Abstract
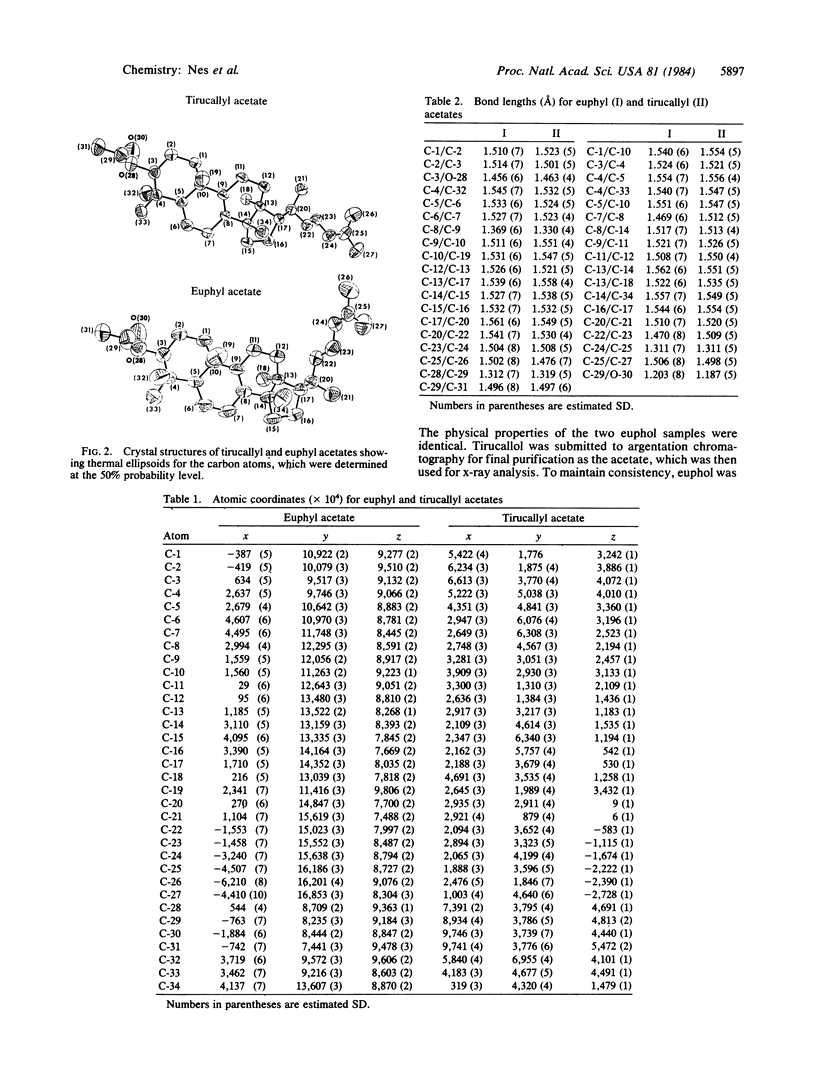
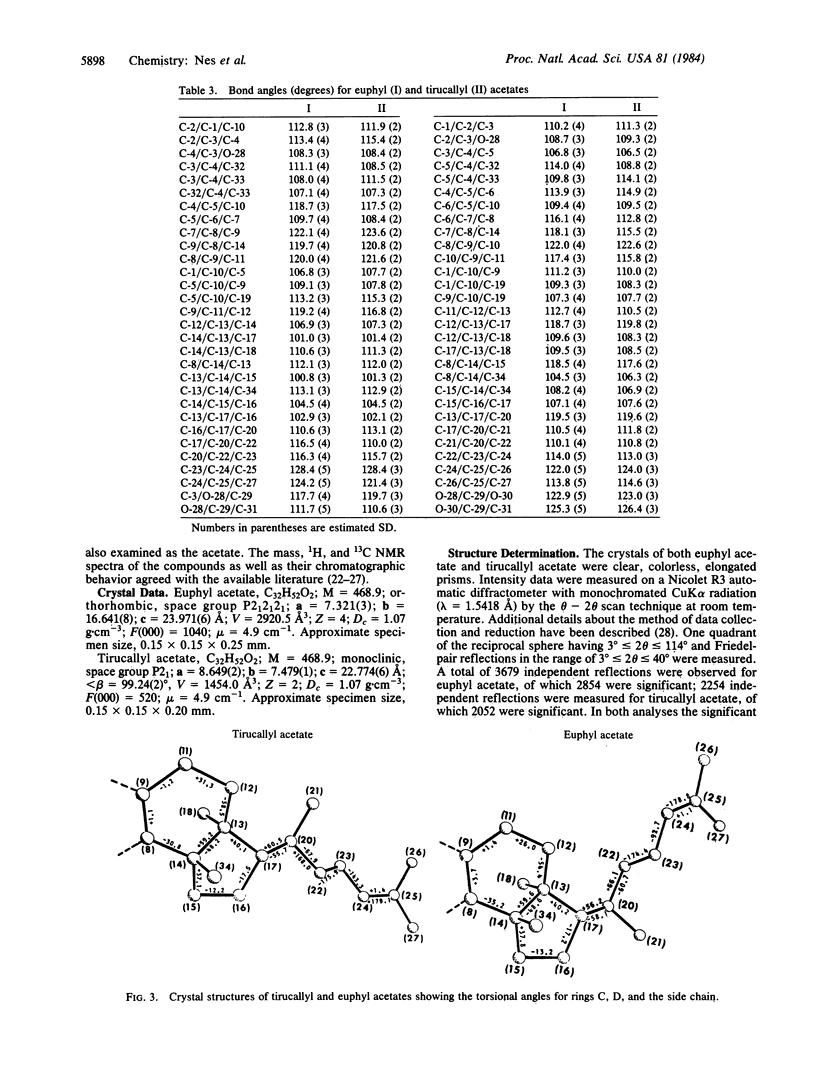
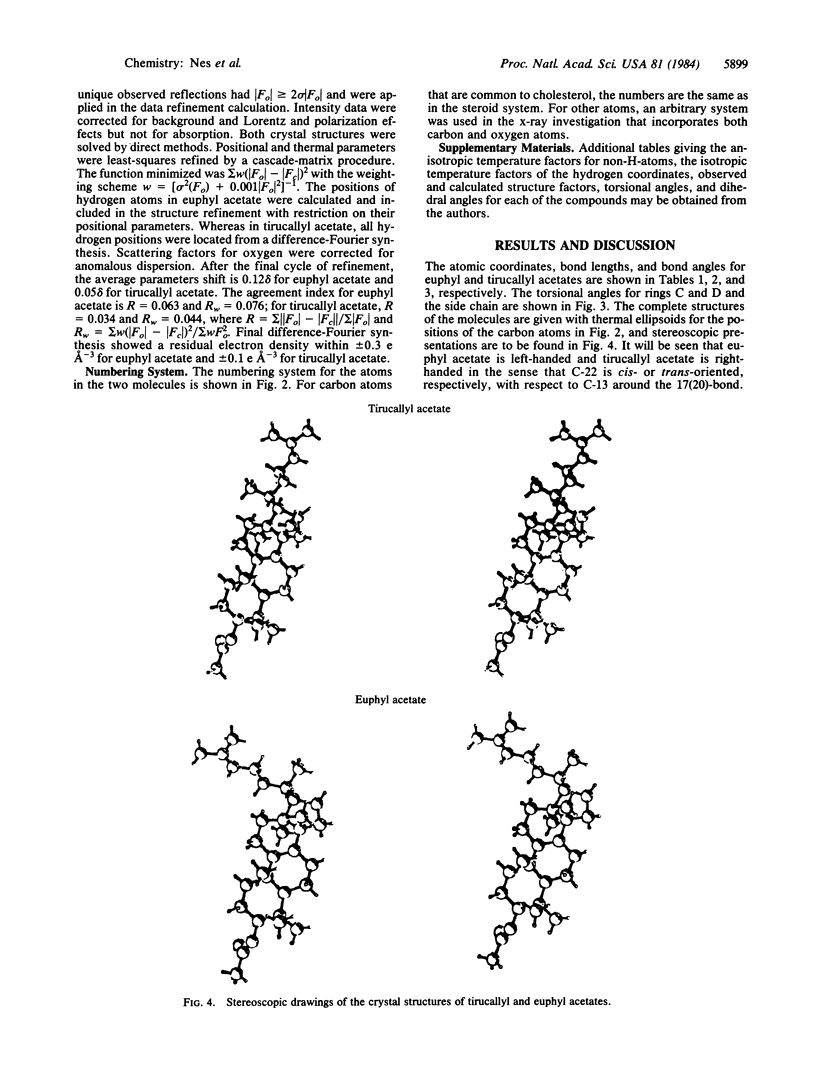
The influence of configuration at C-20 on rotation about the 17(20)-bond in steroids and euphoids was examined by x-ray crystallographic studies of the C-20 epimers euphol and tirucallol. The H atom on C-20 was in back next to C-18 in the crystal structures of both of the compounds, and C-22 was found to be cis-oriented ("left-handed") to C-13 in euphol and trans-oriented to it ("right-handed") in tirucallol. The results, which are consistent with the known left-handed crystal structure of 24(25)-dihydroeuphol and right-handed crystal structure of cholesterol and other natural sterols, lend further credence to the earlier suggestion that rotational isomerism at the 17(20)-bond can arise in C-20 epimers and that there is preference for an arrangement with the 20-H atom adjacent to C-18.
