participant underwent CD4+ T-cell testing, chest radiograph, sputum smear microscopy (two sputa), culture of blood and sputum for mycobacteria, and urine collection. We performed lateral-flow LAM testing immediately after urine collection in accordance with the manufacturer's instructions. We defined sensitivity as the probability of a positive test (2+ or higher) among individuals who exhibited clinical improvement with empiric TB treatment or had a positive culture for M. tuberculosis. Using this case definition, specificity was markedly lower among individuals with CD4+ T-cell counts <50 cells/μL, potentially representing occult TB. Thus, we measured specificity (test result negative or 1+) among individuals with CD4+ T-cell counts of 50 or higher who had neither a positive culture nor clinical response to TB chemotherapy. We performed a retrospective modeling analysis using aggregate data from this study; the parent study was approved by the institutional review boards of the participating institutions, but ethical approval was not required for this model-based analysis.
Modeled Scenarios
Using data from the study above, we constructed a decision-analytic cost-utility model of lateral-flow urine LAM, as used for hospitalized adults with known HIV infection, CD4+ T-cell count <100 cells/mm3 (most patients in this setting have either a known or low CD4 count), and clinical suspicion of TB. We compared a reference scenario in which diagnosis is based on sputum smear, clinical judgment, and the existing array of available additional diagnostic tests, to an alternative scenario in which lateral-flow urine LAM detection is added to that array. We excluded the use of culture in this scenario as medical decisions must be made rapidly in these seriously ill patients. We assumed that the probabilities of positive results using existing diagnostics and urine LAM were independent, and that a positive result with either strategy would trigger treatment for TB. Among those testing negative with existing diagnostics (and LAM, in the intervention scenario), we assumed that a proportion of patients would be treated based on other test results and clinical judgment, and that this proportion was unchanged by the availability of urine LAM detection. We estimated the case-fatality rate of treated TB in this seriously ill population at 20%17 and assumed untreated TB to be uniformly fatal within 1 month. We assumed a life expectancy of 5 years for survivors and patients without TB, based on cohort studies of African populations on antiretroviral therapy. Assuming differential mortality or life expectancy according to LAM positivity status did not change our results of incremental cost-effectiveness, as LAM neither benefitted nor harmed individuals testing negative in our analysis; thus, for parsimony, we assumed that LAM-negative individuals had the same outcomes as those for LAM-positive individuals reported in Table 1. Costs and effectiveness of antiretroviral therapy were not modeled. We assumed that neither the effectiveness of urine LAM detection nor the cost of the lateral-flow assay would vary by country, but that the cost of TB treatment, as well as the willingness-to-pay, would be lower in Uganda. Model parameters are shown in Table 1.
Table 1.
Parameter values.
| Name | Value | Sensitivity Range | Reference |
|---|---|---|---|
| TB Dynamics | |||
| TB Prevalence among suspects with HIV and CD4+ <100 cells/μL | 0.38 | 0.12–0.5 | Study data |
| Probability of death in those with TB and given TB treatment | 0.2 | 0.10–0.30 | 16 |
| Probability of dying from TB treatment toxicities | 5×10−5 | 8.5×10−6−1×10−2 | 26 |
| Characteristics of TB Diagnosis | |||
| Probability of empiric treatment among smear-negative TB cases | 0.53 | 0–0.75 | 27 |
| Probability of empiric treatment among patients without TB | 0.21 | 0–0.5 | 28 |
| LAM Sensitivity | 0.66 | 0.3–1 | Study data,13 |
| LAM Specificity | 0.95 | 0.7–1 | Study data,13 |
| Standard TB Diagnostics Sensitivity | 0.345 | 0.2–0.5 | 29 |
| Standard TB Diagnostics Specificity | 0.998 | 0.848–1 | 29 |
| Disability Weights | |||
