Abstract
Neuropeptides are found in many mammalian CNS neurons where they play key roles in modulating neuronal activity. In contrast to amino acid transmitter release at the synapse, neuropeptide release is not restricted to the synaptic specialization, and after release, a neuropeptide may diffuse some distance to exert its action through a G-protein coupled receptor. Some neuropeptides such as hypocretin/orexin are synthesized only in single regions of the brain, and the neurons releasing these peptides probably have similar functional roles. Other peptides such as neuropeptide Y (NPY) are synthesized throughout the brain, and neurons that synthesize the peptide in one region have no anatomical or functional connection with NPY neurons in other brain regions. Here, I review converging data revealing a complex interaction between slow-acting neuromodulator peptides and fast-acting amino acid transmitters in the control of energy homeostasis, drug addiction, mood and motivation, sleep-wake states, and neuroendocrine regulation.
Just as there are multiple perceptions for the proverbial blind men as to what an elephant is, there are numerous perspectives one can adopt to view neuropeptide modulation in the CNS. Here, I take the view that neuropeptide modulation in the CNS is inextricably linked with fast amino acid GABA and glutamate signaling. Many other viable perspectives exist and are not mutually exclusive. I have used a few examples of peptide secretion and actions which may be representative of many brain regions not discussed herein; many of the examples used here are from the hypothalamus, the part of the brain where neuropeptides have been most thoroughly studied. Many important neuropeptides are not included in the review. Although the focus here is on neuropeptides, some of the mechanisms of release and many of the mechanisms of response to neuropeptides may generalize to other neuromodulators in the brain, including the catecholamines, serotonin, adenosine, endocannabinoids, and neurotrophic factors. Neuropeptides can exert direct effects on neuronal physiology within seconds to minutes, and can also modulate gene expression over the course of hours to days; the focus here is on the direct neurophysiological actions.
The nomenclature of neuropeptides can initially be confusing. Names of CNS neuropeptides often give a historical perspective indicating what the peptide-pioneers initially discovered as the putative function. Since many neuropeptides were discovered in the context of regulation of hormone release, neuropeptide names may bear that functional link. True to its name, somatostatin released into the portal blood supply of the median eminence from nearby hypothalamic neurons can decrease growth hormone secretion from the pituitary gland; on the other hand, the somatostatin-synthesizing neurons in the cortex and hippocampus have no functional relation to hormone regulation. The same is true for thyrotropin releasing hormone in thalamic neurons, and vasopressin and gastrin releasing peptide in circadian clock neurons of the suprachiasmatic nucleus where the neuropeptide names have no bearing on their local function.
Where do peptides act?
Despite strong evidence showing substantive functional roles for many neuropeptides, at the cellular level a number of mysteries remain. Even seemingly straightforward questions can be complicated, such as: How far from a neuronal neuropeptide release site does a peptide act? For the amino acid neurotransmitters GABA, glycine, and glutamate, release occurs to a large degree at a presynaptic active zone, the transmitter diffuses a few tens of nanometers, activates receptors on the postsynaptic neuron, and then the transmitter is rapidly degraded or transported intracellularly. Amino acid transmitters act rapidly at ionotropic receptors, and at very discrete and spatially adjacent synaptic sites. Neuropeptides, in contrast, may be released from many additional release sites not restricted to the synaptic specialization, raising the question of where they act. For example in classic work on the frog sympathetic ganglia, a gonadotropin releasing hormone (GnRH)-like peptide was released by preganglion axons and acted on cells some microns away from the release site (Jan and Jan, 1982). Even in the case of non-synaptic release, a neuropeptide could still act on cells that are postsynaptic to the axon that releases it. For instance, GABAergic neuropeptide Y (NPY) cells of the arcuate nucleus make synaptic contact with other nearby arcuate nucleus neurons that synthesize proopiomelanocortins (POMC); NPY hyperpolarizes the POMC neurons (Cowley et al,2001), and therefore even though NPY may not be released synaptically, it can still exert an inhibitory effect on the cell postsynaptic to its parent axon.
A second possibility that has received considerable attention is that the peptide can diffuse long distances to act far from the release site. Very long distance signaling has been found for a number of neuroactive peptides/proteins. For instance, leptin from adipose tissue, ghrelin from the stomach, and insulin from the pancreas are released a long distance from the brain, but act on receptors within the CNS as signals of energy homeostasis. The blood brain barrier may prohibit entrance into the brain for many blood borne peptides; on the other hand, some regions of the brain such as the median eminence/arcuate nucleus may maintain a weak blood brain barrier which permits blood borne signals to enter the brain. Enhanced transport mechanisms may also exist for facilitating movement of some peptides into the brain. Long distance signaling within the brain has been called volume transmission and there is a substantial body of literature addressing this (Fuxe et al, 2005; 2007; Jansson et al, 2002). Consistent with long distance diffusion, neuropeptide receptors aided by G-protein amplification tend to be sensitive to low nanomolar concentrations of peptide; this compares to the substantially less sensitive ionotropic amino acid receptors that respond to micromolar quantities of GABA or glutamate. Furthermore, some peptides have been suggested to maintain a long extracellular half-life (Ludwig and Leng, 2006), thereby maintaining activity during the temporal window required for diffusion.
In many parts of the brain, the expression patterns of peptide-containing processes and the homologous peptide receptors overlap, consistent with a local action of the neuropeptide. But in a large number of CNS loci, the anatomical expression of a particular peptide and its receptors may be in completely different regions of the brain, as noted in the extensive review of such anatomical mismatches by Herkenham (1987). This peptide-receptor mismatch could be simply a non-functional throwback to some partial preservation of an interaction that was important in the evolutionary past, but is no longer relevant. Alternately, for peptides such as oxytocin, there may be massive release due to the simultaneous activation of a majority of oxytocin neurons within the brain; this can raise the extracellular oxytocin in the area of the supraoptic nucleus to a level 100-fold greater than circulating oxytocin (Ludwig and Leng, 2006), allowing diffusion of a higher concentration of peptide to activate oxytocin receptors at more distant sites than would be possible with asynchronous firing.
Arguing against long distance release and response as a general rule is the fact that a number of neuropeptides, for instance, NPY, dynorphin, or somatostatin, are synthesized and released by many unrelated groups of neurons in different regions of the brain. Any specific role of the peptide relevant to the releasing neuron would be negated if the same peptide from other brain regions was diffusing long distances. Furthermore, peptidases actively break down peptides extracellularly, reducing the effective distance an active peptide may diffuse. Depending on the size, presence of disulfide bonds which increase peptide half-life, amidation, and chemical confirmation of the peptide, peptide half-lives can vary.
Administration of a particular peptide or other modulator into a receptor-rich region of the brain lacking in that particular peptide can generate very selective functional responses, suggesting a functional plausibility to volume transmission. However, neuropeptide receptors simply respond to peptide, and even if the response is specific for a particular brain region or circuit, it may be simply a response of selective circuit activation or inhibition that may not normally occur. A more convincing strategy to show that long distance neuropeptide diffusion may play a functional role would be the use of a receptor antagonist in a region lacking specific peptidergic inputs to show the opposite effect of peptide injection; but even the receptor antagonist strategy can be complicated, as some receptor antagonists may act as inverse agonists, reducing a constitutively active receptor to a level below a normal partially-active state.
For the majority of axons in the CNS that release neuropeptides, I favor a third local diffusion hypothesis-that neuropeptides released by most neurons act locally on cells near the release site, with a distance of action of a few microns. Thus a peptide's action would be on its synaptic partners (even if the peptide is not released at the presynaptic specialization) and on immediately adjacent cells. In part this perspective is based on the low frequency of dense core vesicles in most CNS axons and the hours it would take to replenish released peptides from sites of synthesis in the cell body, making it difficult to achieve a substantial extracellular concentration of neuropeptide needed for a long distance effect. In this context, the relatively slow replenishment of neuropeptide modulators may differ from catecholamine neuromodulators that can be synthesized rapidly within axon terminals to support ongoing release. Furthermore, as determined with ultrastructural analysis, a complex system of astrocytic processes surrounds many axo-dendritic synaptic complexes, and tends to attenuate long-distance transmitter diffusion from many release sites (Fig. 1) (Peters et al, 1991), thereby impeding actions of peptides at far-away targets, and maintaining a higher local extracellular concentration of the peptide. Peters et al credit Ramon y Cajal with favoring the concept that a central function for glia was isolation of neuronal microdomains.
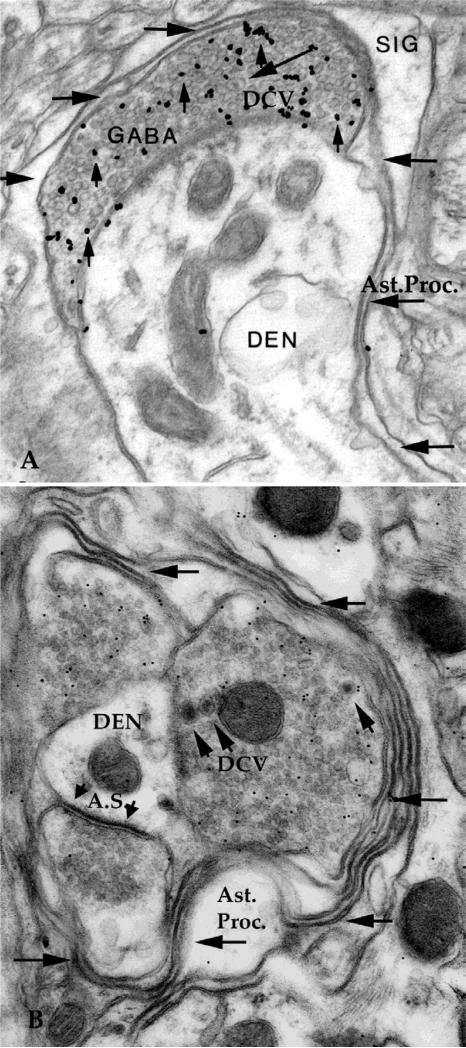
Fig. 1.
A. Astrocytic processes (Ast.Proc.) surround presynaptic axons in contact with central dendrite (DEN). SIG, silver intensified immunogold, DCV, dense core vesicle. Horizontal arrows show astrocytic process surrounding synaptic complex. B. Three boutons contact a central dendrite (DEN). Two GABA boutons are labeled with immunogold, a third bouton makes an asymmetrical synapse (A.S.) typical of glutamate synapses. The synaptic complex is surrounded by several layers of astrocytic processes (Ast.Proc.). Width of micrograph, A 2 um, B 1.7 um.
That peptides released by most neurons may act within a few microns of the release site does not negate the fact that some peptides can be released in large quantities and can act at longer distances. This may be the exception rather than the rule. For instance, considering the multiple subtypes of highly specialized NPY or somatostatin interneurons in the hippocampus or cortex, coupled with the multiple peptide responses reported in nearby cells and the highly specialized functions of different nearby interneurons, often with restricted functional microdomains (Freund and Buzsaki,1996; Bacci et al,2002; Klausberger et al, 2003), it seems most likely that released peptides here act primarily on nearby receptive partners.
Consistent with the local diffusion perspective are findings related to peptides such as pigment dispersing factor (PDF) which plays a key role in regulating circadian rhythms of invertebrates (Im and Taghert,2010; Zhang et al, 2010). Although cells that release PDF project to several regions of the Drosophila brain, the response of the releasing cells to PDF appears to be critical for some aspects of circadian function. Secreted PDF acts on PDF autoreceptors expressed by the releasing lateral-ventral pacemaker neurons to regulate the time of day during which behavioral activity occurs (Choi et al, 2012),
G-protein coupled receptors
Most neuropeptides act by binding to a seven-transmembrane domain G-protein coupled receptor (GPCR). Many hundreds of these receptors have been identified and their normal ligand is known; the ligands for a number of orphan GPCRs have not yet been identified (see Civelli, this issue). Binding to the GPCR induces a conformational change in the receptor, leading to activation of intracellular G-proteins. Many G-proteins exist in an inactive heterotrimeric form consisting of Gα, Gβ, and Gγ. Activation results in an exchange of GDP for GTP at the G-protein's α-subunit, and the dissociation of the G-proteins from the GPCR. Peptide signaling is then amplified by the induction of multiple intracellular signaling pathways that may involve adenylyl cyclase, cAMP, MAPK/ERK, PKA, and phosphorylation of a number of target proteins. Monomeric G-proteins may also play a role in modulating some ion channels and actions of peptides (Murray and O'Connor,2004; Vogler et al, 2008; Thapliyal et al, 2008), and multiple G-protein/effectors have been described for some neuropeptides, for instance GnRH (Gardner and Pawon, 2009). The actions of neuropeptides on GPCRs can also be modulated at the receptor or effector level; for instance, members of the RGS (regulator of G-protein signaling) family of proteins can accelerate activation or deactivation of G-proteins, and may alter receptor-effector coupling (Chuang et al, 1998; Doupnik et al, 2004; Labouebe et al, 2007; Xie and Martemyanov, 2011). The literature on GPCRs is too voluminous to examine here, but has been addressed in some recent reviews (Rosenbaum et al, 2009; Hazell et al, 2012).
Peptide receptors are found heterogeneously distributed throughout the brain, and can be expressed on cell bodies, dendrites, and axon terminals. Some peptides, for instance NPY, activate multiple different receptors expressed by target neurons, whereas others appear to act primarily on a single receptor, for instance kisspeptin acts primarily on GPR54. Our understanding of peptide receptor subcellular localization has lagged behind that of amino acid receptor localization, in part due to questionable specificity of some peptide receptor antisera. Perhaps the clearest picture that emerges of a class of neuronal GPCRs is for metabotropic glutamate receptors (mGluRs). These function similarly to neuropeptide GPCRs, but are activated by glutamate and can act in an excitatory or inhibitory manner. Subcellular localization of mGluRs may provide some insight into the potential localization of neuropeptide GPCRs. Eight different mGluRs have been identified, and interestingly, are expressed in different regions of different neurons. mGluR7, for instance, is often found at the presynaptic active zone (Schoepp,2001) and mGluR4,-7α, and -8α are found on the presynaptic active zone of inhibitory axons, and only those innervating other GABA interneurons but not those innervating excitatory pyramidal cell (Kogo et al,2004). mGluR1α is found on the postsynaptic membrane at the periphery of the synapse active zone (Baude et al,1993); other mGluRs tend to be either pre- or postsynaptic, depending on the expressing neuron and mGluR subtype (Bradley et al, 1996). Future experiments on the ultrastructural localization of neuropeptide receptors may show similar sites of expression at specific regions of the plasma membrane.
Which neurons contain neuropeptides?
The classic view that neuropeptide-containing neurons represented an unusual type of neuron is giving way to the perspective that many, perhaps most neurons in the brain, probably contain some neuropeptide(s) or other neuromodulator in addition to fast-acting amino acid neurotransmitters. In an examination of individual sections containing synaptic boutons with electron microscopy, with the boutons fixed to preserve the dense core of vesicles, some boutons appeared to contain only clear vesicles, others contained clear and DCVs. However, serial ultrathin section reconstruction of GABA-immunogold labeled presynaptic boutons from the paraventricular nucleus demonstrated that every bouton contained at least a few dense core vesicles, suggesting that in addition to a fast amino acid transmitter, most if not all GABAergic axons here also contained some neuromodulator (Decavel and van den Pol,1990). Release and actions of these neuromodulators remains to be demonstrated. Furthermore, because the axons studied contained GABA which is not found in magnocellular neurons, the profiles could not arise from the local oxytocin or vasopressin neurosecretory cells. Similarly, presynaptic boutons showing no immunogold GABA labeling, many of which were probably glutamatergic, also showed a similar frequency of DCVs in boutons, interspersed with small clear vesicles. A complication to the detection of DCVs with electron microscopy is that the dense core can be lost by suboptimal fixation pH, duration, chemistry, and osmotic pressure (Morris and Cannata, 1973), complicating detection in some studies and biasing results toward a false-negative lack of detectable DCVs.
A related question is whether all peptidergic axons also contain a fast amino acid transmitter. Most evidence, including that based on immunocytochemistry, calcium digital imaging, and electrophysiology supports the perspective that the great majority of peptidergic cells also employ fast amino acid transmitters (van den Pol,1991;2003; van den Pol et al,1990; van den Pol and Trombley, 1993; Freund and Buzsaki,1996).