Full text
PDF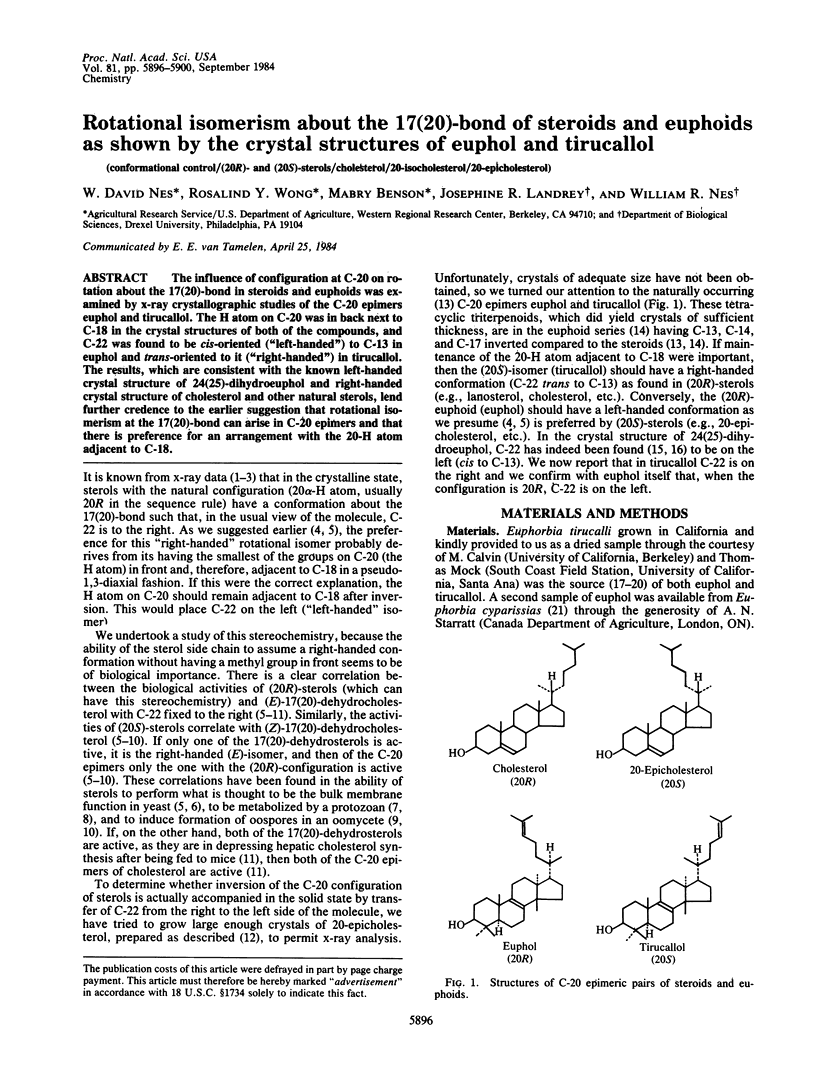

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Caspi E., Duax W. L., Griffin J. F., Moreau J. P., Wittstruck T. A. Letter: An unusual backbone rearrangement. The formation of 5alpha,17alpha-cholest-14-en-3beta-ol acetate from 5alpha-cholest-8(14)-en-3beta-ol acetate. J Org Chem. 1975 Jun 27;40(13):2005–2006. doi: 10.1021/jo00901a034. [DOI] [PubMed] [Google Scholar]
- Duax W. L., Griffin J. F., Rohrer D. C., Weeks C. M. Conformational analysis of sterols: comparison of X-ray crystallographic observations with data from other sources. Lipids. 1980 Sep;15(9):783–792. doi: 10.1007/BF02534032. [DOI] [PubMed] [Google Scholar]
- Erickson K. A., Nes W. R. Inhibition of hepatic cholesterol synthesis in mice by sterols with shortened and stereochemically varied side chains. Proc Natl Acad Sci U S A. 1982 Aug;79(16):4873–4877. doi: 