| HIV+, on ART | 0.167 | 0.142–0.192 | 30 |
| TB | 0.264 | 0.224–0.304 | 30 |
| HIV+, not on ART | 0.505 | 0.429–0.581 | 30 |
| TB treatment | 0.10 | 0.085–0.115 | 30 |
| Life Expectancy (years) | |||
| HIV on ART (WHO Clinical Stage IV) | 5 | 1.5–10 | 22,31–34 |
| HIV acutely ill with TB | 0.0833 | 0.071–0.25 | Assumption |
| Unit Cost (2010 USD) | |||
| TB Treatment, South Africa | $850 | $500–$2000 | 17 |
| TB Treatment, Uganda | $178 | $100–$500 | 35 |
| LAM | $3.50 | $1.00–$30 | 29 |
| Standard TB Diagnostics | $1.58 | $1.34–$1.82 | 8 |
| Discount rate | 0.03 | 0–0.07 | 18 |
Our primary outcome was the incremental cost-effectiveness ratio (ICER), measured from the perspective of the public-sector TB control program and reported in 2010 USD per disability-adjusted life year (DALY) averted. Patient costs were not assessed. We modeled the time horizon as the lifetime of the cohort, assuming that incremental costs would not accrue beyond the period of active TB treatment. Since all costs were reported in 2010 USD, inflation of costs was unnecessary. Future DALYs and costs were discounted at 3% per year.18
Sensitivity and Uncertainty Analysis
We performed one-way sensitivity analyses on all variables across reasonable ranges and three-way sensitivity analysis on the variables to which the primary outcome was most sensitive. When data on sensitivity ranges were not available from the literature, we varied parameters over a range of +/−15% of their base value, using wider ranges for parameters with greater uncertainty. Given that Xpert MTB/RIF, an automated molecular test for TB,19 is being scaled up in South Africa (and, to a lesser extent, in Uganda), we also modeled a scenario in which smear was replaced by Xpert (sensitivity 85%, no change to specificity of the overall diagnostic algorithm) in both arms. To generate 95% uncertainty ranges and cost-effectiveness acceptability curves, we performed a probabilistic uncertainty analysis in which we simultaneously varied all parameters using 10,000 Monte Carlo methods across systematically defined distributions. For variables bounded from 0 to 1, we assumed an underlying beta distribution; for variables bounded from 0 to infinity, we assumed a gamma distribution. Parameters for both types of underlying distribution were derived from the most likely value listed in Table 1, assuming a standard deviation equal to 12.5% of that value. This was an arbitrarily chosen value that standardized the variation of parameter values for the multivariable uncertainty analysis. Analyses were conducted in TreeAge Pro 2011 (TreeAge Software Inc, Williamston MA, USA).
RESULTS
Cost-effectiveness of adding lateral-flow urine LAM
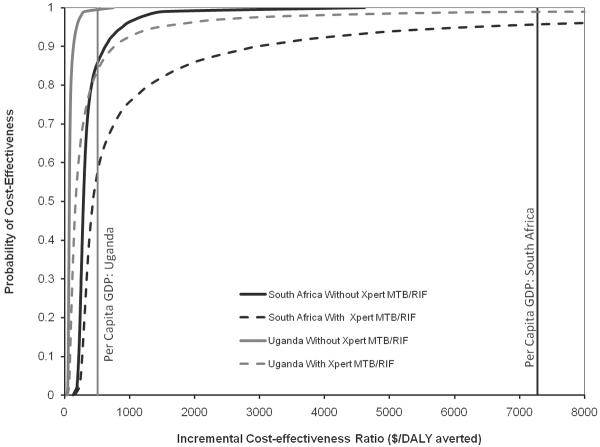
The prevalence of TB was 38% in this cohort of severely immunocompromised, symptomatic individuals. For every 1,000 patients in the reference scenario (no LAM detection), we estimated that 262 of the projected 380 people with culture-positive TB would be appropriately diagnosed and treated, and that 130 patients without TB would be inappropriately treated based on clinical suspicion (Table 2). Addition of lateral-flow urine LAM to this scenario resulted in 80 additional true-positive TB diagnoses (13 of whom nonetheless died) and 25 additional false-positives, at a cost of $79 per individual tested in South Africa and $19 per person tested in Uganda. Of this cost, $3.50 represented the diagnostic test price, and the remainder represented treatment of people who tested positive (either true-positive or false-positive). In South Africa, addition of urine LAM was estimated to avert 224 DALYs at an incremental cost of $353 per DALY averted (95% uncertainty range: $192–$1161). In Uganda, incremental cost-utility was $86 per DALY averted (95% uncertainty range: $49–$239), reflecting lower costs of first-line TB treatment in that country. The probability of acceptability at a per-DALY willingness-to-pay threshold equal to the per-capita gross domestic product (GDP, $7275 in South Africa and $509 in Uganda)20 was >99.8% in both countries (Figure 1, solid lines).