Whereas hypothalamic neurons have long been recognized as utilizing a large number of peptides, other regions of the brain are now being seen as not substantively different in this regard. For instance, in the hippocampus, a region with a rich history in the study of fast GABA and glutamate transmission, a plethora of neuropeptides are synthesized, particularly by GABAergic inhibitory interneurons, including neuropeptide Y, somatostatin, vasoactive intestinal polypeptide, cholecystokinin, dynorphin, enkephalin, neurokinin B, and substance P (Acsády et al,1996;2000; Billova et al,2007; Antonucci et al,2012; Dun et al,1994; Bering et al,1997; Freund and Buzsáki,1996). Hippocampal pyramidal cells, often used as a primary model for the study of glutamatergic neurons, are reported to express peptides, for instance cholecystokinin (Wyeth et al, 2012) particularly in models of brain disease such as epilepsy. From an evolutionary perspective, peptide synthesis in invertebrates may give us clues as to the parallel in vertebrates. In Aplysia, every identified motorneuron was found to contain one or more of a number of different peptide modulators (Church and Lloyd,1991).
Neuropeptide release
Scientists have a keen insight into the temporal sequence and many of the molecules involved in the release of fast neurotransmitters at presynaptic specializations (Sudhof, 2012). Release of neuropeptides, mostly from non-synaptic sites, has received considerably less attention than fast transmitter release; neuropeptide release from dense core vesicles (DCVs) may require a unique set of proteins that regulate transport and release (Sieburth et al, 2005; 2007). Mammalian neuropeptide release has been most thoroughly investigated in the neurohypophysis where axons arising from magnocellular neurons of the hypothalamic paraventricular and supraoptic nuclei converge to release vasopressin or oxytocin into the vascular system. Vasopressin plays a key role in water homeostasis and water reabsorption in the kidney, and oxytocin acts to evoke milk release during lactation. The neurohypophysis provides a good model to study release, as it contains a high density of large axon terminals filled with large (180-200 nm diameter) dense core neurosecretory vesicles, providing a relatively high and measurable amount of peptide release. Classical work here has shown that the amount of neuropeptide released per spike increases with spike frequency up to a point (Dreifuss et al, 1971; Gainer et al, 1986) and that spike bursts followed by intervals of silence are particularly effective at releasing oxytocin or vasopressin (Dutton and Dyball, 1977; Bicknell and Leng, 1981; Cazalis et al, 1985). A mechanism that has been reported to underlie this enhanced-release phenomenon is the increase in cytoplasmic calcium in axon terminals induced by spike bursts which may be a key to the enhanced probability of DCV exocytosis (Bondy et al, 1987; Jackson et al, 1991; Muschol and Salzberg, 2000).
Although the neurohypophysis provides a useful model for studying neuropeptide release, there are some serious differences between peptide release from large neurohypophyseal boutons filled with large neurosecretory vesicles and peptide release from the more common small axon terminals that may possess medium size (100 nm diameter) neuropeptide-containing DCVs; large DCVs have been estimated to contain 60,000 (Dreifuss,1975) or 85,000 (Nordmann and Morris,1984) molecules of oxytocin or vasopressin. The volume of the medium size DCVs in most neurons is roughly about 1/8 of the volume of the large DCV in magnocellular neurons, suggesting a similarly reduced peptide content (Fig. 2). Most axons that release neuropeptides contain only a small number of DCVs that show no preferential localization near presynaptic specializations, in contrast to glutamate- or GABA-containing small clear vesicles that tend to congregate in the active zone near the synaptic specialization (Fig. 2, 3). Unlike the small clear vesicles that can be re-filled with amino acid transmitter by vesicular transporters locally within the axonal bouton, neuropeptides are synthesized on the rough endoplasmic reticulum, and loaded into DCVs that are generated in the Golgi apparatus of the cell body, and DCVs must be transported down long thin axons for release at sites distant from the cell body. The relatively small number of DCVs in axon terminals of most neurons suggests that neuropeptide release from boutons in the CNS is under considerably different spatial and temporal constraints than release from the neurohypophysis. If the small number of DCVs in a single CNS bouton undergo exocytosis, it may be at least several hours before replenishment.
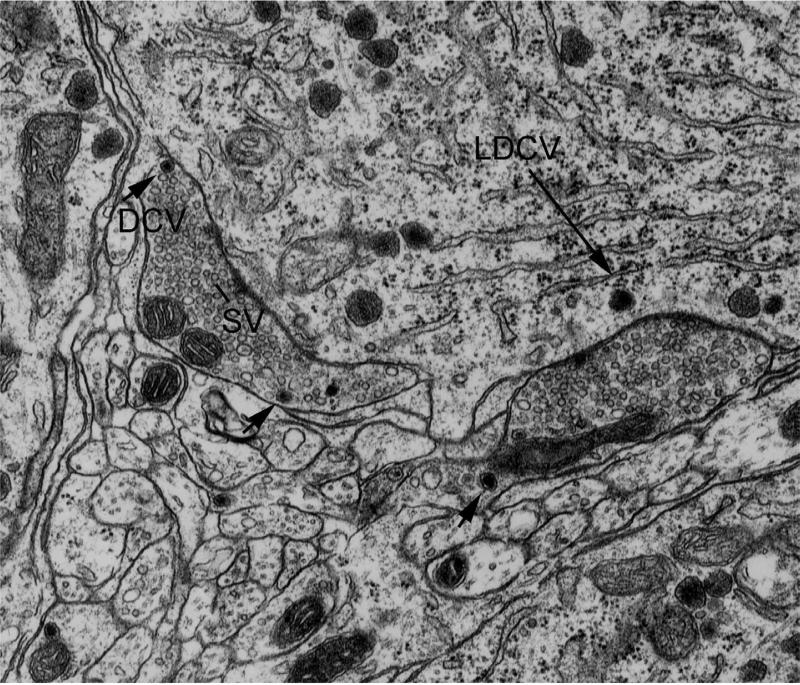
Fig. 2.
Two axons make symmetrical-type synaptic contact with a magnocellular neuron. The presynaptic axons contain a few dense core vesicles (DCV, arrowheads) and many small clear synaptic vesicles (SV). In the postsynaptic neuron, a large dense core vesicle (LDCV) is shown by long arrow. Width of micrograph, 4 um.
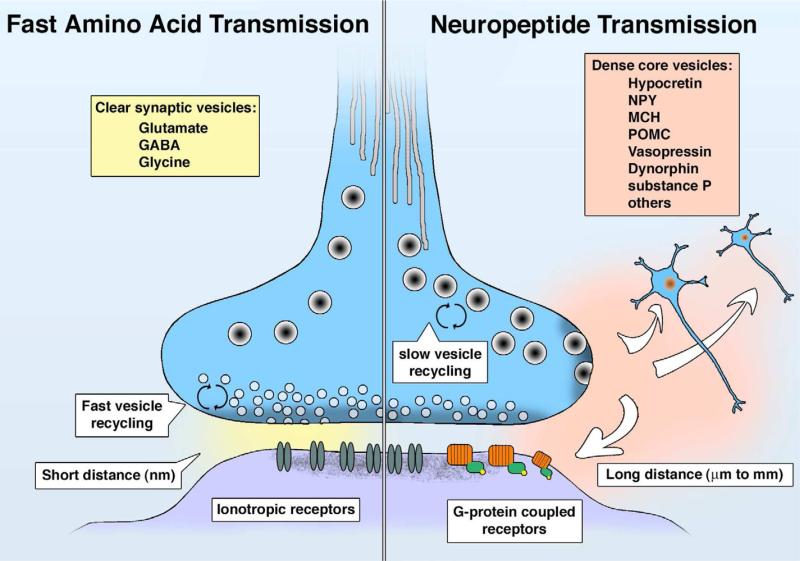
Fig. 3.
Comparison of fast amino acid synaptic transmission (left) and slower neuropeptide transmission (right).
Invertebrate neurons have proven useful for the study of vesicle transport and release (Church et al, 1993; Whim and Lloyd,1992). Recent imaging evidence in invertebrate neurons suggests that neuropeptide-containing DCVs are transported in a seemingly inefficient manner, and shuttle back and forth between the cell body and distal axon terminal. These DCVs move in an anterograde direction on microtubules with the motor kinesin-3 (Barkus et al,2008), and then switch to dynein for a ride in the retrograde direction back to the axon initial segment where the direction again may be reversed, with only a minority of DCVs moving into boutons during each trip (Wong et al, 2012). In mammalian trigeminal ganglion neurons in vitro, neuronal stimulation reduced anterograde velocity of DCVs, and increased DCV pausing. As determined with pHluorin, DCV membrane fusion and release occurred throughout the axon and in axonal growth cones (Sobota et al, 2010).
A question that often arises when confronted with a peptide-immunoreactive axon in apparent contact with another cell is whether the axon makes a synapse with its putative partner, theoretically therefore increasing the potential role of the peptide. But maybe it is irrelevant if the immunoreactive axon makes that synapse if peptides are released at non-synaptic sites and generally diffuse a few microns to activate nearby cells.
Why is peptide release difficult to study? Much of what we know about fast transmitter release arises from the electrophysiological response to the released transmitter. Glutamate and GABA both generate a very rapid msec response at the postsynaptic specialization that can be easily detected as a shift in voltage or current recorded from the postsynaptic neuron. In contrast to the fast amino acid transmitters, as noted above, the amount of neuropeptide released by a single bouton in the CNS may be small, but even more importantly, the response of receptive cells is relatively slow, on the order of many seconds to minutes, making it difficult to correlate peptide release with a response, and difficult to use quantal analysis of single or multiple exocytotic events. Additionally, the rise time of a glutamate- or GABA- mediated synaptic event is relatively fast, facilitating the segregation of individual events, whereas the rise time for a neuromodulator is much slower. Finally, whereas a single spike may release GABA or glutamate, peptide release may require a higher level of activity, further confounding the study of stimulus response relationships. Capacitance recordings have also proven useful to study fusion of large DCVs and small clear vesicles in magnocellular axon terminals of isolated neurohypophyses (Klyachko and Jackson, 2002) and in isolated magnocellular neuron cell bodies (de Kock et al, 2003).
Peptides can be genetically labeled with a fluorescent reporter such as GFP and examined microscopically, assuming controls are used to ensure that the reporter does not alter peptide transport and release (Lang et al, 1997; Burke et al,1997). Release of fast transmitters has been studied with lipophilic dyes such as FM1-43 to detect dye internalization upon vesicle fusion (Ryan and Smith, 1995) and with a number of interesting genetically encoded agents, for instance pHluorin, a GFP variant with pH sensitivity (Pan and Ryan,2012; Ariel and Ryan, 2010; Kim and Ryan,2010), but these approaches have been used only to a limited degree in the study of peptide release from boutons in the CNS (e.g., Fuenzalida et al, 2011).
One promising approach in the mammalian CNS is the use of the invertebrate neuropeptide FMRF that directly opens an ion channel resulting in an inward Na+ current, independent of G-protein coupling (Lingueglia et al, 1995). The FMRF peptide and its receptor can be expressed in mammalian cells to study fast responses to released peptide (Whim and Moss, 2001). This FMRF approach has been employed to study neuropeptide release from secretory endocrine cells, including pancreatic beta cells (Whim, 2011) and adrenal chromaffin cells where co-release of neuropeptide and catecholamines from single vesicles was reported (Whim, 2006).
Another dimension of neuropeptide release is whether it is constitutive (ongoing) or actively regulated. Ongoing release may result in desensitization of receptors, and a decrease in response amplitude, or it may result in a chronically active receptor. Different responses have been found with slow and fast release of brain derived neurotrophic factor (BDNF). Acute activation by BDNF of the TrkB receptor resulted in developing hippocampal neuron neurite elongation, whereas sustained activation was more likely to initiate neurite branching, and the two modes of release also differentially regulated expression of Homer1 and Arc (Ji et al, 2010).
Dendritic release of neuropeptides
Peptides may be released locally by the somatodendritic complex. Based on ultrastructural analysis of omega membrane-fusion/ release figures in fixed mammalian supraoptic nucleus after high K+ or calcium ionophore A23187 stimulation, suggestive evidence of neuropeptide exocytosis was found occasionally at the presynaptic and perisynaptic membrane, but more often independent of synaptic specializations, and was found in the cell body, dendrites, axonal boutons, and axon shafts (Morris and Pow,1991). Neuropeptide release from the somatodendritic complex of magnocellular neurons may provide a unique insight into release mechanisms and peptide signaling in general. Again, the neurosecretory cells of the supraoptic nucleus of the hypothalamus (Fig. 4) provide a model system in which to study dendritic release. The model is aided by the high level of neuropeptide synthesized by magnocellular neurons, the presence of a large number of large peptide-containing DCVs in the dendrites, and key to the interpretation of many of the results, the probable absence of local axon terminals originating from magnocellular neurosecretory cells. Magnocellular axons project primarily to non-synaptic terminals in the neurohypophysis. In the paraventricular nucleus but not in the supraoptic nucleus, parvocellular neurons also synthesize oxytocin and vasopressin; axons from these parvocellular neurons do not target the neurohypophysis, but instead make synaptic contact with other CNS neurons in the brain and spinal cord (Hosoya and Matsushita, 1979; Sawchenko and Swanson, 1982; Swanson and Kuypers, 1980).
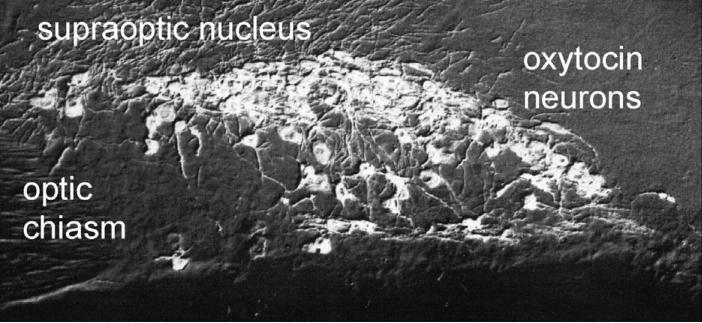
Fig. 4.
This micrograph shows the oxytocin neurons of the hypothalamic supraoptic nucleus. Width of micrograph, 350 um.
Increases in action potential frequency generally enhance release of neuropeptides from both axons and dendrites. A key ion in release of both fast amino acid transmitters and peptides is calcium; peptide release may require a greater increase in cytoplasmic calcium, and possibly greater neuronal activity, than needed for amino acid secretion (Tallent, 2008). Depolarization of the membrane potential activates voltage-gated calcium channels, leading to calcium influx through the plasma membrane, and initiation of vesicle release. Several lines of evidence suggest the intriguing possibility that dendritic release may be regulated in a manner independent from axonal release under some circumstances. In part, differences in release may be dependent on different sets of ion channels in axons and dendrites. For instance, different calcium channels may underlie dynorphin release from hippocampal dendrites and axons; activation of L-type calcium channels enhanced release from dendrites, but not axons (Simmons et al, 1995). Depolarization-mediated oxytocin release from supraoptic neuron dendrites was dependent primarily on N-type calcium channels and to a lesser extent, P/Q channels; other calcium channels played no substantive role in mature oxytocin neurons (Tobin et al, 2011; Hirasawa et al, 2001). In parallel, calcium channel-independent mechanisms differ between axons and cell body; the endoplasmic reticulum plays a role in calcium regulation/ peptide release from the oxytocin cell body, but does not appear to play a substantive role in the axon terminal. Differences between axon terminals and cell bodies may also be found in systems that reduce calcium, including mitochondria, plasma membrane calcium pumps, and sodium/calcium exchangers (Dayanithi et al, 2012). Finally, ATP coreleased from magnocellular neurons exerts different feedback effects on axon terminals and cell bodies, potentially differentially regulating peptide release, in part due to different sets of ATP receptors on axons and cell bodies (Lemos et al, 2012).