10.1073/pnas.79.16.4873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T., Tamura T., Matsumoto T. Sterols, methylsterols, and triterpene alcohols in three Theaceae and some other vegetable oils. Lipids. 1974 Mar;9(3):173–184. doi: 10.1007/BF02532689. [DOI] [PubMed] [Google Scholar]
- Nes W. D., Patterson G. W., Bean G. A. Effect of Steric and Nuclear Changes in Steroids and Triterpenoids on Sexual Reproduction in Phytophthora cactorum. Plant Physiol. 1980 Nov;66(5):1008–1011. doi: 10.1104/pp.66.5.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nes W. R., Joseph J. M., Landrey J. R., Behzadan S., Conner R. L. Steric effects at C-20 and C-24 on the metabolism of sterols by Tetrahymena pyriformis. J Lipid Res. 1981 Jul;22(5):770–777. [PubMed] [Google Scholar]
- Nes W. R., Joseph J. M., Landrey J. R., Conner R. L. The steric requirements for the metabolism of sterols by Tetrahymena pyriformis. J Biol Chem. 1978 Apr 10;253(7):2361–2367. [PubMed] [Google Scholar]
- Nes W. R., Sekula B. C., Nes W. D., Adler J. H. The functional importance of structural features of ergosterol in yeast. J Biol Chem. 1978 Sep 10;253(17):6218–6225. [PubMed] [Google Scholar]
- Nes W. R., Varkey T. E. (Z)-17(20)-dehydrocholesterol. A new sterol with C-21 and C-22 spatially fixed. J Org Chem. 1976 Oct 15;41(21):3429–3433. doi: 10.1021/jo00883a023. [DOI] [PubMed] [Google Scholar]
- Nes W. R., Varkey T. E., Krevitz K. The stereochemistry of sterols at C-20 and its biosynthetic implications. J Am Chem Soc. 1977 Jan 5;99(1):260–262. doi: 10.1021/ja00443a054. [DOI] [PubMed] [Google Scholar]
- Pinto W. J., Lozano R., Sekula B. C., Nes W. R. Stereochemically distinct roles for sterol in Saccharomyces cerevisiae. Biochem Biophys Res Commun. 1983 Apr 15;112(1):47–54. doi: 10.1016/0006-291x(83)91795-3. [DOI] [PubMed] [Google Scholar]