Table 2.
Cost-Utility of Lateral-Flow Urine LAM Detection
| Country and Diagnostic Strategy | Cohort Sizea | TB Cases | TB Cases Treated | False-Positives Treated | DALYs | DALYs averted | Cost (2010 US$) | Incremental Cost | Incremental Cost-Effectiveness ($/DALY) |
|---|---|---|---|---|---|---|---|---|---|
| South Africa | |||||||||
| Existing Diagnostics | 1000 | 380 | 262 | 130 | 1491 | (ref) | $299,000 | (ref) | (ref) |
| Existing Diagnostics + Urine LAM | 1000 | 380 | 342 | 155 | 1267 | 224 | $378,000 | $79,000 | $353 |
| Uganda | |||||||||
| Existing Diagnostics | 1000 | 380 | 262 | 130 | 1491 | (ref) | $64,000 | (ref) | (ref) |
| Existing Diagnostics + Urine LAM | 1000 | 380 | 342 | 155 | 1267 | 224 | $83,000 | $19,000 | $208 |
DALY, disability-adjusted life year; LAM, lipoarabinomannan; TB, tuberculosis
Figure 1. Cost-effectiveness Acceptability Curves for Lateral-Flow Urine LAM Detection.
Curves show the probability of cost-effectiveness (cost per DALY averted lower than the value on the x-axis) of urine LAM, over 10,000 Monte Carlo simulations. Black lines show cost-effectiveness in South Africa (where TB treatment is more expensive); grey lines represent Uganda (where TB treatment is cheaper). Solid lines utilize a baseline in which smear and other non-bacteriological diagnostics (e.g., chest X-ray) are available, but not Xpert MTB/RIF; dotted lines assume that Xpert MTB/RIF is also available.
Sensitivity analysis
In one-way sensitivity analysis, incremental cost-utility was most sensitive to life expectancy after TB cure, assay specificity, cohort TB prevalence, and cost of TB treatment (Figure 2). Given the importance of the former three variables, we conducted a corresponding three-way sensitivity analysis (Figure 3). Under the reference-case assumption of test specificity (95%), lateral-flow urine LAM was cost-effective in South Africa (at a willingness-to-pay of $7275 per DALY averted) for hospitalized cohorts in which TB prevalence was as low as 5.0% even when assuming life expectancy of 1.5 years after TB cure (Figure 3A), and also in cohorts for whom the probability of death despite TB treatment was as high as 97%. Similarly, under a “worst-case” two-way sensitivity analysis in which we assumed a test specificity of 87% and probability of death from drug side effects of 1%, the cost of the lateral-flow urine LAM assay was $509/DALY averted. Results in Uganda were qualitatively similar to those in South Africa, although the cost-effectiveness ratios were lower (data not shown).
Figure 2. Sensitivity Analysis: Cost-Utility of Lateral-Flow Urine LAM Detection in South Africa.
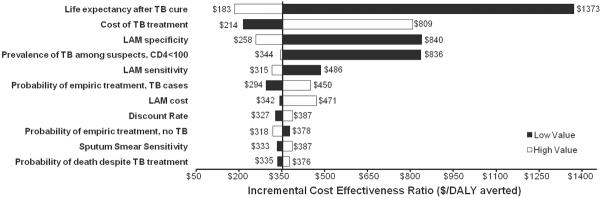
The vertical line represents the base-case scenario ($353 per DALY averted, see also Table 2). The black bars represent the incremental cost-effectiveness ratio at the low value of the sensitivity range for each parameter (Table 1), and the white bars represent the same ratio at the high value of the range.
Figure 3. Three-way Sensitivity Analysis: Effect of Assay Specificity, TB prevalence, and Life Expectancy after TB cure in South Africa.