Calcium can also be released into the cytoplasm from intracellular stores, particularly the endoplasmic reticulum. Oxytocin, or other agents such as thapsigargin that induce calcium release from intracellular stores into the cytoplasm, can directly evoke dendritic release of oxytocin or vasopressin independent of action potentials (Lambert et al, 1994; Ludwig et al, 2002). Release of intracellular calcium can also prime the system for enhanced release upon subsequent increases in electrical activity (Ludwig et al, 2002; Ludwig and Leng, 2006). Oxytocin receptor activation induces phospholipase C resulting in production of IP3 and subsequent release of calcium from the endoplasmic reticulum. This priming enhances the subsequent release of oxytocin, potentially related to actin-dependent movement of peptide-containing granules toward the plasma membrane (Tobin and Ludwig, 2007; Leng et al, 2008). Priming of oxytocin-laden DCVs, in part by movement of the DCVs to a position closer to the plasma membrane, allows a substantial amplification of oxytocin release with subsequent electrical activity. Interestingly, priming with thapsigargin can increase the K+ mediated depolarization-induced oxytocin release for an extended period of 90 minutes (Ludwig et al, 2002). Priming of DCV release has been studied outside the brain, particularly in pituitary cells that synthesize luteinizing hormone; axonal release of gonadotropin releasing hormone (GnRH) from preoptic neurons into the portal blood supply of the median eminence primes the luteinizing hormone cells by multiple mechanisms to show an enhanced release in response to subsequent GnRH stimulation (Leng et al, 2008; Fink, 1995).
Differential expression of proteins involved in exocytosis in dendrites and axon terminals may also account for differences in release. In magnocellular axon terminals in the neurohypophysis, VAMP-2, SNAP-25, and syntaxin-1 are found near oxytocin and vasopressin-containing dense core vesicles; in contrast, the dendrites of the same cell type contain syntaxin-1, but SNAP-25, VAMP-2 and synaptotagmin-1 show no colocalization with oxytocin or vasopressin (Tobin et al, 2012). Synthesis of neuropeptides generally occurs in the cell body, but has also been reported in dendrites. Dendritic synthesis of neuromodulators such as BDNF has been suggested as playing a critical role in the development of cortical dendritic spines (Kaneko et al, 2012), and in responses of hypothalamic neurons to leptin to control energy homeostasis (Liao et al, 2012).
What function would a dendritically released neuropeptide play? The most probable role would be that the neuropeptide acts to signal other nearby neurons to either increase or decrease activity. In the olfactory bulb, most of the neurons, including mitral, periglomerular, and granule cells possess dendrites that release either GABA or glutamate at presynaptic specializations (Shepherd et al, 2004). Many of the presynaptic dendrites are organized in a reciprocal manner; for instance, mitral cell dendritic release of glutamate activates a presynaptic granule cell dendrite that releases GABA back onto the mitral cell, resulting in feedback inhibition. In contrast, most dendrites in the brain are not presynaptic to other cells, and dendritic release of peptides appears to be independent of synaptic specializations. Non-synaptic release of oxytocin or vasopressin could serve to recruit or inhibit neighboring cells, or to synchronize activity. Oxytocin receptors are expressed by oxytocin neurons (Freund-Mercier et al, 1994), and vasopressin receptors by vasopressin cells (Hurbin et al, 2002). During lactation, oxytocin is released in an orchestrated burst where many or most oxytocin neurons fire rapidly for a brief period of about a second (Amstrong and Hatton, 2006; Leng et al, 2008). Intermittent bursts of oxytocin release may prevent oxytocin receptors in the mammary gland from desensitizing if oxytocin levels were to remain at statically raised levels. The burst of oxytocin potentially appears to be dependent on dendritic release of oxytocin that primes the cells for subsequent massive oxytocin release induced by an increase in spike frequency, as described above.
Dendritically released peptides can act to initiate retrograde signals to modulate subsequent release of fast amino acid neurotransmitters from local axons. Oxytocin released by magnocellular cell bodies and dendrites reduces presynaptic glutamate and GABA release; although this was initially thought to be mediated by presynaptic peptide receptors, it appears more likely that oxytocin release activates receptors on oxytocin cells, resulting in release of an endocannabinoid that diffuses in a retrograde direction to activate CB1 receptors on presynaptic axons and thereby reducing fast transmitter release (Kombian et al, 1997;2002;Hirasawa et al, 2001; Hirasawa et al, 2004; Leng et al,2008). Oxytocin release appears to be obligatory to achieve this presynaptic inhibition after depolarization of oxytocin neurons (Hirasawa et al, 2004). Blockade of synaptic activity transiently isolates oxytocin cells from external influences, potentially amplifying local cellular interactions. Here, the modulatory peptide that activates endocannabinoid secretion is released from dendrites to inhibit nearby presynaptic axons; a key feature of endocannabinoid modulation in many brain regions is release from the postsynaptic neuron and retrograde diffusion to modulate fast transmitter release from a presynaptic axon, resulting in either short or long-lasting attenuation of GABA or glutamate release by depression of presynaptic calcium, increase in potassium conductance, or direct inhibition of vesicle fusion (Lovinger,2008). In vasopressin neurons, depolarization-induced release of endocannabinoids also attenuated presynaptic GABA synaptic activity by a calcium-dependent mechanism; in addition, induced release of vasopressin reduced IPSC frequency by a second cannabinoid-independent mechanism (Wang and Armstrong 2012).
Neurons in the preoptic/septal area synthesize GnRH. These neurons may also release peptide from their dendrites to orchestrate activity of other nearby GnRH neurons. Studies on fetal primate GnRH neurons found FM 1-43 labeling increased in cell body and dendrites with increased activity, and suggested colocalization of FM1-43 with GnRH immunoreactivity (Fuenzalida et al, 2011); further corroboration with imaging of mature neuron somatodendritic release from live GnRH cells would complement the histology.
The magnocellular neurosecretory neurons provide a good model in which to study dendritic release of peptides, but as with axonal release, these cells contain a substantially greater number of peptide-containing DCVs, probably by a couple of orders of magnitude, than other peptide- releasing neurons that do not maintain a prominent projection to the median eminence or neurohypophysis. Whether other neurons with more modest expression of peptides follow the same model of dendritic release is possible, but merits further exploration.
Neuropeptide modulation of GABA and glutamate synaptic actions
Most fast synaptic activity in the brain is due to synaptic release of excitatory glutamate or inhibitory GABA or glycine. Modulation of fast amino acid synaptic activity is a key target of CNS neuropeptides. Classically, signaling in regions of the brain such as the hypothalamus involved in homeostatic regulation have been seen as being based on direct peptidergic actions. A number of early reviews on the transmitters of the hypothalamus either ignored GABA and glutamate, or included only a brief mention of them. In contrast, signaling in higher regions of the brain such as the hippocampus and cortex were seen primarily as being based on GABA and glutamate transmission, with less consideration of neuropeptide modulators. This dichotomy has shown a strong convergence in recent years, with a greater appreciation of fast transmitters in the more vegetative regions of the brain, and more inclusion of neuropeptide modulation in higher brain regions. Although peptide action in the CNS is not restricted to modulation of fast synaptic activity, most actions of peptides do alter GABA or glutamate signaling at post- or presynaptic sites. Figure 5 shows an electrophysiological example of an excitatory peptide, TRH, that enhanced the activity of local presynaptic GABA neurons, resulting in an increased inhibition in GABAergic MCH neurons.
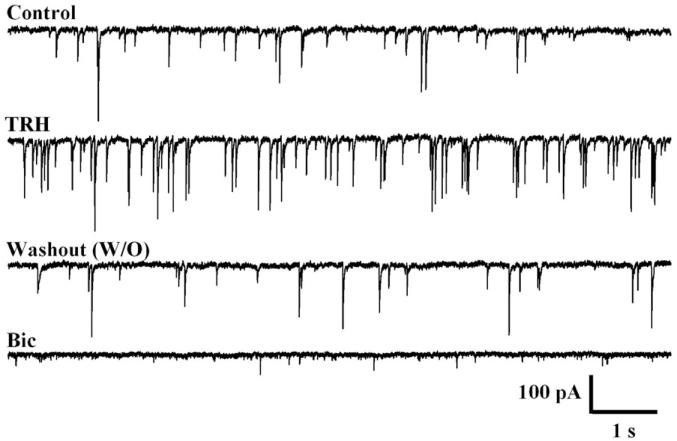
Fig. 5.
Whole cell recording of GABA-mediated synaptic currents. TRH substantially increased the frequency of the IPSCs recorded in GABAergic MCH neurons, which recovered after peptide washout. The GABAA receptor antagonist bicuculline (BIC) blocked the synaptic currents. TRH had no effect on miniature PSCs. Recordings were done in the presence of AP5 and CNQX to block responses to synaptically-released glutamate. The recording pipette contained a high concentration of Cl−, resulting in GABA-mediated inward currents. (From Zhang and van den Pol,2012).
That peptides can play key roles in CNS function is shown in experiments where genes coding for peptides were deleted. Knocking out the POMC peptides resulted in an increase in food intake and obesity, consistent with the view that these cells play an anorexigenic role in energy homeostasis (Yaswen et al, 1999); injections of alpha MSH agonists reversed the obesity. Knockout of the MC4 receptor also results in obesity in rodents (Huszar et al, 1997). In parallel, severe human obesity can be caused by mutations in genes coding for POMC or its melanocortin receptors (Hager et al, 1998; Yeo et al,2000; Krude et al,2003; Mencarelli et al, 2012).
An intriguing example of the importance of amino acid transmitters in cells considered as primarily peptidergic is shown by recent work on the inhibitory NPY/AgRP neuron. These cells play a key orexigenic role in food intake. As noted above, injections of either NPY or AgRP into the hypothalamic area increase food intake (Clark et al, 1984; Woods et al,1998; Marsh et al, 1998). Selective activation of the NPY/AgRP neuron with DREADD (designer receptors exclusively activated by designer drugs; Rogan and Roth, 2011) receptors increased feeding and reduced energy expenditure (Krashes et al, 2011). Hunger and ghrelin evoke a long-lasting increase in glutamatergic activity to the AgRP neurons, and leptin reverses the increased activity suggesting an on/off activation of glutamate input to AgRP neurons is important in regulating activity and energy homeostasis (Yang et al, 2011). In genetic knock-out mice, various neuroactive substances have been deleted from the NPY/AgRP neuron. Surprisingly, the loss of NPY or its receptor, or AgRP did not evoke a substantive change in feeding phenotype (Palmiter et al, 1998; Qian et al, 2002; Erickson et al, 1996). However, selective loss of AgRP/NPY neurons in the adult led to a cessation of feeding and death (Luquet et al, 2005; Gropp et al, 2005), suggesting that NPY and AgRP, while important modulators of food intake, are only part of the transmitter puzzle regulating energy homeostasis, and that other substances released by the AgRP/NPY neurons are critical for survival, as examined below:
The other piece of the transmitter puzzle synthesized by AgRP/NPY neurons is GABA. Loss of GABA input to the parabrachial nucleus (PBN) appears to be essential for the severe drop in food intake and death that results from ablation of the AgRP/NPY neuron. Increasing GABA receptor activation in the PBN (Wu et al, 2009), or reducing excitatory input to the PBN from the nucleus of the solitary tract (Wu et al, 2012) both enhanced food intake and survival. Suppression of glutamate excitation in the PBN reversed starvation caused by AgRP/NPY neuron ablation, and increased food intake in otherwise normal mice (Wu et al, 2012). Together these data suggest that peptidergic signaling in the AgRP/NPY cells plays a modulatory role in food intake, but that the fast amino acid transmitter plays a key role in some aspects of the function of these cells that cannot be ignored.
AgRP/NPY neurons also project to the paraventricular nucleus; in a complex series of experiments based in part on selective optogenetic activation and inactivation, AgRP/NPY axonal projections to oxytocin neurons were found to be critical for stimulation of feeding elicited from activation of AgRP/NPY cells. Both NPY and GABA inhibition of paraventricular oxytocin cells contributed to the initiation of feeding (Atasoy et al, 2012); the role of the GABA projection from the AgRP/NPY neuron to the PBN was interpreted in the context of visceral malaise.
Another independent line of work has shown that knocking out glutamate neurotransmission from SF1 neurons of the hypothalamic ventromedial nucleus disturbs glucose regulation and causes mice to suffer from hypoglycemia during fasting, and to have defective responses to insulin-induced hypoglycemia (Tong et al, 2007).
Modulation of GABA and glutamate release by neuropeptides
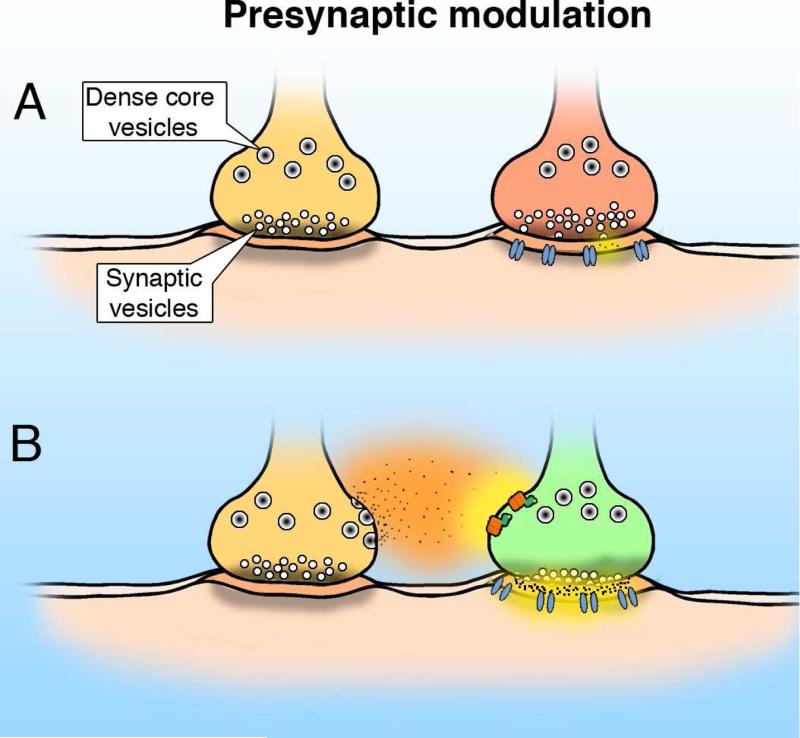
One substantial mechanism underlying neuropeptide modification of neuronal activity is the modulation of neurotransmitter release by direct peptide actions on the axon terminal (Miller, 1998; Willis, 2006). Some peptides, for instance NPY (Colmers et al, 1988), somatostatin (López-Huerta et al, 2012; Tallent and Siggins, 1997), and dynorphin, tend to reduce transmitter release, whereas others such as hypocretin (van den Pol et al, 1998) or glucagon-like peptide 1 (Acuna-Goycolea and van den Pol, 2004) enhance release probability. Neuropeptide receptors are found on both glutamate and GABA axon terminals (Fig. 6). In some regions of the brain, presynaptic modulation has been suggested as the primary or only role of some neuropeptides. NPY, for instance, acts to a large degree by inhibiting neurotransmitter release from excitatory CA3 neurons in the hippocampus; NPY had no detectable effect on either the active or passive membrane properties of CA3 pyramidal neuron cell bodies, but reduced release of glutamate from axons of these cells that terminated on CA1 pyramidal cells by a mechanism based on reduction of calcium influx into the axon terminal (Colmers et al, 1988). In the dentate gyrus, NPY Y2 receptors expressed on axon terminals inhibited glutamate or GABA release, and NPY Y1 receptors on granule cells mediated a cellular inhibition (Sperk et al, 2007). Insight into a potential function of hippocampal NPY is provided by NPY gene knockout (KO) mice which maintained normal electrophysiological activity in the hippocampus, but showed poor recovery after induction of limbic seizures with the glutamate agonist kainate, which caused death in the majority of NPY-KO mice compared with little death in normal mice treated with similar doses of kainate (Baraban et al, 1997). Similarly, NPY Y5-receptor KO mice were also more sensitive to kainate- induced seizures (Marsh et al, 1999a). Together these data support the view that NPY may function as an endogenous anticonvulsant, in part by attenuating glutamate release.
Fig. 6.
A. A schematic representation of two axons both in synaptic contact with a common dendrite. B. Release of neuropeptide from the bouton on the left diffuses laterally to the other axon and enhances release of the fast amino acid transmitter.
