Abstract
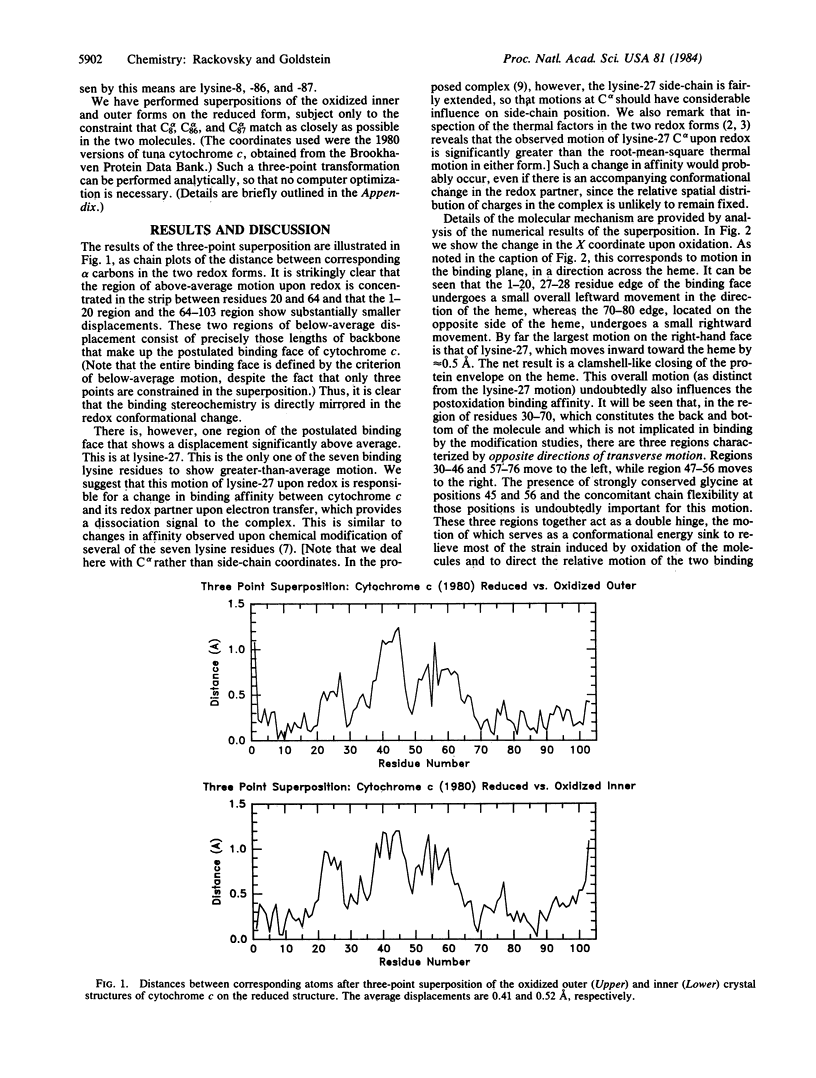
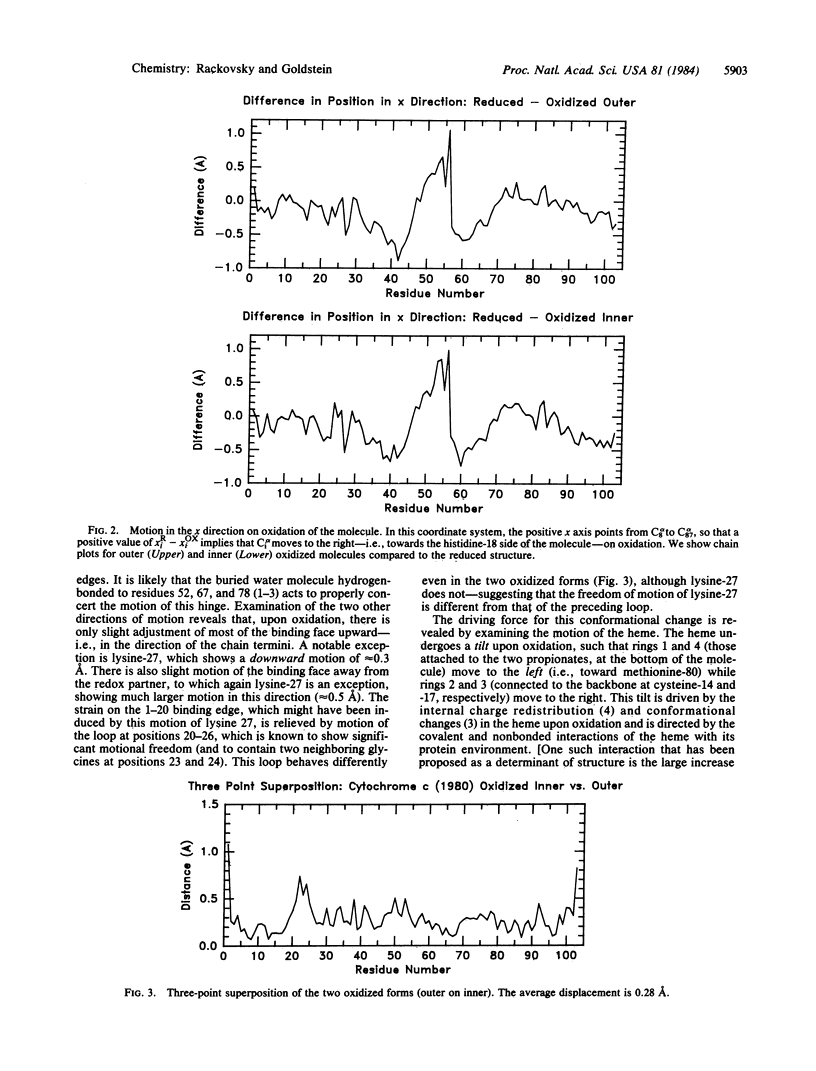
The relationship between the crystal structures of oxidized and reduced tuna cytochrome c has been reexamined by a superposition method motivated by recent studies of the cytochrome c-cytochrome c peroxidase complex. It is shown that the observed structural changes precisely reflect the binding face suggested by chemical modification studies. It is further suggested that the large observed motion of lysine-27 and a smaller overall motion of the two binding edges constitute a redox binding-affinity switch and that the driving force for the conformational change of the protein is provided by the internal conformational change and charge redistribution of the heme, which cause it to tilt, under the influence of covalent and nonbonded interactions, within its protein envelope. A picture is presented of the molecule as an electron storage/transfer machine with three elements--a binding module, an electron storage module, and a conformational energy-storage module.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dickerson R. E. Cytochrome c and the evolution of energy metabolism. Sci Am. 1980 Mar;242(3):137–153. [PubMed] [Google Scholar]
- Juillerat M., Parr G. R., Taniuchi H. A biologically active, three-fragment complex of horse heart cytochrome c. J Biol Chem. 1980 Feb 10;255(3):845–853. [PubMed] [Google Scholar]
- Kang C. H., Brautigan D. L., Osheroff N., Margoliash E. Definitaion of cytochrome c binding domains by chemical modification. Reaction of carboxydinitrophenyl- and trinitrophenyl-cytochromes c with baker's yeast cytochrome c peroxidase. J Biol Chem. 1978 Sep 25;253(18):6502–6510. [PubMed] [Google Scholar]
- Makinen M. W., Schichman S. A., Hill S. C., Gray H. B. Heme-heme orientation and electron transfer kinetic behavior of multisite oxidation-reduction enzymes. Science. 1983 Nov 25;222(4626):929–931. doi: 10.1126/science.6415814. [DOI] [PubMed] [Google Scholar]