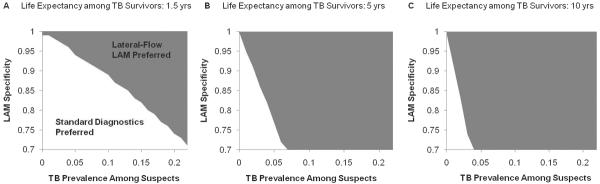
Cost-utility of lateral-flow urine LAM, according to specificity, TB prevalence, and life expectancy after TB cure in South Africa. Grey shading indicates that the existing diagnostic strategy (without Xpert MTB/RIF) would be preferred at a willingness to pay (WTP) of $7,275, while white shading indicates that addition of lateral-flow urine LAM would be preferred at this WTP threshold. Thus, for example, assuming a life expectancy of 1.5 years and assay specificity of 95%, lateral-flow urine LAM would be the preferred testing strategy in South Africa if deployed in populations with a probability of finding active TB that is greater than 5% (panel A, transition from white to grey).
Xpert MTB/RIF scenario
Assuming that sputum smear was replaced by Xpert MTB/RIF increased the incremental cost of urine LAM detection to $731 per DALY averted in South Africa (95% uncertainty range: $234–$12,397) and $208 in Uganda (95% uncertainty range $113–$2847). The probability of acceptability at a per-DALY willingness-to-pay threshold equal to the per-capita GDP of South Africa was 95.8%, and in Uganda was 84%.
DISCUSSION
In this population of hospitalized, severely immunocompromised, HIV-infected African adults, the addition of lateral-flow urine LAM testing to standard TB diagnostics detected an additional 80 cases of TB at an incremental cost per DALY averted of $353 in South Africa and $86 in Uganda. Relative to widely-used standards (e.g., per-capita GDP), diagnosis of TB using lateral-flow urine LAM is likely to be cost-effective in these populations. Although cost-utility was sensitive to life expectancy after TB cure, cost of TB treatment, LAM specificity, and the prevalence of TB, it remained below the per-capita GDP of both countries despite variation across a wide range of parameter estimates.
Findings of the diagnostic accuracy of urine LAM detection in this study are similar to those reported elsewhere.13,21 Lateral-flow urine LAM testing is a rapid point-of-care test, allowing for timely administration of TB therapy. However, patients with positive LAM results also tend to be the most ill, and there is currently no empirical evidence demonstrating that diagnosis of TB using urine LAM results in a survival benefit. Although the effectiveness of urine LAM detection depends on its ability to improve survival due to earlier diagnosis, patients who die also incur fewer TB treatment costs – which account for the majority of costs in our analysis. Thus, even though the total effectiveness (i.e., DALYs averted) of lateral-flow urine LAM detection varies closely with the projected survival of people who receive an early diagnosis, the cost-effectiveness (i.e., cost per DALY averted) of urine LAM does not depend strongly on offering a large survival benefit. We estimated that the cost of lateral-flow urine LAM detection per DALY averted would remain under South Africa's per-capita GDP even if it prevented death in only 3% of TB patients. Importantly, however, the question of whether a diagnostic test falls under a universal threshold of cost-effectiveness may be less important to decision-making than the determination of whether that test is more cost-effective than other available options. Future studies could directly compare the cost-effectiveness of the urine LAM assay directly with other HIV- and TB-related health interventions (e.g., Xpert MTB/RIF) to determine which interventions provide the most health impact per dollar invested.
In evaluating the determinants of cost-effectiveness for the lateral-flow urine LAM assay, treatment of patients without TB (i.e., false-positives) had great influence. For example, we estimated that, for every 1,000 patients in South Africa, $3,500 would be spent on materials for the lateral-flow assay, versus $21,250 to treat 25 false-positive cases. Additional empirical studies evaluating assay specificity under routine conditions and the mortality benefit provided by a positive test are necessary to better specify both the effectiveness and cost-effectiveness of lateral-flow urine LAM detection for TB diagnosis.