In contrast to its presynaptic actions on CA3 axons, NPY acted primarily on cellular Y1 receptors to inhibit basolateral amygdala neurons by suppressing a hyperpolarization-activated depolarizing Ih current that is a mixed cation current (Giesbrecht et al, 2010).
Activation of presynaptic axonal peptide receptors can alter transmitter release in a number of ways: alter voltage-gated calcium channels, change potassium channel conductance, change the phosphorylation state of a channel or channel-related protein, or alter the actions of proteins involved in vesicle movement or membrane fusion. Calcium plays a key role in transmitter/neuropeptide release. Activation of voltage-gated calcium channels increases cytoplasmic calcium by influx from the extracellular space, and enhances neuropeptide release. Calcium release from intracellular stores can also enhance neuropeptide release (Shakiryanova et al, 2011) as described above, and can potentially be achieved in the absence of membrane potential depolarization (Ludwig and Leng, 2006).
Many examples of peptides that alter GABA or glutamate release presynaptically by modulation of cytoplasmic calcium exist. For instance, MCH reduces calcium influx through L, N, and P/Q type calcium channels, and presynaptically reduces release of glutamate and GABA (Gao and van den Pol, 2001; 2002). In the suprachiasmatic nucleus (SCN), nociceptin (orphanin FQ) acts presynaptically to reduce glutamate release from the retinohypothalamic tract by a mechanism based on attenuation of N-type calcium currents, and to a lesser degree P/Q type calcium currents; because the retinal ganglion cells have been eliminated by brain slice preparation, the peptide actions could not have been on the glutamatergic cell body (Gompf et al, 2005). Similarly, nociceptin acts presynaptically to reduce GABA release in the central amygdala (Roberto and Siggins, 2006). Excitatory hippocampal mossy fibers release dynorphin, which results in heterosynaptic inhibition of glutamate release from other hippocampal mossy fibers, and inhibits hippocampal long term potentiation (LTP) (Weisskopf et al, 1993), and is dependent on calcium regulation, but not on a specific L, N, or P calcium channel (Castillo et al, 1996). The long duration of the dynorphin-induced effect on LTP was suggested to be due to slow dynorphin clearance from the extracellular space.
Peptide release from an axon can potentially feed back on the releasing axon to depress or enhance release of fast amino acid transmitters. Mu opioid neuropeptides are released by POMC neurons, and these peptides reduce release of fast amino acid transmitters from POMC axons (Dicken et al, 2012). Peptides released at a particular location can act at multiple pre- and postsynaptic sites to modulate the activity of multiple effectors. NPY acts pre- and postsynaptically to inhibit hypocretin neurons and the axons that terminate on POMC neurons (Fig. 7; Fu et al,2004). Hypocretin-2 enhances glutamate release by presynaptic actions in the ventral tegmental area, a region of the brain involved in reward and motivation, and also potentiates NMDA receptor actions in the postsynaptic cell through activation of protein kinase C (Borgland et al, 2008).
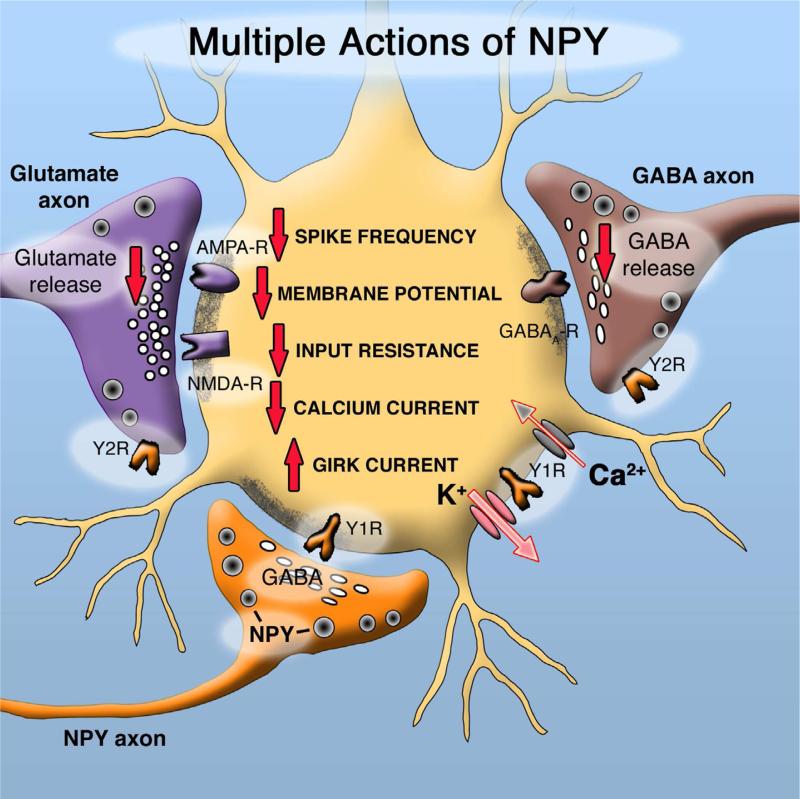
Fig. 7.
NPY inhibits glutamatergic hypocretin cells by multiple mechanisms. NPY acts on Y1 postsynaptic receptors to inhibit the hypocretin cell, and acts on Y2 presynaptic receptors to attenuate release of GABA and glutamate. Different actions of NPY are shown by the red arrows, with a downward arrow indicating a decrease, and upward arrow an increase. These NPY axons also contain GABA. Based on Fu et al, 2004; Horvath et al,1999. NPY probably has similar effect on many of its target neurons, including the POMC cell (Cowley et al, 2001), although the subset of NPY receptors expressed in cell bodies or terminals may differ from cell to cell.
Peptides can modulate a number of different channels or transporters that regulate neuronal activity and spike probability, including sodium channels, nonselective cation channels, sodium-calcium exchangers, and voltage-dependent calcium channels. Many inhibitory neuropeptides reduce GABA or glutamate release by activating G-protein coupled inwardly rectifying K+ (GIRK) channels, also called Kir3 channels. GIRK channels have become increasingly recognized as playing important roles in both normal brain processes, and in disease states (Lüscher and Slesinger, 2010). Different GIRK channels arise from the heteromeric assembly of different subunits (Lujan et al, 2009); after Gi/Go activation, Gβ and Gγ bind to the GIRK channel, resulting in hyperpolarization and inhibition. GPCR kinases can block GPCR function by phosphorylation-mediated internalization of the receptor; recent evidence suggests that the GPCR kinases can also directly and rapidly inactivate GIRK channels by competitively binding Gβ and Gγ subunits, thereby reducing GIRK channel activity (Raveh et al, 2010). Neuropeptides that inhibit neuronal activity by activating GIRK channels include NPY, somatostatin, opioid neuropeptides including dynorphin and met-enkephalin and others (Nakatsuka et al, 2008; Nassirpour et al, 2010; Li and van den Pol, 2008). On the other hand, excitatory neuropeptides such as substance P (Koike-Tani et al, 2005) and hypocretin (Hoang et al, 2003) also act on GIRK channels, but inhibit the GIRK current to increase neuronal activity.
Coupling of receptors to ion channels may be different in different processes of the same cell. For instance, mu opioid receptors that respond to met-enkephalin and other related opioid peptides often show fast desensitization of GIRK currents (Williams et al, 2001). Mu opioid receptor responses desensitize rapidly in the POMC cell body; in contrast, mu receptor responses are resistant to desensitization in the context of reducing GABA release from presynaptic axon terminals synapsing with the recorded cell (Pennock et al, 2012).
Role of colocalized neuropeptides with similar or opposing neuronal actions
Many neurons contain multiple neuropeptides (Hokfelt et al, 1986; 1990; Kofitsch et al, 1985; Zupanc, 1996). For example, neurons of the hypothalamic arcuate nucleus that utilize the catecholamine neuromodulator dopamine to inhibit prolactin release from the adenohypophysis also employ the amino acid transmitter GABA, and additionally synthesize a number of different neuropeptides including neurotensin, galanin, growth hormone releasing factor (GRF), met-enkephalin, leu-enkephalin, and dynorphin (Everitt et al, 1986). Single cells utilizing GABA and dopamine also contained galanin and either GRF or neurotensin; whether single dopamine cells contain all the peptides listed here remains to be determined. Synthesis of multiple neuroactive agents is not restricted to the hypothalamus. Single hippocampal interneurons synthesize GABA, NPY, and somatostatin, all with inhibitory actions. Neuropeptides are sequestered in DCVs, whereas the fast amino acid transmitters are found in small clear vesicles. Although we generally assume that peptides cosynthesized by a neuron are also stored and released from the same DCVs, there is limited evidence that different DCVs in a single neuron may contain different concentrations of multiple peptides (Zupanc, 1996). For instance, vasopressin and galanin have been reported to show differential expression in different DCVs in the same cell (Landry et al, 2003). Outside the brain, cells of the anterior pituitary synthesize both luteinizing hormone and follicle cell stimulating hormone, and there is good evidence for differential regulation of synthesis and release (Fink, 1995). One clear manner in which different peptides can be released differentially by the same neuron is by differential regulation of synthesis. Peptides coded by different genes have different regulatory elements and respond to different sets of transcription factors. Therefore, differential synthesis, potentially in response to neuromodulators that alter transcription of different genes selectively, may allow a change in the proportion of released peptides over time.
Multiple peptides with similar actions in single neurons
In some neurons, multiple active peptides are cleaved from the same precursor. One example of this is proopiomelanocortin which is cleaved by peptidases into a number of potentially neuroactive peptides including beta-endorphin, α-MSH, γ-MSH, corticotropin and others. POMC cells may also synthesize galanin-like peptide and cocaine and amphetamine related transcript (CART); all three of these may modulate food intake (Elmquist, 2001; Gundlach, 2002).
Colocalization of peptides with related functions, but with independent synthetic pathways is also not uncommon. Neurons in the hypothalamic arcuate nucleus that synthesize NPY and AgRP are a good example of this. NPY exerts direct inhibitory actions through a number of NPY receptors. AgRP is thought to act in a less direct inhibitory manner, by blocking the excitatory actions of POMC derived α-MSH on the MC4 melanocortin receptors (Cone,2005), although actions of AgRP independent of the MC4 receptor have also been described (Marsh et al,1999; Fu and van den Pol,2008). Intrahypothalamic application of either NPY or AgRP increases food intake, indicating that both peptides play a positive role in energy homeostasis (Schwartz et al., 2000; Seeley and Woods, 2003). Use of multiple neuroactive peptides could enhance the response of target cells expressing the multiple relevant receptors. Alternately, one peptide could activate homotypic receptors on one cell, and the second could activate relevant receptors on a different target cell. Co-synthesized peptides are probably also co-released.
Another mechanism of peptide collaboration is based on competition for peptidases. For instance, the actions of substance P on EPSCs in the parabrachial nucleus were enhanced by calcitonin gene related peptide (CGRP) by a mechanism based on CGRP-mediated attenuation of the activity of extracellular peptidases that inactivate substance P, apparently by competition for the peptidases (Saleh et al, 1996). Furthermore, use of the peptidase inhibitor phosphoramidon increased the amplitude of the substance P effect by about ten-fold, suggesting a normally rapid breakdown of substance P. The expression and release of peptidases is another dimension of the regulation of the half-life of neuropeptides that merits consideration relating to the range of efficacy of peptide actions.
Opposing peptides in single cells
From different precursor proteins, neurons may also synthesize neuropeptides that can exert opposing actions at the cellular level. Dynorphin is an opioid neuropeptide which acts at kappa Gi/Go-coupled opioid receptors, leading to cellular inhibition (Chavkin et al, 1982) by presynaptic inhibition, activation of K+ currents, or attenuation of voltage-gated calcium channels. An electrophysiological example of dynorphin attenuation of calcium current is shown in figure 8. A number of different excitatory neurons release this inhibitory peptide. Dynorphin is colocalized with vasopressin in magnocellular neurosecretory neurons, with excitatory hypocretin/orexin neurons in the lateral hypothalamus, with excitatory kisspeptin and neurokinin B in the arcuate nucleus (Goodman et al, 2007), and in glutamatergic granule cells in the hippocampus (Simmons et al,1995).
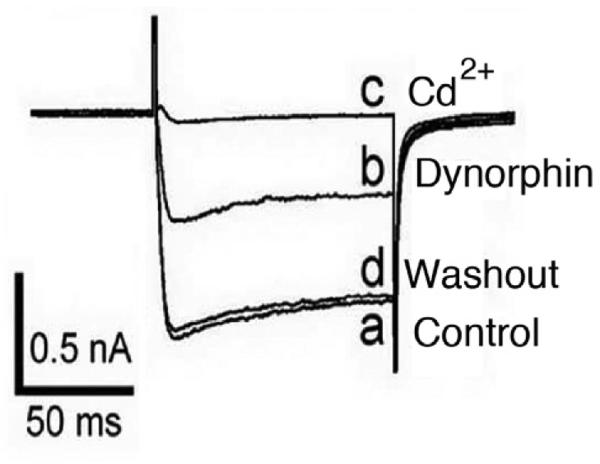
Fig. 8.
Dynorphin attenuates voltage-gated calcium current. A command depolarization of a glutamatergic hypocretin neuron caused a calcium influx (a). Dynorphin reduced the calcium current (b), which recovered after peptide washout. Cd2+ completely blocked the current, consistent with it being calcium-mediated. From Li and van den Pol,2006.
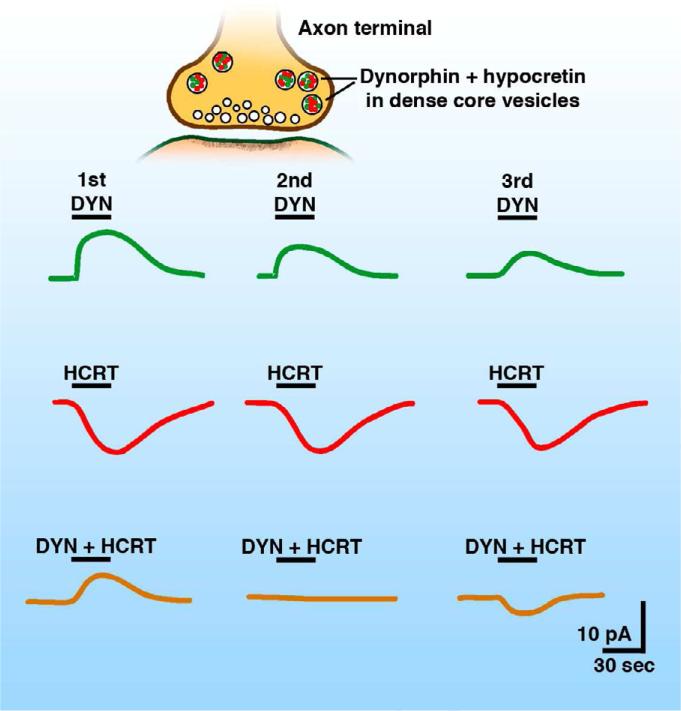
Hypothalamic hypocretin neurons are critical for cognitive arousal and normal sleep and wake cycles in mammals, and they also play a role in drug addiction. In humans, the loss of hypocretin neurons results in the neurological syndrome narcolepsy, characterized by excessive day-time sleepiness (Burgess and Scammel, 2012; Chemelli et al, 1999; Lin et al, 1999). In the hypocretin-dynorphin neuron, both peptides are synthesized by the same neurons in rodents and humans (Chou et al, 2001; Crocker et al, 2005) and released, probably simultaneously (Li and van den Pol, 2006) from long axons that terminate in a large number of regions of the brain and spinal cord (Peyron et al, 1998; van den Pol, 1999). Receptors for hypocretin (Sakura et al, 1998) and dynorphin (DePaoli et al, 1994) are expressed widely through the CNS. Hypocretin plays an excitatory role (de Lecea et al, 1998; van den Pol et al, 1998) through Gq coupled receptors (Sakurai et al, 1998). In hypocretin-receptive neurons, hypocretin depolarizes the membrane potential, increases spike frequency, increases intracellular calcium, and increases GABA or glutamate release presynaptically (van den Pol et al, 1998; 2002; Li et al, 2002). Why would a neuron release neuromodulators of opposing actions? There are a number of possibilities. One possibility is that at the site of release, cells may express receptors for only one of the peptides, and therefore respond to only that peptide. Peptides with opposing actions can also act synergistically. Hypocretin evokes a direct excitation of arcuate nucleus NPY cells; dynorphin inhibits GABA release onto NPY cells by acting on presynaptic opioid receptors thereby reducing synaptic inhibition and facilitating the excitatory direct actions of hypocretin (Li and van den Pol, 2006). Thus, the opposing peptides released from the same axon act on different cells to synergistically increase activity of one of the responding cells.