- Pettigrew G. Mapping an electron transfer site on cytochrome c. FEBS Lett. 1978 Feb 1;86(1):14–16. doi: 10.1016/0014-5793(78)80087-8. [DOI] [PubMed] [Google Scholar]
- Poulos T. L., Freer S. T., Alden R. A., Edwards S. L., Skogland U., Takio K., Eriksson B., Xuong N., Yonetani T., Kraut J. The crystal structure of cytochrome c peroxidase. J Biol Chem. 1980 Jan 25;255(2):575–580. [PubMed] [Google Scholar]
- Poulos T. L., Kraut J. A hypothetical model of the cytochrome c peroxidase . cytochrome c electron transfer complex. J Biol Chem. 1980 Nov 10;255(21):10322–10330. [PubMed] [Google Scholar]
- Rieder R., Bosshard H. R. Cytochrome bc1 and cytochrome oxidase can bind to the same surface domain of the cytochrome c molecule. FEBS Lett. 1978 Aug 15;92(2):223–226. doi: 10.1016/0014-5793(78)80759-5. [DOI] [PubMed] [Google Scholar]
- Salemme F. R. An hypothetical structure for an intermolecular electron transfer complex of cytochromes c and b5. J Mol Biol. 1976 Apr 15;102(3):563–568. doi: 10.1016/0022-2836(76)90334-x. [DOI] [PubMed] [Google Scholar]
- Salemme F. R. Structure and function of cytochromes c. Annu Rev Biochem. 1977;46:299–329. doi: 10.1146/annurev.bi.46.070177.001503. [DOI] [PubMed] [Google Scholar]
- Takano T., Dickerson R. E. Conformation change of cytochrome c. I. Ferrocytochrome c structure refined at 1.5 A resolution. J Mol Biol. 1981 Nov 25;153(1):79–94. doi: 10.1016/0022-2836(81)90528-3. [DOI] [PubMed] [Google Scholar]
- Takano T., Dickerson R. E. Conformation change of cytochrome c. II. Ferricytochrome c refinement at 1.8 A and comparison with the ferrocytochrome structure. J Mol Biol. 1981 Nov 25;153(1):95–115. doi: 10.1016/0022-2836(81)90529-5. [DOI] [PubMed] [Google Scholar]
- Takano T., Dickerson R. E. Redox conformation changes in refined tuna cytochrome c. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6371–6375. doi: 10.1073/pnas.77.11.6371. [DOI] [PMC free article] [PubMed] [Google Scholar]