In addition to specificity, the cost-effectiveness of the LAM assay also depends on characteristics of the population in which it is used. Deployment of this assay in a population with lower TB prevalence and less immunosuppression (e.g., outpatients) might result in more false positives but might also provide more years of life to individuals with TB who were promptly treated. Clinical evaluation of urine LAM detection in outpatient settings might provide additional insights. Rapid initiation of HIV treatment is also essential if the benefit of urine LAM testing is to be optimized. In our sensitivity analysis, we found that the cost of LAM testing per DALY averted rose threefold when life expectancy after TB cure was decreased from 5 years to 1.45 years, as might be expected if antiretroviral coverage were incomplete.22,23
As with any modeling analysis, there are limitations to this study. We did not model reductions in transmission, though effects on TB transmission are likely to be small in this setting of high background mortality. Our data (e.g., TB prevalence, assay specificity, willingness-to-pay thresholds) may not generalize to countries with fewer resources or to the outpatient setting. For the sake of simplicity, we focused only on the costs and effectiveness associated with the lateral-flow urine LAM assay; the cost of HIV treatment was therefore not included. Inclusion of these costs might lower the cost-utility of urine LAM, as antiretroviral therapy – which would be recommended for all survivors in this analysis – is generally less cost-effective than TB diagnosis and treatment.24 Our determination of LAM specificity was based on the population with CD4>50 cells/μL, as false-negative TB culture results (the “gold standard”) were felt to be less likely in this subgroup; additional adjudication of all results has confirmed this specificity estimate (data not shown). Nevertheless, we did conduct sensitivity analysis for a specificity estimate as low as 70%, under which the LAM assay remained cost-effective. We also assumed that a negative LAM result would not affect clinical decision-making in this seriously ill population. To the extent that physicians would be less likely to treat TB cases with negative LAM results (i.e., false-negatives), our analysis may overestimate effectiveness; however, to the extent that true-negatives would also not be treated, we may also overestimate incremental costs. Finally, we assume that survival will improve for TB patients who are appropriately treated on the basis of a positive LAM test. Preliminary data suggest that patients testing positive with urine LAM may have higher background mortality, such that the survival benefit associated with an accurate diagnosis may be lower than expected.25
In conclusion, we found that lateral-flow detection of LAM in urine among HIV-infected, severely immunocompromised, hospitalized African adults is likely to be both an effective and cost-effective strategy for diagnosis of TB in high-burden settings, whether low income (Uganda) or upper-middle income (South Africa). Urine LAM represents the first true point-of-care diagnostic test for TB and likely provides an important and cost-effective benefit to this narrowly selected patient population, although this cannot be confirmed unless further studies demonstrate a survival benefit. Due to the narrow indication of this assay, broader control of the TB epidemic will depend will depend on our ability to develop novel develop novel technologies that that improve TB diagnosis in more general more general populations as well.
ACKNOWLEDGMENT
This project has been funded in part with United States Federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services, under Contract No: HHSN272200900050C.
References
- 1.Diedrich CR, Flynn JL. HIV-1/mycobacterium tuberculosis coinfection immunology: how does HIV-1 exacerbate tuberculosis? Infect. Immun. 2011;79(4):1407–1417. doi: 10.1128/IAI.01126-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bock NN, Jensen PA, Miller B, Nardell E. Tuberculosis infection control in resource-limited settings in the era of expanding HIV care and treatment. J. Infect. Dis. 2007;196(Suppl 1):S108–113. doi: 10.1086/518661. [DOI] [PubMed] [Google Scholar]
- 3.Getahun H, Gunneberg C, Granich R, Nunn P. HIV infection-associated tuberculosis: the epidemiology and the response. Clin. Infect. Dis. 2010;50(Suppl 3):S201–207. doi: 10.1086/651492. [DOI] [PubMed] [Google Scholar]