Differential desensitization could also play a role in the response to opposing peptides in responding cells expressing both receptor types. The initial effect, or effect of low level release, may favor one peptide, whereas more protracted release, or a high level release, may ultimately favor the other peptide. Repeated application of dynorphin to voltage-clamped melanin concentrating hormone (MCH) cells resulted in substantially attenuated second and third outward (inhibitory) currents; in contrast, repeated application of hypocretin showed substantially less attenuation of its evoked inward currents (Fig. 9). Repeated co-application of dynorphin + hypocretin therefore resulted in an initial outward (hyperpolarizing) current, but shifted to an inward current (depolarizing) with repeated coapplication (Li and van den Pol, 2006). Thus, in this example, low levels of co-release might favor a modest inhibition, whereas high levels of co-release may ultimately favor excitation. A related possibility is that two opposing peptides could act with different time-courses either due to different latencies or durations of action, and therefore one peptide may truncate the effect of the other primarily during the overlap of the two time-courses. Here, I focus on two opposing neuroactive substances; however, many cells contain more. For instance, a recent paper found that channelrhodopsin-evoked glutamate release from hypocretin cells was a critical substance released for controlling the activity of postsynaptic histamine neurons (Schone et al, 2012).
Fig. 9.
Inhibitory dynorphin and excitatory hypocretin are synthesized and released together. Serial application of dynorphin evokes an outward current (green) with an amplitude that desensitizes and deceases substantially with repeated exposure. Serial application of hypocretin evokes an inward current (red) with only modest desensitization. Combined application of dynorphin + hypocretin (orange) gives an initial outward current, then little current, and then an inward current. Based on Li and van den Pol,2006.
Another role for opposing peptide signaling would be in feedback regulation of release of peptides from the same or neighboring release sites. In vasopressin neurosecretory cells, dynorphin is coreleased with vasopressin locally by the somatodendritic complex, and serves a key role in feedback inhibition of vasopressin cells (Brown et al, 2004a, b), in part by inhibition of plateau potentials required for spike bursts; vasopressin exerts a rapid inhibitory action, whereas dynorphin shows a slower inhibitory action resulting in a gradual inhibition during successive spikes of a burst. Dynorphin expression is increased during periods of dehydration, and so continues to provide a feedback inhibition even while spike frequency is increased to counter dehydration effects by increasing vasopressin release (Scott et al, 2009). Dynorphin also reduces transmitter release from presynaptic glutamate axons (Iremonger and Bains, 2009). The dual effect of direct inhibition of release from the parent cell or its similar neighbors, and presynaptic reduction in excitatory transmitter stimulation, serve a similar role allowing dynorphin to depress activity by multiple converging mechanisms. Actions of dynorphin in attenuating hippocampal mossy fiber glutamate release are discussed below.
Kisspeptin is synthesized by cells of the median hypothalamus, and the peptide modulates the activity of GnRH neurons and regulates reproduction and onset of puberty (Kauffman et al, 2007; Han et al, 2005). Mutations of the GPR54, the kisspeptin receptor, block puberty and cause infertility in rodents and humans (Dungan et al, 2006; Smith and Clarke, 2007). Dynorphin and neurokinin B colocalize with kisspeptin in many mammals (Goodman et al, 2007); dynorphin is proposed to act back on the releasing kisspeptin neurons to synchronize and shape pulsatile release patterns of kisspeptin (Navarro et al, 2009; Wakabayashi et al, 2010).
Astrocytes and peptides
Although we generally think of neuroactive peptides as being synthesized by and exerting effects on neurons, the focus of this review, glial cells may also employ neuropeptide signaling and express receptors for neuromodulators in the CNS (Azmitia et al, 1996; Kimelberg, 1988; Tasker et al, 2012). For instance, one class of olfactory ensheathing glia that accompanies the olfactory nerve from the olfactory mucosa into the olfactory bulb shows very high levels of NPY expression (Ubink et al, 1994; Ubink and Hokfelt, 2000); NPY may act here as a trophic factor to promote olfactory receptor neuron maturation and survival (Doyle et al, 2012). Schwann cell precursors also express NPY, and this expression is lost during postnatal development (Ubink and Hokfelt, 2000). NPY may also be released by astrocytes. Ramamoorthy and Whim (2008) employed NPY-bound red fluorescent protein to show glutamate-agonist mediated NPY secretion from cortical astrocytes.
Astrocytes in many brain regions express functionally active vasopressin receptors (Brinton et al, 1998; Jurzak et al, 1995; Kozneiwska and Romaniuk,2008). Peptide-responsive astrocytes can show fairly rapid activity-dependent structural plasticity which may allow a further dimension of modulation of neuropeptide actions and diffusion (Miyata et al, 2001; Theodosis et al, 2008), including potential selective restriction of peptide diffusion from a release site. For instance, during periods of dehydration or lactation, astrocytic processes may retract to reduce segregation of synaptic complexes in the paraventricular and supraoptic nuclei, thereby increasing neuron to neuron membrane apposition, and potentially enhancing diffusion of neuropeptides away from release sites (Hatton et al, 1984; Oliet et al, 2004; Tasker et al, 2012).
Conclusion
At the cellular level, peptides enhance or attenuate neuronal activity by modulating the activity of a number of different ion channels, and by increasing or decreasing GABA or glutamate release by direct actions on peptide receptors on presynaptic axons. There are many unanswered, or even unaddressed, questions relating to peptide release and response throughout the brain. A key question relates to the release and response to neuropeptides in the majority of neurons that synthesize moderate or small amounts of peptide; do these cells follow in the footsteps of the magnocellular neurosecretory neurons that synthesize large amounts of peptide and have been the subject of intense scrutiny? Or do the neurons that synthesize substantially more modest amounts of neuropeptide possess a different subset of defining features of release and induced response?
With many neurons containing fast transmitters in addition to one (or more) slower neuromodulator peptides, many questions arise as to the relative role and contribution of the different neuroactive substances in single cell types, both from a perspective of ion channel regulation, and from a more global view of the neuron's general functional assignment in the brain. Where a particular neuropeptide acts relative to its release site, both at the cellular and subcellular peptide receptor level, is another important question that, although difficult to address, will provide a critical link to understand the role of neuropeptides at a functional level.
Acknowledgements
I am indebted to Dr Guido Wollmann for generous help with some of the figures, and Drs.M. Whim, W.E. Armstrong, Q. Pittman, M.N. Nitabach, J. Paglino, X. Zhang, and J. Davis for suggestions on the manuscript. Neuromodulation work in the lab is supported by the US National Institute of Health NS48476, NS079274, and DK084052.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acuna-Goycolea C, van den Pol A. Glucagon-like peptide 1 excites hypocretin/orexin neurons by direct and indirect mechanisms: implications for viscera-mediated arousal. J Neurosci. 2004;24:8141–8152. doi: 10.1523/JNEUROSCI.1607-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acsády L, Arabadzisz D, Freund TF. Correlated morphological and neurochemical features identify different subsets of vasoactive intestinal polypeptide-immunoreactive interneurons in rat hippocampus. Neuroscience. 1996;73:299–315. doi: 10.1016/0306-4522(95)00610-9. [DOI] [PubMed] [Google Scholar]
- Acsády L, Katona I, Martínez-Guijarro FJ, Buzsáki G, Freund TF. Unusual target selectivity of perisomatic inhibitory cells in the hilar region of the rat hippocampus. J Neurosci. 2000;20:6907–6919. doi: 10.1523/JNEUROSCI.20-18-06907.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonucci F, Alpár A, Kacza J, Caleo M, Verderio C, Giani A, Martens H, Chaudhry FA, Allegra M, Grosche J, Michalski D, Erck C, Hoffmann A, Harkany T, Matteoli M, Härtig W. Cracking down on inhibition: selective removal of GABAergic interneurons from hippocampal networks. J Neurosci. 2012;32:1989–2001. doi: 10.1523/JNEUROSCI.2720-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariel P, Ryan TA. Optical mapping of release properties in synapses. Front Neural Circuits 4: art. 2010;18:1–10. doi: 10.3389/fncir.2010.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong WE, Hatton GI. The puzzle of pulsatile oxytocin secretion during lactation: some new pieces. Am J Physiol. 2006;291:R26–R28. doi: 10.1152/ajpregu.00879.2005. [DOI] [PubMed] [Google Scholar]
- Atasoy D, Betley JN, Su HH, Sternson SM. Deconstruction of a neural circuit for hunger. Nature. 2012 doi: 10.1038/nature11270. [Epub ahead of print doi:10.1038/nature11270] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azmitia EC, Gannon PJ, Kheck NM, Whitaker-Azmitia PM. Cellular localization of the 5-HT1A receptor in primate brain neurons and glial cells. Neuropsychopharm. 1996;14:35–46. doi: 10.1016/S0893-133X(96)80057-1. [DOI] [PubMed] [Google Scholar]
- Bacci A, Huguenard JR, Prince DA. Differential modulation of synaptic transmission by neuropeptide Y in rat neocortical neurons. Proc Natl Acad Sci USA. 2002;99:17125–17130. doi: 10.1073/pnas.012481899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baraban SC, Hollopeter G, Erickson JC, Schwartzkroin PA, Palmiter RD. Knock-out mice reveal a critical antiepileptic role for neuropeptide Y. J Neurosci. 1997;17:8927–8936. doi: 10.1523/JNEUROSCI.17-23-08927.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkus RV, Klyachko O, Horiuchi D, Dickson BJ, Saxton WM. Identification of an axonal kinesin-3 motor for fast anterograde vesicle transport that facilitates retrograde transport of neuropeptides. Mol Biol Cell. 2008;19:274–283. doi: 10.1091/mbc.E07-03-0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baude A, Nusser Z, Roberts JD, Mulvihill E, McIlhinney RA, Somogyi P. The metabotropic glutamate receptor (mGluR1 alpha) is concentrated at perisynaptic membrane of neuronal subpopulations as detected by immunogold reaction. Neuron. 1993;11:771–787. doi: 10.1016/0896-6273(93)90086-7. [DOI] [PubMed] [Google Scholar]
- Bering R, Draguhn A, Diemer NH, Johansen FF. Ischemia changes the coexpression of somatostatin and neuropeptide Y in hippocampal interneurons. Exp Brain Res. 1997;115:423–429. doi: 10.1007/pl00005712. [DOI] [PubMed] [Google Scholar]
- Bicknell RJ, Leng G. Relative efficiency of neural firing patterns for vasopressin release in vitro. Neuroendocr. 1981;33:295–299. doi: 10.1159/000123248. [DOI] [PubMed] [Google Scholar]
- Billova S, Galanopoulou AS, Seidah NG, Qiu X, Kumar U. Immunohistochemical expression and colocalization of somatostatin, carboxypeptidase-E and prohormone convertases 1 and 2 in rat brain. Neurosci. 2007;147:403–418. doi: 10.1016/j.neuroscience.2007.04.039. [DOI] [PubMed] [Google Scholar]
- Bondy CA, Gainer H, Russell JT. Effects of stimulus frequency and potassium channel blockade on the secretion of vasopressin and oxytocin from the neurohypophysis. Neuroendocrinol. 1987;46:258–267. doi: 10.1159/000124829. [DOI] [PubMed] [Google Scholar]
- Borgland SL, Storm E, Bonci A. Orexin B/hypocretin 2 increases glutamatergic transmission to ventral tegmental area neurons. Eur J Neurosci. 2008;28:1545–1556. doi: 10.1111/j.1460-9568.2008.06397.x. [DOI] [PubMed] [Google Scholar]
- Bradley SR, Levey AI, Hersch SM, Conn PJ. Immunocytochemical localization of group III metabotropic glutamate receptors in the hippocampus with subtype-specific antibodies. J Neurosci. 1996;16:2044–2056. doi: 10.1523/JNEUROSCI.16-06-02044.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinton RD, Yamazaki R, Gonzalez CM, O'Neill K, Schreiber SS. Vasopressin-induction of the immediate early gene, NGFI-A, in cultured hippocampal glial cells. Brain Res Mol Brain Res. 1998;57:73–85. doi: 10.1016/s0169-328x(98)00069-2. [DOI] [PubMed] [Google Scholar]
- Brown CH, Ludwig M, Leng G. Temporal dissociation of the feedback effects of dendritically co-released peptides on rhythmogenesis in vasopressin cells. Neuroscience. 2004;124:105–111. doi: 10.1016/j.neuroscience.2003.11.038. [DOI] [PubMed] [Google Scholar]
- Brown CH, Bourque CW. Autocrine feedback inhibition of plateau potentials terminates phasic bursts in magnocellular neurosecretory cells of the rat supraoptic nucleus. J Physiol. 2004;557:949–960. doi: 10.1113/jphysiol.2004.063818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess CR, Scammell TE. Narcolepsy: neural mechanisms of sleepiness and cataplexy. J Neurosci. 2012;32:12305–12311. doi: 10.1523/JNEUROSCI.2630-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke NV, Han W, Li D, Takimoto K, Watkins SC, Levitan ES. Neuronal peptide release is limited by secretory granule mobility. Neuron. 1997;19:1095–1102. doi: 10.1016/s0896-6273(00)80400-6. [DOI] [PubMed] [Google Scholar]
- Castillo PE, Salin PA, Weisskopf MG, Nicoll RA. Characterizing the site and mode of action of dynorphin at hippocampal mossy fiber synapses in the guinea pig. J Neurosci. 1996;16:5942–5950. doi: 10.1523/JNEUROSCI.16-19-05942.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazalis M, Dayanithi G, Nordmann JJ. The role of patterned burst and interburst interval on the excitation-coupling mechanism in the isolated rat neural lobe. J Physiol. 1985;369:45–60. doi: 10.1113/jphysiol.1985.sp015887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavkin C, James IF, Goldstein A. Dynorphin is a specific endogenous ligand of the kappa opioid receptor. Science. 1982;215:413–415. doi: 10.1126/science.6120570. [DOI] [PubMed] [Google Scholar]
- Chemelli RM, Willie JT, Sinton CM, Elmquist JK, Scammell T, Lee C, Richardson JA, Williams SC, Xiong Y, Kisanuki Y, Fitch TE, Nakazato M, Hammer RE, Saper CB, Yanagisawa M. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;98:437–451. doi: 10.1016/s0092-8674(00)81973-x. [DOI] [PubMed] [Google Scholar]
- Choi C, Cao G, Tanenhaus AK, McCarthy E, Jung M, Schleyer W, Shang Y, Rosbash M, Yin JCP, Nitabach MN. Autoreceptor control of peptide/neurotransmitter corelease from PDF neurons determines allocation of circadian activity in Drosophila. Cell Reports. 2012;2:1–13. doi: 10.1016/j.celrep.2012.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou TC, Lee CE, Lu J, Elmquist JK, Hara J, Willie JT, Beuckmann CT, Chemelli RM, Sakurai T, Yanagisawa M, Saper CB, Scammell TE. Orexin (hypocretin) neurons contain dynorphin. J Neurosci 21 RC168. 2001:1–6. doi: 10.1523/JNEUROSCI.21-19-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang HH, Yu M, Jan YN, Jan LY. Evidence that the nucleotide exchange and hydrolysis cycle of G proteins causes acute desensitization of G-protein-gated inward rectifier K+ channels. Proc. Natl. Acad. Sci. USA. 1998;95:11727–11732. doi: 10.1073/pnas.95.20.11727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church PJ, Lloyd PE. Expression of diverse neuropeptide cotransmitters by identified motor neurons in Aplysia. J Neurosci. 1991;11:618–625. doi: 10.1523/JNEUROSCI.11-03-00618.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church PJ, Whim MD, Lloyd PE. Modulation of neuromuscular transmission by conventional and peptide transmitters released from excitatory and inhibitory motor neurons in Aplysia. J Neurosci. 1993;13:2790–2800. doi: 10.1523/JNEUROSCI.13-07-02790.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark JT, Kalra PS, Crowley WR, Kalra SP. Neuropeptide Y and human pancreatic polypeptide stimulate feeding behavior in rats. Endocrinology. 1984;115:427–429. doi: 10.1210/endo-115-1-427. [DOI] [PubMed] [Google Scholar]
- Colmers WF, Lukowiak K, Pittman QJ. Neuropeptide Y action in the rat hippocampal slice: site and mechanism of presynaptic inhibition. J Neurosci. 1988;8:3827–3837. doi: 10.1523/JNEUROSCI.08-10-03827.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cone RD. Anatomy and regulation of the central melanocortin system. Nature Neurosci. 2005;8:571–578. doi: 10.1038/nn1455. [DOI] [PubMed] [Google Scholar]
- Cowley MA, Smart JL, Rubinstein M, Cerdán MG, Diano S, Horvath TL, Cone RD, Low MJ. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature. 2001;411:480–484. doi: 10.1038/35078085. [DOI] [PubMed] [Google Scholar]
- Crocker A, Espana RA, Papadopoulou M, Saper CB, Faraco J, Sakurai T, Honda M, Mignot E, Scammell TE. Concomitant loss of dynorphin, NARP, and orexin in narcolepsy. Neurology. 2005;65:1184–1188. doi: 10.1212/01.wnl.0000168173.71940.ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayanithi G, Forostyak O, Ueta Y, Verkhratsky A, Toescu EC. Segregation of calcium signalling mechanisms in magnocellular neurones and terminals. Cell Calcium. 2012;51:293–299. doi: 10.1016/j.ceca.2012.02.002. [DOI] [PubMed] [Google Scholar]
- Decavel C, van den Pol AN. GABA: a dominant neurotransmitter in the hypothalamus. J Comp Neurol. 1990;302:1019–1037. doi: 10.1002/cne.903020423. [DOI] [PubMed] [Google Scholar]
- de Kock CP, Wierda KD, Bosman LW, Min R, Koksma JJ, Mansvelder HD, Verhage M, Brussaard AB. Somatodendritic secretion in oxytocin neurons is upregulated during the female reproductive cycle. J Neurosci. 2003;23:2726–2734. doi: 10.1523/JNEUROSCI.23-07-02726.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lecea L, Kilduff TS, Peyron C, Gao XB, Foye PE, Danielson PE, Fukuhara C, Battenberg ELF, Gautvik VT, Bartlett II FS, Frankel WN, van den Pol AN, Bloom FE, Gautvik KM, Sutcliffe JG. The hypocretins: two hypothalamic peptides with neuroexcitatory activity. Proc Natl Acad Sci USA. 1998;95:322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePaoli AM, Hurley KM, Yasada K, Reisine T, Bell G. Distribution of kappa opioid receptor mRNA in adult mouse brain: an in situ hybridization histochemistry study. Mol Cell Neurosci. 1994;5:327–335. doi: 10.1006/mcne.1994.1039. [DOI] [PubMed] [Google Scholar]
- Dicken MS, Tooker RE, Hentges ST. Regulation of GABA and glutamate release from proopiomelanocortin neuron terminals in intact hypothalamic networks. J Neurosci. 2012;32:4042–4048. doi: 10.1523/JNEUROSCI.6032-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doupnik CA, Jaen C, Zhang Q. Measuring the modulatory effects of RGS proteins on GIRK channels. Methods Enzymol. 2004;389:131–154. doi: 10.1016/S0076-6879(04)89009-8. 2004. [DOI] [PubMed] [Google Scholar]
- Doyle KL, Hort YJ, Herzog H, Shine J. Neuropeptide Y and peptide YY have distinct roles in adult mouse olfactory neurogenesis. J Neurosci Res. 2012;90:1126–1135. doi: 10.1002/jnr.23008. [DOI] [PubMed] [Google Scholar]
- Dreifuss JJ, Kalnins I, Kelly JS, Ruf K. Action potentials and release of neurohypophysial hormones in vitro. J Physiol. 1971;215:805–817. doi: 10.1113/jphysiol.1971.sp009499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreifuss JJ. A review on neurosecretory granules: their contents and mechanisms of release. Ann N Y Acad Sci. 1975;248:184–201. doi: 10.1111/j.1749-6632.1975.tb34185.x. [DOI] [PubMed] [Google Scholar]
- Dun NJ, Dun SL, Wong RK, Förstermann U. Colocalization of nitric oxide synthase and somatostatin immunoreactivity in rat dentate hilar neurons. Proc Natl Acad Sci USA. 1994;91:2955–2959. doi: 10.1073/pnas.91.8.2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dungan HM, Clifton DK, Steiner RA. Minireview: kisspeptin neurons as central processors in the regulation of gonadotropin-releasing hormone secretion. Endocrinology. 2006;147:1154–1158. doi: 10.1210/en.2005-1282. [DOI] [PubMed] [Google Scholar]
- Dutton A, Dyball REJ. Phasic firing enhances vasopressin release from the rat neurohypophysis. J Physiol. 1977;290:433–440. doi: 10.1113/jphysiol.1979.sp012781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmquist JK. Hypothalamic pathways underlying the endocrine, autonomic, and behavioral effects of leptin. Physiol Behav. 2001;74:703–708. doi: 10.1016/s0031-9384(01)00613-8. [DOI] [PubMed] [Google Scholar]
- Erickson JC, Clegg KE, Palmiter RD. Sensitivity to leptin and susceptibility to seizures of mice lacking neuropeptide Y. Nature. 1996;381:415–421. doi: 10.1038/381415a0. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Meister B, Hökfelt T, Melander T, Terenius L, Rökaeus A, Theodorsson-Norheim E, Dockray G, Edwardson J, Cuello C, et al. The hypothalamic arcuate nucleus-median eminence complex: immunohistochemistry of transmitters, peptides and DARPP-32 with special reference to coexistence in dopamine neurons. Brain Res. 1986;396:97–155. doi: 10.1016/s0006-8993(86)80192-5. [DOI] [PubMed] [Google Scholar]
- Fink G. The self-priming effect of LHRH: a unique servomechanism and possible cellular model for memory. Front Neuroendocrinol. 1995;16:183–190. doi: 10.1006/frne.1995.1006. [DOI] [PubMed] [Google Scholar]
- Freund TF, Buzsáki G. Interneurons of the hippocampus. Hippocampus. 1996;6:347–470. doi: 10.1002/(SICI)1098-1063(1996)6:4<347::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Freund-Mercier MJ, Stoeckel ME, Klein MJ. Oxytocin receptors on oxytocin neurones: histoautoradiographic detection in the lactating rat. J Physiol (Lond) 1994;480:155–161. doi: 10.1113/jphysiol.1994.sp020349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu LY, Acuna-Goycolea C, van den Pol AN. Neuropeptide Y inhibits hypocretin/orexin neurons by multiple presynaptic and postsynaptic mechanisms: tonic depression of the hypothalamic arousal system. J Neurosci. 2004;24:8741–8751. doi: 10.1523/JNEUROSCI.2268-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu LY, van den Pol AN. Agouti-related peptide and MC3/4 receptor agonists both inhibit excitatory hypothalamic ventromedial nucleus neurons. J Neurosci. 2008;28:5433–5449. doi: 10.1523/JNEUROSCI.0749-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuxe K, Rivera A, Jacobsen KX, Höistad M, Leo G, Horvath TL, Staines W, De la Calle A, Agnati LF. Dynamics of volume transmission in the brain. Focus on catecholamine and opioid peptide communication and the role of uncoupling protein 2. J Neural Transm. 2005;112:65–76. doi: 10.1007/s00702-004-0158-3. [DOI] [PubMed] [Google Scholar]
- Fuxe K, Dahlström A, Höistad M, Marcellino D, Jansson A, Rivera A, Diaz-Cabiale Z, Jacobsen K, Tinner-Staines B, Hagman B, Leo G, Staines W, Guidolin D, Kehr J, Genedani S, Belluardo N, Agnati LF. From the Golgi-Cajal mapping to the transmitter-based characterization of the neuronal networks leading to two modes of brain communication: wiring and volume transmission. Brain Res Rev. 2007;55:17–54. doi: 10.1016/j.brainresrev.2007.02.009. [DOI] [PubMed] [Google Scholar]
- Fuenzalida LC, Keen KL, Terasawa E. Colocalization of FM1-43, Bassoon, and GnRH-1: GnRH-1 release from cell bodies and their neuroprocesses. Endocrinol. 2011;152:4310–4321. doi: 10.1210/en.2011-1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gainer H, Wolfe SA, Jr, Obaid AL, Salzberg BM. Action potentials and frequency-dependent secretion in the mouse neurohypophysis. Neuroendocrinology. 1986;43:557–563. doi: 10.1159/000124582. [DOI] [PubMed] [Google Scholar]
- Gao XB, van den Pol AN. Melanin-concentrating hormone depresses L-, N-, and P/Q-type voltage-dependent calcium channels in rat lateral hypothalamic neurons. J Physiol. 2002;542:273–286. doi: 10.1113/jphysiol.2002.019372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao XB, van den Pol AN. Melanin concentrating hormone depresses synaptic activity of glutamate and GABA neurons from rat lateral hypothalamus. J Physiol. 2001;533:237–252. doi: 10.1111/j.1469-7793.2001.0237b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner S, Pawson AJ. Emerging targets of the GnRH receptor: novel interactions with Wnt signalling mediators. Neuroendocrinology. 2009;89:241–251. doi: 10.1159/000165377. [DOI] [PubMed] [Google Scholar]
- Giesbrecht CJ, Mackay JP, Silveira HB, Urban JH, Colmers WF. Countervailing modulation of Ih by neuropeptide Y and corticotrophin-releasing factor in basolateral amygdala as a possible mechanism for their effects on stress-related behaviors. J Neurosci. 2010;30:16970–16982. doi: 10.1523/JNEUROSCI.2306-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gompf HS, Moldavan MG, Irwin RP, Allen CN. Nociceptin/orphanin FQ (N/OFQ) inhibits excitatory and inhibitory synaptic signaling in the suprachiasmatic nucleus (SCN). Neuroscience. 2005;132:955–965. doi: 10.1016/j.neuroscience.2004.11.057. [DOI] [PubMed] [Google Scholar]
- Goodman RL, Lehman MN, Smith JT, Coolen LM, de Oliveira CV, Jafarzadehshirazi MR, Pereira A, Iqbal J, Caraty A, Ciofi P, Clarke IJ. Kisspeptin neurons in the arcuate nucleus of the ewe express both dynorphin A and neurokinin B. Endocrinology. 2007;148:5752–5760. doi: 10.1210/en.2007-0961. [DOI] [PubMed] [Google Scholar]
- Gropp E, Shanabrough M, Borok E, Xu AW, Janoschek R, Buch T, Plum L, Balthasar N, Hampel B, Waisman A, Barsh GS, Horvath TL, Brüning JC. Agouti-related peptide-expressing neurons are mandatory for feeding. Nat Neurosci. 2005;8:1289–1291. doi: 10.1038/nn1548. [DOI] [PubMed] [Google Scholar]
- Gundlach AL. Galanin/GALP and galanin receptors: role in central control of feeding, body weight/obesity and reproduction? Eur J Pharmacol. 2002;440:255–268. doi: 10.1016/s0014-2999(02)01433-4. [DOI] [PubMed] [Google Scholar]
- Hager J, Dina C, Francke S, Dubois S, Houari M, Vatin V, Vaillant E, Lorentz N, Basdevant A, Clement K, Guy-Grand B, Froguel P. A genome-wide scan for human obesity genes reveals a major susceptibility locus on chromosome 10. Nat Genet. 1998;20:304–308. doi: 10.1038/3123. [DOI] [PubMed] [Google Scholar]
- Han SK, Gottsch ML, Lee KJ, Popa SM, Smith JT, Jakawich SK, Clifton DK, Steiner RA, Herbison AE. Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J Neurosci. 2005;25:11349–11356. doi: 10.1523/JNEUROSCI.3328-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatton GI, Perlmutter LS, Salm AK, Tweedle CD. Dynamic neuronal-glial interactions in hypothalamus and pituitary: implications for control of hormone synthesis and release. Peptides. 1984;5(Suppl 1):121–138. doi: 10.1016/0196-9781(84)90271-7. [DOI] [PubMed] [Google Scholar]
- Hazell GG, Hindmarch CC, Pope GR, Roper JA, Lightman SL, Murphy D, O'Carroll AM, Lolait SJ. G protein-coupled receptors in the hypothalamic paraventricular and supraoptic nuclei--serpentine gateways to neuroendocrine homeostasis. Front Neuroendocrinol. 2012;33:45–66. doi: 10.1016/j.yfrne.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herkenham M. Mismatches between neurotransmitter and receptor localizations in brain: observations and implications. Neuroscience. 1987;23:1–38. doi: 10.1016/0306-4522(87)90268-5. [DOI] [PubMed] [Google Scholar]
- Hoang QV, Bajic D, Yanagisawa M, Nakajima S, Nakajima Y. Effects of orexin (hypocretin) on GIRK channels. J Neurophysiol. 2003;90:693–702. doi: 10.1152/jn.00001.2003. [DOI] [PubMed] [Google Scholar]
- Hirasawa M, Kombian SB, Pittman QJ. Oxytocin retrogradely inhibits evoked, but not miniature, EPSCs in the rat supraoptic nucleus: role of N- and P/Q-type calcium channels. J Physiol. 2001;532:595–607. doi: 10.1111/j.1469-7793.2001.0595e.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirasawa M, Schwab Y, Natah S, Hillard CJ, Mackie K, Sharkey KA, Pittman QJ. Dendritically released transmitters cooperate via autocrine and retrograde actions to inhibit afferent excitation in rat brain. J Physiol. 2004;559:611–624. doi: 10.1113/jphysiol.2004.066159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hökfelt T, Holets VR, Staines W, Meister B, Melander T, Schalling M, Schultzberg M, Freedman J, Bjorklund H, Olson L, Lindh B, Elfvin L, Lundberg JM, Lindgren JA, Samuelsson B, Pernow B, Terenius L, Post C, Everitt B, Goldstein M. Coexistence of neuronal messengers-an overview. Prog Brain Res. 1986;68:33–70. doi: 10.1016/s0079-6123(08)60230-7. [DOI] [PubMed] [Google Scholar]
- Hökfelt T, Meister B, Villar MJ, Ceccatelli S, Corts R, Schalling M, Everitt B. Colocalization of messenger substances with special reference to the hypothalamic arcuate and paraventricular nuclei. Prog Clin Biol Res. 1990;342:257–264. [PubMed] [Google Scholar]
- Horvath TL, Diano S, van den Pol AN. Synaptic interaction between hypocretin (orexin) and neuropeptide Y cells in the rodent and primate hypothalamus: a novel circuit implicated in metabolic and endocrine regulations. J Neurosci. 1999;19:1072–1087. doi: 10.1523/JNEUROSCI.19-03-01072.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosoya Y, Matsushita M. Identification and distribution of the spinal and hypophyseal projection neurons in the paraventricular nucleus of the rat. A light and electron microscopic study with the horseradish peroxidase method. Exp Brain Res. 1979;35:315–331. doi: 10.1007/BF00236618. [DOI] [PubMed] [Google Scholar]
- Hurbin A, Orcel H, Alonso G, Moos F, Rabie A. The vasopressin receptors colocalize with vasopressin in the magnocellular neurons of the rat supraoptic nucleus and are modulated by water balance. Endocrinology. 2002;143:456–466. doi: 10.1210/endo.143.2.8643. [DOI] [PubMed] [Google Scholar]