- 4.Lawn SD, Wood R. Tuberculosis in Antiretroviral Treatment Services in Resource-Limited Settings: Addressing the Challenges of Screening and Diagnosis. J Infect Dis. 2011;204(suppl_4):S1159–S1167. doi: 10.1093/infdis/jir411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anandaiah A, Dheda K, Keane J, et al. Novel Developments in the Epidemic of Human Immunodeficiency Virus and Tuberculosis Coinfection. Am. J. Respir. Crit. Care Med. 2011;183(8):987–997. doi: 10.1164/rccm.201008-1246CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cox JA, Lukande RL, Lucas S, et al. Autopsy causes of death in HIV-positive individuals in sub-Saharan Africa and correlation with clinical diagnoses. AIDS Rev. 2010;12(4):183–194. [PubMed] [Google Scholar]
- 7.Dowdy DW, O'Brien MA, Bishai D. Cost-effectiveness of novel diagnostic tools for the diagnosis of tuberculosis. Int. J. Tuberc. Lung Dis. 2008;12(9):1021–1029. [PubMed] [Google Scholar]
- 8.Vassall A, van Kampen S, Sohn H, et al. Rapid Diagnosis of Tuberculosis with the Xpert MTB/RIF Assay in High Burden Countries: A Cost-Effectiveness Analysis. PLoS Med. 2011;8(11):e1001120. doi: 10.1371/journal.pmed.1001120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andrews JR, Lawn SD, Rusu C, et al. The cost-effectiveness of routine tuberculosis screening with Xpert MTB/RIF prior to initiation of antiretroviral therapy in South Africa: a model-based analysis. [Accessed April 4, 2012];AIDS (London, England) 2012 doi: 10.1097/QAD.0b013e3283522d47. Available at: http://www.ncbi.nlm.nih.gov/pubmed/22333751. [DOI] [PMC free article] [PubMed]
- 10.Hughes R, Wonderling D, Li B, Higgins B. The cost effectiveness of Nucleic Acid Amplification Techniques for the diagnosis of tuberculosis. Respir Med. 2012;106(2):300–307. doi: 10.1016/j.rmed.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 11.Minion J, Leung E, Talbot E, et al. Diagnosing tuberculosis with urine lipoarabinomannan: systematic review and meta-analysis. Eur. Respir. J. 2011;38(6):1398–1405. doi: 10.1183/09031936.00025711. [DOI] [PubMed] [Google Scholar]
- 12.Chan J, Fan XD, Hunter SW, Brennan PJ, Bloom BR. Lipoarabinomannan, a possible virulence factor involved in persistence of Mycobacterium tuberculosis within macrophages. Infect Immun. 1991;59(5):1755–1761. doi: 10.1128/iai.59.5.1755-1761.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lawn SD, Kerkhoff AD, Vogt M, Wood R. Diagnostic accuracy of a low-cost, urine antigen, point-of-care screening assay for HIV-associated pulmonary tuberculosis before antiretroviral therapy: a descriptive study. [Accessed December 1, 2011];The Lancet Infectious Diseases. doi: 10.1016/S1473-3099(11)70251-1. Available at: http://www.sciencedirect.com/science/article/pii/S1473309911702511. [DOI] [PMC free article] [PubMed]
- 14.Shah M, Martinson NA, Chaisson RE, et al. Quantitative analysis of a urine-based assay for detection of lipoarabinomannan in patients with tuberculosis. J. Clin. Microbiol. 2010;48(8):2972–2974. doi: 10.1128/JCM.00363-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boehme C, Molokova E, Minja F, et al. Detection of mycobacterial lipoarabinomannan with an antigen-capture ELISA in unprocessed urine of Tanzanian patients with suspected tuberculosis. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2005;99(12):893–900. doi: 10.1016/j.trstmh.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 16.Dorman S, Manabe Y, Nicol M, et al. Accuracy of Determine LAM lateral flow test for diagnosis of TB in HIV+ adults: interim results of a multicenter study. 19th Conference on Retroviruses and Opportunistic Infections; Seattle. 2012. Abstract 149aLB. [Google Scholar]
- 17.World Health Organization [Accessed February 22, 2012];Global tuberculosis control 2011. Available at: http://www.who.int/tb/publications/global_report/en/.
- 18.Russell LB, Gold MR, Siegel JE, Daniels N, Weinstein MC. The role of cost-effectiveness analysis in health and medicine. Panel on Cost-Effectiveness in Health and Medicine. JAMA. 1996;276(14):1172–1177. [PubMed] [Google Scholar]
- 19.Boehme CC, Nicol MP, Nabeta P, et al. Feasibility, diagnostic accuracy, and effectiveness of decentralised use of the Xpert MTB/RIF test for diagnosis of tuberculosis and multidrug resistance: a multicentre implementation study. Lancet. 2011;377(9776):1495–1505. doi: 10.1016/S0140-6736(11)60438-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.South Africa | Data. The World Bank; [Accessed March 22, 2012]. Anon. Available at: http://data.worldbank.org/country/south-africa. [Google Scholar]
- 21.Shah M, Variava E, Holmes CB, et al. Diagnostic accuracy of a urine lipoarabinomannan test for tuberculosis in hospitalized patients in a High HIV prevalence setting. J. Acquir. Immune Defic. Syndr. 2009;52(2):145–151. doi: 10.1097/QAI.0b013e3181b98430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Actuarial Society of South Africa [Accessed February 8, 2012];ASSA2008 AIDS and Demographic Model. Available at: http://aids.actuarialsociety.org.za/ASSA2008-Model-3480.htm.