- Huszar D, Lynch CA, Fairchild-Huntress V, Dunmore JH, Fang Q, Berkemeier LR, Gu W, Kesterson RA, Boston BA, Cone RD, Smith FJ, Campfield LA, Burn P, Lee F. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell. 1997;88:131–141. doi: 10.1016/s0092-8674(00)81865-6. [DOI] [PubMed] [Google Scholar]
- Im SH, Taghert PH. PDF receptor expression reveals direct interactions between circadian oscillators in Drosophila. J. Comp. Neurol. 2010;518:1925–1945. doi: 10.1002/cne.22311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iremonger KJ, Bains JS. Retrograde opioid signaling regulates glutamatergic transmission in the hypothalamus. J Neurosci. 2009;29:7349–7358. doi: 10.1523/JNEUROSCI.0381-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson MB, Konnerth A, Augustine GJ. Action potential broadening and frequency dependent facilitation of calcium signals in pituitary nerve terminals. Proc Natl Acad Sci USA. 1991;88:380–384. doi: 10.1073/pnas.88.2.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan LY, Jan YN. Peptidergic transmission in sympathetic ganglia of the frog. J Physiol. 1982;327:219–246. doi: 10.1113/jphysiol.1982.sp014228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansson A, Descarries L, Cornea-Herbert V, Riad M, Verge D, Bancila M, Agnati LF, Fuxe K. Transmitter-receptor mismatches in central dopamine, serotonin, and neuropeptide systems. Further evidence for volume transmission. In: Walz W, editor. The neuronal environ-ment: brain homeostasis in health and disease. Humana Press; Totowa, NJ: 2002. pp. 83–101. [Google Scholar]
- Ji Y, Lu Y, Yang F, Shen W, Tang TT, Feng L, Duan S, Lu B. Acute and gradual increases in BDNF concentration elicit distinct signaling and functions in neurons. Nat Neurosci. 2010;13:302–309. doi: 10.1038/nn.2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurzak M, Müller AR, Gerstberger R. Characterization of vasopressin receptors in cultured cells derived from the region of rat brain circumventricular organs. Neuroscience. 1995;65:1145–1159. doi: 10.1016/0306-4522(94)00539-h. [DOI] [PubMed] [Google Scholar]
- Kaneko M, Xie Y, An JJ, Stryker MP, Xu B. Dendritic BDNF synthesis is required for late-phase spine maturation and recovery of cortical responses following sensory deprivation. J Neurosci. 2012;32:4790–4802. doi: 10.1523/JNEUROSCI.4462-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauffman AS, Clifton DK, Steiner RA. Emerging ideas about kisspeptin- GPR54 signaling in the neuroendocrine regulation of reproduction. Trends Neurosci. 2007;30:504–511. doi: 10.1016/j.tins.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Kim SH, Ryan TA. CDK5 serves as a major control point in neurotransmitter release. Neuron. 2010;67:797–809. doi: 10.1016/j.neuron.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimelberg H. Glial Cell Receptors. Raven Press; New York: 1988. [Google Scholar]
- Klausberger T, Magill PJ, Márton LF, Roberts JD, Cobden PM, Buzsáki G, Somogyi P. Brain-state- and cell-type-specific firing of hippocampal interneurons in vivo. Nature. 2003;421:844–848. doi: 10.1038/nature01374. [DOI] [PubMed] [Google Scholar]
- Klyachko VA, Jackson MB. Capacitance steps and fusion pores of small and large-dense-core vesicles in nerve terminals. Nature. 2002;418:89–92. doi: 10.1038/nature00852. [DOI] [PubMed] [Google Scholar]
- Kofitsch G, Zamir N, Helke CJ, Savitt JM, Jacobowitz DM. Corticotropin releasing factor-like immunoreactivity in sensory ganglia and capsaicin sensitive neurons of the rat central nervous system: colocalization with other neuropeptides. Peptides. 1985;6:307–318. doi: 10.1016/0196-9781(85)90057-9. [DOI] [PubMed] [Google Scholar]
- Kogo N, Dalezios Y, Capogna M, Ferraguti F, Shigemoto R, Somogyi P. Depression of GABAergic input to identified hippocampal neurons by group III metabotropic glutamate receptors in the rat. Eur J Neurosci. 2004;19:2727–2740. doi: 10.1111/j.0953-816X.2004.03394.x. [DOI] [PubMed] [Google Scholar]
- Koike-Tani M, Collins JM, Kawano T, Zhao P, Zhao Q, Kozasa T, Nakajima S, Nakajima Y. Signal transduction pathway for the substance P-induced inhibition of rat Kir3 (GIRK) channel. J Physiol. 2005;564:489–500. doi: 10.1113/jphysiol.2004.079285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kombian SB, Mouginot D, Pittman QJ. Dendritically released peptides act as retrograde modulators of afferent excitation in the supraoptic nucleus in vitro. Neuron. 1997;19:903–912. doi: 10.1016/s0896-6273(00)80971-x. [DOI] [PubMed] [Google Scholar]
- Kombian SB, Hirasawa M, Mouginot D, Pittman QJ. Modulation of synaptic transmission by oxytocin and vasopressin in the supraoptic nucleus. Prog Brain Res. 2002;139:235–246. doi: 10.1016/s0079-6123(02)39020-4. [DOI] [PubMed] [Google Scholar]
- Kozniewska E, Romaniuk K. Vasopressin in vascular regulation and water homeostasis in the brain. J Physiol Pharmacol 59 Suppl. 2008;8:109–116. [PubMed] [Google Scholar]
- Krashes MJ, Koda S, Ye C, Rogan SC, Adams AC, Cusher DS, Maratos-Flier E, Roth BL, Lowell BB. Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. J Clin Invest. 2011;121:1424–1428. doi: 10.1172/JCI46229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krude H, Biebermann H, Gruters A. Mutations in the human proopiomelanocortin gene. Ann N Y Acad Sci. 2003;994:233–239. doi: 10.1111/j.1749-6632.2003.tb03185.x. [DOI] [PubMed] [Google Scholar]
- Kupfermann I. Functional studies of cotransmission. Physiol Rev. 1991;71:683–732. doi: 10.1152/physrev.1991.71.3.683. [DOI] [PubMed] [Google Scholar]
- Labouèbe G, Lomazzi M, Cruz HG, Creton C, Luján R, Li M, Yanagawa Y, Obata K, Watanabe M, Wickman K, Boyer SB, Slesinger PA, Lüscher C. RGS2 modulates coupling between GABAB receptors and GIRK channels in dopamine neurons of the ventral tegmental area. Nat Neurosci. 2007;10:1559–1568. doi: 10.1038/nn2006. [DOI] [PubMed] [Google Scholar]
- Lambert RC, Dayanithi G, Moos FC, Richard P. A rise in the intracellular Ca2+ concentration of isolated rat supraoptic cells in response to oxytocin. J Physiol. 1994;478:275–287. doi: 10.1113/jphysiol.1994.sp020249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landry M, Vila-Porcile E, Hökfelt T, Calas A. Differential routing of coexisting neuropeptides in vasopressin neurons. Eur J Neurosci. 2003;17:579–589. [PubMed] [Google Scholar]
- Lang T, Wacker I, Steyer J, Kaether C, Wunderlich I, Soldati T, Gerdes H, Almers W. Ca2+-triggered peptide secretion in single cells imaged with green fluorescent protein and evanescent-wave microscopy. Neuron. 1997;18:857–863. doi: 10.1016/s0896-6273(00)80325-6. [DOI] [PubMed] [Google Scholar]
- Lemos JR, Ortiz-Miranda SI, Cuadra AE, Velázquez-Marrero C, Custer EE, Dad T, Dayanithi G. Modulation/physiology of calcium channel sub-types in neurosecretory terminals. Cell Calcium. 2012;51:284–292. doi: 10.1016/j.ceca.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng G, Caquineau C, Ludwig M. Priming in oxytocin cells and in gonadotrophs. Neurochem Res. 2008;33:668–677. doi: 10.1007/s11064-007-9500-0. [DOI] [PubMed] [Google Scholar]
- Li Y, van den Pol AN. Differential target-dependent actions of coexpressed inhibitory dynorphin and excitatory hypocretin/orexin neuropeptides. J Neurosci. 2006;26:13037–13047. doi: 10.1523/JNEUROSCI.3380-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, van den Pol AN. Mu-opioid receptor-mediated depression of the hypothalamic hypocretin/orexin arousal system. J Neurosci. 2008;28:2814–2819. doi: 10.1523/JNEUROSCI.5447-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Gao XB, Sakurai T, van den Pol AN. Hypocretin/Orexin excites hypocretin neurons via a local glutamate neuron-A potential mechanism for orchestrating the hypothalamic arousal system. Neuron. 2002;36:1169–1181. doi: 10.1016/s0896-6273(02)01132-7. [DOI] [PubMed] [Google Scholar]
- Liao GY, An JJ, Gharami K, Waterhouse EG, Vanevski F, Jones KR, Xu B. Dendritically targeted Bdnf mRNA is essential for energy balance and response to leptin. Nat Med. 2012;18:564–571. doi: 10.1038/nm.2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L, Faraco J, Li R, Kadotani H, Rogers W, Lin X, Qiu X, de Jong PJ, Nishino S, Mignot E. The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell. 1999;98:365–376. doi: 10.1016/s0092-8674(00)81965-0. [DOI] [PubMed] [Google Scholar]
- Lingueglia E, Champigny G, Lazdunski M, Barbry P. Cloning of the amiloride-sensitive FMRFamide peptide-gated sodium channel. Nature. 1995;378:730–733. doi: 10.1038/378730a0. [DOI] [PubMed] [Google Scholar]
- López-Huerta VG, Blanco-Hernández E, Bargas J, Galarraga E. Presynaptic modulation by somatostatin in the rat neostriatum is altered in a model of parkinsonism. J Neurophysiol. 2012 doi: 10.1152/jn.00244.2012. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Lovinger DM. Presynaptic modulation by endocannabinoids. Handb Exp Pharmacol. 2008;184:435–477. doi: 10.1007/978-3-540-74805-2_14. [DOI] [PubMed] [Google Scholar]
- Ludwig M, Leng G. Dendritic peptide release and peptide-dependent behaviours. Nat Rev Neurosci. 2006;7:126–136. doi: 10.1038/nrn1845. [DOI] [PubMed] [Google Scholar]
- Ludwig M, Sabatier N, Bull PM, Landgraf R, Dayanithi G, Leng G. Intracellular calcium stores regulate activity-dependent neuropeptide release from dendrites. Nature. 2002;418:85–89. doi: 10.1038/nature00822. [DOI] [PubMed] [Google Scholar]
- Luján R, Maylie J, Adelman JP. New sites of action for GIRK and SK channels. Nature Rev Neurosci. 2009;10:475–480. doi: 10.1038/nrn2668. [DOI] [PubMed] [Google Scholar]
- Luquet S, Perez FA, Hnasko TS, Palmiter RD. NPY/AgRP neurons are essential for feeding in adult mice but can be ablated in neonates. Science. 2005;310:683–685. doi: 10.1126/science.1115524. [DOI] [PubMed] [Google Scholar]
- Lüscher C, Slesinger PA. Emerging roles for G protein-gated inwardly rectifying potassium (GIRK) channels in health and disease. Nat Rev Neurosci. 2010;11:301–315. doi: 10.1038/nrn2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh DJ, Hollopeter G, Kafer KE, Palmiter RD. Role of the Y5 neuropeptide Y receptor in feeding and obesity. Nat Med. 1998;4:718–721. doi: 10.1038/nm0698-718. [DOI] [PubMed] [Google Scholar]
- Marsh DJ, Miura GI, Yagaloff KA, Schwartz MW, Barsh GS, Palmiter RD. Effects of neuropeptide Y deficiency on hypothalamic agouti-related protein expression and responsiveness to melanocortin analogues. Brain Res. 1999;848:66–77. doi: 10.1016/s0006-8993(99)01962-9. [DOI] [PubMed] [Google Scholar]
- Marsh DJ, Baraban SC, Hollopeter G, Palmiter RD. Role of the Y5 neuropeptide Y receptor in limbic seizures. Proc Natl Acad Sci U S A. 1999a;96:13518–13523. doi: 10.1073/pnas.96.23.13518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mencarelli M, Zulian A, Cancello R, Alberti L, Gilardini L, Di Blasio AM, Invitti C. A novel missense mutation in the signal peptide of the human POMC gene: a possible additional link between early-onset type 2 diabetes and obesity. Eur J Hum Genet. 2012 doi: 10.1038/ejhg.2012.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RJ. Presynaptic receptors. Annu Rev Pharmacol Toxicol. 1998;38:201–227. doi: 10.1146/annurev.pharmtox.38.1.201. [DOI] [PubMed] [Google Scholar]
- Miyata S, Takamatsu H, Maekawa S, Matsumoto N, Watanabe K, Kiyohara T, Hatton GI. Plasticity of neurohypophysial terminals with increased hormonal release during dehydration: ultrastructural and biochemical analyses. J Comp Neurol. 2001;434:413–427. doi: 10.1002/cne.1184. [DOI] [PubMed] [Google Scholar]
- Morris JF, Cannata MA. Ultrastructural preservation of the dense core of posterior pituitary neurosecretory granules and its implications for hormone release. J Endocrinol. 1973;57:517–529. doi: 10.1677/joe.0.0570517. [DOI] [PubMed] [Google Scholar]
- Morris JF, Pow DV. Widespread release of peptides in the central nervous system: quantitation of tannic acid-captured exocytoses. Anat Rec. 1991;231:437–445. doi: 10.1002/ar.1092310406. [DOI] [PubMed] [Google Scholar]
- Murray HJ, O'Connor JJ. A role for monomeric G-proteins in synaptic plasticity in the rat dentate gyrus in vitro. Brain Res. 2004;1000:85–91. doi: 10.1016/j.brainres.2003.11.044. [DOI] [PubMed] [Google Scholar]
- Muschol M, Salzberg BM. Dependence of transient and residual calcium dynamics on action-potential patterning during neuropeptide secretion. J Neurosci. 2000;20:6773–6780. doi: 10.1523/JNEUROSCI.20-18-06773.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatsuka T, Fujita T, Inoue K, Kumamoto E. Activation of GIRK channels in substantia gelatinosa neurones of the adult rat spinal cord: a possible involvement of somatostatin. J Physiol. 2008;586:2511–2522. doi: 10.1113/jphysiol.2007.146076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassirpour R, Bahima L, Lalive AL, Lüscher C, Luján R, Slesinger PA. Morphine- and CaMKII-dependent enhancement of GIRK channel signaling in hippocampal neurons. J Neurosci. 2010;30:13419–13430. doi: 10.1523/JNEUROSCI.2966-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro VM, Gottsch ML, Chavkin C, Okamura H, Clifton DK, Steiner RA. Regulation of gonadotropin-releasing hormone secretion by kisspeptin/dynorphin/neurokinin B neurons in the arcuate nucleus of the mouse. J Neurosci. 2009;29:11859–11866. doi: 10.1523/JNEUROSCI.1569-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordmann JJ, Morris JF. Method for quantitating the molecular content of a subcellular organelle: hormone and neurophysin content of newly formed and aged neurosecretory granules. Proc Natl Acad Sci U S A. 1984;81:180–184. doi: 10.1073/pnas.81.1.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliet SH, Piet R, Poulain DA, Theodosis DT. Glial modulation of synaptic transmission: insights from the supraoptic nucleus of the hypothala-mus. Glia. 2004;47:258–267. doi: 10.1002/glia.20032. [DOI] [PubMed] [Google Scholar]
- Pan PY, Ryan TA. Calbindin controls release probability in ventral tegmental area dopamine neurons. Nat Neurosci. 2012 doi: 10.1038/nn.3099. [Epub ahead of print doi: 10.1038/nn.3099] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmiter RD, Erickson JC, Hollopeter G, Baraban SC, Schwartz MW. Life without neuropeptide Y. Recent Prog Horm Res. 1998;53:163–169. [PubMed] [Google Scholar]
- Pennock RL, Dicken MS, Hentges ST. Multiple inhibitory G-Protein-coupled receptors resist acute desensitization in the presynaptic but not postsynaptic compartments of neurons. J Neurosci. 2012;32:10192–10200. doi: 10.1523/JNEUROSCI.1227-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A, Palay SL, Webster H. The fine structure of the nervous system. Oxford Univ Press; 1991. pp. 1–494. [Google Scholar]
- Qian S, Chen H, Weingarth D, Trumbauer ME, Novi DE, Guan X, Yu H, Shen Z, Feng Y, Frazier E, Chen A, Camacho RE, Shearman LP, Gopal-Truter S, MacNeil DJ, Van der Ploeg LH, Marsh DJ. Neither agouti-related protein nor neuropeptide Y is critically required for the regulation of energy homeostasis in mice. Mol Cell Biol. 2002;22:5027–5035. doi: 10.1128/MCB.22.14.5027-5035.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramamoorthy P, Whim MD. Trafficking and fusion of neuropeptide Y-containing dense-core granules in astrocytes. J Neurosci. 2008;28:13815–13827. doi: 10.1523/JNEUROSCI.5361-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raveh A, Cooper A, Guy-David L, Reuveny E. Nonenzymatic rapid control of GIRK channel function by a G protein-coupled receptor kinase. Cell. 2010;143:750–760. doi: 10.1016/j.cell.2010.10.018. [DOI] [PubMed] [Google Scholar]
- Roberto M, Siggins GR. Nociceptin/orphanin FQ presynaptically decreases GABAergic transmission and blocks the ethanol-induced increase of GABA release in central amygdala. Proc Natl Acad Sci USA. 2006;103:9715–9720. doi: 10.1073/pnas.0601899103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogan SC, Roth BL. Remote control of neuronal signaling. Pharmacol Rev. 2011;63:291–315. doi: 10.1124/pr.110.003020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum DM, Rasmussen SG, Kobilka BK. The structure and function of G-protein-coupled receptors. Nature. 2009;459:356–363. doi: 10.1038/nature08144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan TA, Smith SJ. Vesicle pool mobilization during action potential firing at hippocampal synapses. Neuron. 1995;14:983–989. doi: 10.1016/0896-6273(95)90336-4. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richardson JA, Kozlowski GP, Wilson S, Arch JR, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- Saleh TM, Kombian SB, Zidichouski JA, Pittman QJ. Peptidergic modulation of synaptic transmission in the parabrachial nucleus in vitro: importance of degradative enzymes in regulating synaptic efficacy. J Neurosci. 1996;16:6046–6055. doi: 10.1523/JNEUROSCI.16-19-06046.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawchenko PE, Swanson LW. Immunohistochemical identification of neurons of the paraventricular nucleus of the hypothalamus that project to the medulla or to the spinal cord in the rat. J Comp Neurol. 1982;205:260–272. doi: 10.1002/cne.902050306. [DOI] [PubMed] [Google Scholar]
- Schoepp DD. Unveiling the functions of presynaptic metabotropic glutamate receptors in the central nervous system. J Pharmacol Exp Ther. 2001;299:12–20. [PubMed] [Google Scholar]
- Schwartz MW, Woods SC, Porte Jr D, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404:661–671. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- Scott V, Bishop VR, Leng G, Brown CH. Dehydration-induced modulation of kappa-opioid inhibition of vasopressin neurone activity. J Physiol. 2009;587:5679–5689. doi: 10.1113/jphysiol.2009.180232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley RJ, Woods SC. Monitoring of stored and available fuel by the CNS: implications for obesity. Nat Rev Neurosci. 2003;4:901–909. doi: 10.1038/nrn1245. [DOI] [PubMed] [Google Scholar]
- Shakiryanova D, Zettel GM, Gu T, Hewes RS, Levitan ES. Synaptic neuropeptide release induced by octopamine without Ca2+ entry into the nerve terminal. Proc Natl Acad Sci USA. 2011;108:4477–4481. doi: 10.1073/pnas.1017837108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd GM, Chen WR, Greer CA. Olfactory bulb. In: Shepherd GM, editor. In Synaptic organization of the brain. Oxford Univ Press; 2004. pp. 165–216. [Google Scholar]
- Schone C, Cao ZH, Apergis-Schoute J, Adamantidis A, Sakurai T, Burdakov D. Optogenetic probing of fast glutamatergic transmission from hypocretin/orexin to histamine neurons in situ. J. Neurosci. 2012;32:12437–12443. doi: 10.1523/JNEUROSCI.0706-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieburth D, Ch'ng Q, Dybbs M, Tavazoie M, Kennedy S, Wang D, Dupuy D, Rual JF, Hill DE, Vidal M, Ruvkun G, Kaplan JM. Systematic analysis of genes required for synapse structure and function. Nature. 2005;436:510–517. doi: 10.1038/nature03809. [DOI] [PubMed] [Google Scholar]
- Sieburth D, Madison JM, Kaplan JM. PKC-1 regulates secretion of neuropeptides. Nat Neurosci. 2007;10:49–57. doi: 10.1038/nn1810. [DOI] [PubMed] [Google Scholar]
- Simmons ML, Terman GW, Gibbs SM, Chavkin C. L-type calcium channels mediate dynorphin neuropeptide release from dendrites but not axons of hippocampal granule cells. Neuron. 1995;14:1265–1272. doi: 10.1016/0896-6273(95)90273-2. [DOI] [PubMed] [Google Scholar]
- Smith JT, Clarke IJ. Kisspeptin expression in the brain: Catalyst for the initiation of puberty. Rev Endocr Metab Disord. 2007;8:1–9. doi: 10.1007/s11154-007-9026-4. [DOI] [PubMed] [Google Scholar]
- Sperk G, Hamilton T, Colmers WF. Neuropeptide Y in the dentate gyrus. Prog Brain Res. 2007;163:285–297. doi: 10.1016/S0079-6123(07)63017-9. [DOI] [PubMed] [Google Scholar]
- Sudhof TC. The presynaptic active zone. Neuron. 2012;75:11–25. doi: 10.1016/j.neuron.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LW, Kuypers HG. The paraventricular nucleus of the hypothalamus: Cytoarchitectonic subdivisions and the organiza- tion of projections to the pituitary, dorsal vagal complex and spinal cord as demonstrated by the retrograde fluorescence double labeling methods. J Comp Neurol. 1980;194:555–570. doi: 10.1002/cne.901940306. [DOI] [PubMed] [Google Scholar]
- Tallent MK, Siggins GR. Somatostatin depresses excitatory but not inhibitory neurotransmission in rat CA1 hippocampus. J Neurophysiol. 1997;78:3008–3018. doi: 10.1152/jn.1997.78.6.3008. [DOI] [PubMed] [Google Scholar]
- Tallent MK. Presynaptic inhibition of glutamate release by neuropeptides: use-dependent synaptic modification. Results Probl Cell Differ. 2008;44:177–200. doi: 10.1007/400_2007_037. [DOI] [PubMed] [Google Scholar]
- Tasker JG, Oliet SH, Bains JS, Brown CH, Stern JE. Glial regulation of neuronal function: from synapse to systems physiology. J Neuroendocrinol. 2012;24:566–576. doi: 10.1111/j.1365-2826.2011.02259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thapliyal A, Bannister RA, Hanks C, Adams BA. The monomeric G proteins AGS1 and Rhes selectively influence Galphai-dependent signaling to modulate N-type (CaV2.2) calcium channels. Am J Physiol Cell Physiol. 2008;295:C1417–C1426. doi: 10.1152/ajpcell.00341.2008. [DOI] [PubMed] [Google Scholar]
- Theodosis DT, Poulain DA, Oliet SH. Activity-dependent structural and functional plasticity of astrocyte-neuron interactions. Physiol Rev. 2008;88:983–1008. doi: 10.1152/physrev.00036.2007. [DOI] [PubMed] [Google Scholar]
- Tobin V, Ludwig M. The role of the actin cytoskeleton in oxytocin and vasopressin release from rat supraoptic nucleus neurones. J Physiol. 2007;582:1337–1348. doi: 10.1113/jphysiol.2007.132639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin VA, Douglas AJ, Leng G, Ludwig M. The involvement of voltage-operated calcium channels in somato-dendritic oxytocin release. PLoS One. 2011;6:e25366. doi: 10.1371/journal.pone.0025366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin V, Schwab Y, Lelos N, Onaka T, Pittman QJ, Ludwig M. Expression of exocytosis proteins in rat supraoptic nucleus neurones. J Neuroendocrinol. 2012;24:629–641. doi: 10.1111/j.1365-2826.2011.02237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong Q, Ye C, McCrimmon RJ, Dhillon H, Choi B, Kramer MD, Yu J, Yang Z, Christiansen LM, Lee CE, Choi CS, Zigman JM, Shulman GI, Sherwin RS, Elmquist JK, Lowell BB. Synaptic glutamate release by ventromedial hypothalamic neurons is part of the neurocircuitry that prevents hypoglycemia. Cell Metab. 2007;5:383–393. doi: 10.1016/j.cmet.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubink R, Halasz N, Zhang X, Dagerlind A, Hökfelt T. Neuropeptide tyrosine is expressed in ensheathing cells around the olfactory nerves in the rat olfactory bulb. Neuroscience. 1994;60:709–726. doi: 10.1016/0306-4522(94)90499-5. [DOI] [PubMed] [Google Scholar]
- Ubink R, Hökfelt T. Neuropeptide Y expression in Schwann cell precursors. Glia. 2000;32:71–83. [PubMed] [Google Scholar]
- van den Pol AN. Hypothalamic hypocretin (orexin): robust innervation of the spinal cord. J Neurosci. 1999;19:3171–3182. doi: 10.1523/JNEUROSCI.19-08-03171.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Pol AN, Gao XB, Obrietan K, Kilduff TS, Belousov AB. Presynaptic and postsynaptic actions and modulation of neuroendocrine neurons by a new hypothalamic peptide, hypocretin/orexin. J Neurosci. 1998;18:7962–7971. doi: 10.1523/JNEUROSCI.18-19-07962.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Pol AN, Ghosh PK, Liu RJ, Li Y, Aghajanian GK, Gao XB. Hypocretin (orexin) enhances neuron activity and cell synchrony in developing mouse GFP-expressing locus coeruleus. J Physiol. 2002;541:169–185. doi: 10.1113/jphysiol.2002.017426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Pol AN, Wuarin JP, Dudek FE. Glutamate, the dominant excitatory transmitter in neuroendocrine regulation. Science. 1990;250:1276–1278. doi: 10.1126/science.1978759. [DOI] [PubMed] [Google Scholar]
- van den Pol AN. Glutamate and aspartate immunoreactivity in hypothalamic presynaptic axons. J Neurosci. 1991;11:2087–2101. doi: 10.1523/JNEUROSCI.11-07-02087.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Pol AN, Trombley PQ. Glutamate neurons in hypothalamus regulate excitatory transmission. J Neurosci. 1993;13:2829–2836. doi: 10.1523/JNEUROSCI.13-07-02829.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Pol AN. Weighing the role of hypothalamic feeding neurotransmitters. Neuron. 2003;40:1059–1061. doi: 10.1016/s0896-6273(03)00809-2. [DOI] [PubMed] [Google Scholar]
- Vögler O, Barceló JM, Ribas C, Escribá PV. Membrane interactions of G proteins and other related proteins. Biochim Biophys Acta. 2008;1778:1640–1652. doi: 10.1016/j.bbamem.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Weisskopf MG, Zalutsky RA, Nicoll RA. The opioid peptide dynorphin mediates heterosynaptic depression of hippocampal mossy fibre synapses and modulates long-term potentiation. Nature. 1993;362:423–427. doi: 10.1038/362423a0. [DOI] [PubMed] [Google Scholar]
- Wakabayashi Y, Nakada T, Murata K, Ohkura S, Mogi K, Navarro VM, Clifton DK, Mori Y, Tsukamura H, Maeda K, Steiner RA, Okamura H. Neurokinin B and dynorphin A in kisspeptin neurons of the arcuate nucleus participate in generation of periodic oscillation of neural activity driving pulsatile gonadotropin-releasing hormone secretion in the goat. J Neurosci. 2010;30:3124–3132. doi: 10.1523/JNEUROSCI.5848-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Armstrong WE. Tonic regulation of GABAergic synaptic activity on vasopressin neurones by cannabinoids. J Neuroendocrinol. 2012;24:664–673. doi: 10.1111/j.1365-2826.2011.02239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whim MD, Moss GW. A novel technique that measures peptide secretion on a millisecond timescale reveals rapid changes in release. Neuron. 2001;30:37–50. doi: 10.1016/s0896-6273(01)00261-6. [DOI] [PubMed] [Google Scholar]
- Whim MD, Lloyd PE. Modulation of peptide release from single identified Aplysia neurons in culture. J Neurosci. 1992;12:3545–3553. doi: 10.1523/JNEUROSCI.12-09-03545.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whim MD. Pancreatic beta cells synthesize neuropeptide Y and can rapidly release peptide co-transmitters. PLoS One. 2011;6:e19478. doi: 10.1371/journal.pone.0019478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whim MD. Near simultaneous release of classical and peptide cotransmitters from chromaffin cells. J Neurosci. 2006;26:6637–6642. doi: 10.1523/JNEUROSCI.5100-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JT, Christie MJ, Manzoni O. Cellular and synaptic adaptations mediating opioid dependence. Physiol Rev. 2001;81:299–343. doi: 10.1152/physrev.2001.81.1.299. [DOI] [PubMed] [Google Scholar]
- Willis WD. John Eccles’ studies of spinal cord presynaptic inhibition. Prog Neurobiol. 2006;78:189–214. doi: 10.1016/j.pneurobio.2006.02.007. [DOI] [PubMed] [Google Scholar]
- Wong MY, Zhou C, Shakiryanova D, Lloyd TE, Deitcher DL, Levitan ES. Neuropeptide delivery to synapses by long-range vesicle circulation and sporadic capture. Cell. 2012;148:1029–1038. doi: 10.1016/j.cell.2011.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SC, Seeley RJ, Porte D, Jr, Schwartz MW. Signals that regulate food intake and energy homeostasis. Science. 1998;280:1378–1383. doi: 10.1126/science.280.5368.1378. [DOI] [PubMed] [Google Scholar]
- Wu Q, Clark MS, Palmiter RD. Deciphering a neuronal circuit that mediates appetite. Nature. 2012;483:594–597. doi: 10.1038/nature10899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Boyle MP, Palmiter RD. Loss of GABAergic signaling by AgRP neurons to the parabrachial nucleus leads to starvation. Cell. 2009;137:1225–1234. doi: 10.1016/j.cell.2009.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyeth MS, Zhang N, Houser CR. Increased cholecystokinin labeling in the hippocampus of a mouse model of epilepsy maps to spines and glutamatergic terminals. Neuroscience. 2012;202:371–383. doi: 10.1016/j.neuroscience.2011.11.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie K, Martemyanov KA. Control of striatal signaling by g protein regulators. Front Neuroanat 5,Art. 2011;14:1–14. doi: 10.3389/fnana.2011.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Atasoy D, Su HH, Sternson SM. Hunger states switch a flip-flop memory circuit via a synaptic ampk-dependent positive feedback loop. Cell. 2011;146:992–1003. doi: 10.1016/j.cell.2011.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaswen L, Diehl N, Brennan MB, Hochgeschwender U. Obesity in the mouse model of proopiomelanocortin deficiency responds to peripheral melanocortin. Nat Med. 1999;5:1066–1070. doi: 10.1038/12506. [DOI] [PubMed] [Google Scholar]
- Yeo GS, Farooqi IS, Challis BG, Jackson RS, O'Rahilly S. The role of melanocortin signalling in the control of body weight: evidence from human and murine genetic models. QJM. 2000;93:7–14. doi: 10.1093/qjmed/93.1.7. [DOI] [PubMed] [Google Scholar]
- Zhang L, Chung BY, Lear BC, Kilman VL, Liu Y, Mahesh G, Meissner RA, Hardin PE, Allada R. DN1(p) circadian neurons coordinate acute light and PDF inputs to produce robust daily behavior in Drosophila. Curr. Biol. 2010;20:591–599. doi: 10.1016/j.cub.2010.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, van den Pol AN. Thyrotropin-releasing hormone (TRH) inhibits melanin-concentrating hormone neurons: Implications for TRH-mediated anorexic and arousal actions. J Neurosci. 2012;32:3032–3043. doi: 10.1523/JNEUROSCI.5966-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zupanc GKH. Peptidergic transmission: From morphological correlates to functional implications. Micron. 1996;27:35–91. doi: 10.1016/0968-4328(95)00028-3. [DOI] [PubMed] [Google Scholar]