- 23.Manosuthi W, Chottanapand S, Thongyen S, Chaovavanich A, Sungkanuparph S. Survival rate and risk factors of mortality among HIV/tuberculosis-coinfected patients with and without antiretroviral therapy. J. Acquir. Immune Defic. Syndr. 2006;43(1):42–46. doi: 10.1097/01.qai.0000230521.86964.86. [DOI] [PubMed] [Google Scholar]
- 24.Bongaarts J, Over M. Public health. Global HIV/AIDS policy in transition. Science. 2010;328(5984):1359–1360. doi: 10.1126/science.1191804. [DOI] [PubMed] [Google Scholar]
- 25.Talbot E, Munseri P, Teixeira P, et al. Test Characteristics of Urinary Lipoarabinomannan and Predictors of Mortality among Hospitalized HIV-Infected Tuberculosis Suspects in Tanzania. PLoS ONE. 2012;7(3):e32876. doi: 10.1371/journal.pone.0032876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shang P, Xia Y, Liu F, et al. Incidence, Clinical Features and Impact on Anti-Tuberculosis Treatment of Anti-Tuberculosis Drug Induced Liver Injury (ATLI) in China. PLoS ONE. 2011;6(7):e21836. doi: 10.1371/journal.pone.0021836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Field N, Murray J, Wong ML, et al. Missed opportunities in TB diagnosis: a TB process-based performance review tool to evaluate and improve clinical care. BMC Public Health. 2011;11:127. doi: 10.1186/1471-2458-11-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martinson NA, Karstaedt A, Venter WF, et al. Causes of death in hospitalized adults with a premortem diagnosis of tuberculosis: an autopsy study. AIDS. 2007;21(15):2043–2050. doi: 10.1097/QAD.0b013e3282eea47f. [DOI] [PubMed] [Google Scholar]
- 29.Lawn SD, Ayles H, Egwaga S, et al. Potential utility of empirical tuberculosis treatment for HIV-infected patients with advanced immunodeficiency in high TB-HIV burden settings [Unresolved issues] The International Journal of Tuberculosis and Lung Disease. 2011;15(3):287–295. [PubMed] [Google Scholar]
- 30. [Accessed March 2, 2012];WHO | The global burden of disease: 2004 update. Anon. Available at: http://www.who.int/healthinfo/global_burden_disease/2004_report_update/en/index.html.
- 31.Mills EJ, Bakanda C, Birungi J, et al. Mortality by baseline CD4 cell count among HIV patients initiating antiretroviral therapy: evidence from a large cohort in Uganda. AIDS. 2011;25(6):851–855. doi: 10.1097/QAD.0b013e32834564e9. [DOI] [PubMed] [Google Scholar]
- 32.Brinkhof MWG, Boulle A, Weigel R, et al. Mortality of HIV-infected patients starting antiretroviral therapy in sub-Saharan Africa: comparison with HIV-unrelated mortality. PLoS Med. 2009;6(4):e1000066. doi: 10.1371/journal.pmed.1000066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.May M, Boulle A, Phiri S, et al. Prognosis of patients with HIV-1 infection starting antiretroviral therapy in sub-Saharan Africa: a collaborative analysis of scale-up programmes. Lancet. 2010;376(9739):449–457. doi: 10.1016/S0140-6736(10)60666-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abdool Karim SS, Naidoo K, Grobler A, et al. Integration of antiretroviral therapy with tuberculosis treatment. N. Engl. J. Med. 2011;365(16):1492–1501. doi: 10.1056/NEJMoa1014181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.WHO [Accessed June 7, 2012];Tuberculosis Finance Profile-Uganda. Available at: https://extranet.who.int/sree/Reports?op=Replet&name=%2FWHO_HQ_Reports%2FG2%2FPROD%2FEXT%2FTBFinancingCountryProfile&ISO2=UG&outtype=html.