Abstract
Background
Few comparative effectiveness studies of treatment strategies of the use of antihypertensive therapeutic classes in hypertension control have been assessed in a primary care environment.
Objectives
1) To compare the effectiveness of common antihypertensive therapeutic classes initiated as monotherapy in control of hypertension; and 2) To compare the effectiveness of fixed-dose combinations (FDC), free-equivalent combinations (FEC) and monotherapy on hypertension control.
Design
Observational comparative effectiveness analyses of data electronically extracted from the electronic health records.
Participants
The study population consisted of 8,676 patients with an incident prescription for an antihypertensive agent out of 79,176 patients receiving antihypertensive therapy under care in 33 geographically diverse primary care clinics.
Main Measures
Reductions in systolic (SBP) and diastolic (DBP) blood pressure and rates of JNC7 goal attainment.
Key Results
There were small, clinically insignificant differences in BP reductions between the monotherapy classes. Higher rates of BP control were obtained when patients were initiated on an angiotensin-converting-enzyme inhibitor than a thiazide or thiazide-like diuretic (47.8% vs. 39.9%) or a beta-blocker versus a thiazide (45.9% vs. 39.9%). Patients initiated on FDCs had significantly larger reductions in BP than patients initiated on FECs (−17.3 vs. −12.0 mm Hg SBP; −10.1 vs. −6.0 mm Hg DBP) or monotherapy (−17.3 vs. −13.6 mm Hg SBP; −10.1 vs. −7.9 mm Hg DBP). Rates of JNC7 goal attainment also were better for FDCs than FECs (57.2% vs. 42.5%) and for FDCs versus monotherapy (57.2% vs. 44.9%).
Conclusions
Patients initiated on ACEIs and Beta-blockers had slightly higher rates of BP control. The use of FDCs as initial therapy is more effective in the control of hypertension than monotherapy or FECs.
INTRODUCTION
About one third of US adults (76.4 million)1 have hypertension, which is strongly associated with an increased risk of major adverse cardiovascular events (MACE), while treatment of hypertension has been shown to reduce that risk2–4. However, only about half of hypertensive patients have control of their blood pressure1, which leaves a substantial proportion of the population at an increased, but modifiable, risk of MACE.
Monotherapy is the recommended initial approach for reducing blood pressure, except for Stage II hypertension (≥160/100)5. While some individuals may achieve control of their blood pressure to guideline-recommended levels on a single medication, 63% of 12,210 patients with a 5-year visit in the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) required two or more agents6. Another strategy for treating hypertension use of combination therapy either as a fixed-dose combination (FDC), which combines two active agents into a single pill, or free-equivalent combination (FEC), which is the prescribing of the corresponding single-agent pills separately. Several efficacy trials have previously shown combination therapy to be more effective than monotherapy in achieving blood pressure control, but we have found no randomized control trials (RCT) that explicitly evaluated differences in efficacy between the two combination strategies7–9. Other studies, however, have shown that patients on FDCs have greater adherence and persistence to medication use compared to patients on FEC10–11.
The objectives of the present study were: 1) To assess the comparative effectiveness of several antihypertensive therapeutic classes initiated as monotherapy, and 2) To compare the effectiveness of the initial use of three treatment strategies (monotherapy, FDC and FEC) in hypertensive patients receiving care in a diverse primary care setting.
METHODS
Data Source
This study was conducted using data from primary care clinics participating in the DARTNet (Distributed Ambulatory Research in Therapeutics Network) collaborative, a federated network of electronic health record (EHR) data, which has as one of its objectives the facilitation of observational comparative effectiveness research12–14. DARTNet, in collaboration with QED Clinical, Inc., d/b/a CINA (http://www.cina-us.com/), has developed data extraction, transformation, and loading (ETL) processes, which allow aggregation of data from disparate EHRs into a limited database. All data were imported nightly from the organization EHR to a relational clinical data repository (CDR) located behind the firewall of each organization. The CINA software used for ETL were tools that were already in place and being used by each organization to produce point-of-care clinical decision support reports and population management reports.
Data validation was largely the responsibility of CINA as the ETL vendor in place at each organization prior to the initiation of this project. Because CINA provides software tools that utilize data from the CDR in the course of clinical care and decision-making, CINA has several processes in place to ensure the reliability and validity of the data that is contained within the CDR. Data reliability testing by CINA includes the following: 1) Patient-level sampling comparing the data imported into the CDR with the source data as it is represented in the EHR; 2) Daily use in clinical practice of the data in the CDR through the point-of-care clinical decision support tool and population management tools provided by CINA; and 3) Data reliability testing with each data extraction for research analysis.
The DARNet Cardiovascular Risk Reduction Learning Community (CRRLC) was designed to provide patient-specific, point-of-care clinical decision support and audit with feedback on national guidelines (JNC7) for the control of blood pressure (unpublished report). The CRRLC limited data set obtained from the DARTNet collaborative was also used for the current study which includes 33 primary care clinics from 10 health organizations and approximately 154 clinicians providing care to over one quarter million patients.
Data use agreements for access to the limited data set were obtained from each organization and a waiver of informed consent and HIPAA authorization were approved by the American Academy of Family Physician’s IRB and the Colorado Multiple Institutional Review Board (COMIRB) of the University of Colorado Denver.
Data Collection and Cleaning
The data used in the present analysis included patient demographics, height, weight, blood pressure, comorbidities (ICD-9 codes from the problem lists and reasons for visit), medications, laboratory data, and dates of encounters between August 2001 and August 2011. Data on patient race/ethnicity and medication dosing frequency were sparsely populated and were not included in our analyses.
For continuous variables, physiologically implausible values were identified by clinicians examining the distributions of the variables. These consensus-derived, physiologically implausible values were systolic blood pressure <50 or >260 mm Hg, diastolic blood pressure <0 or >200 mm Hg, height <45 or >90 inches, weight <50 or >500 lbs, and serum creatinine <0.2 or >20 mg/dl and were excluded in the current study. The proportion of values deleted varied from 0.005% for systolic blood pressure to 0.5% for serum creatinine. Additionally, height and weight were missing in 4.2% and 0.3% of patients, respectively, and were replaced with gender specific mean height (females 63.9 and males 69.8 inches) and weight (females 178.9 and males 212.9 lbs).
Definitions
A diagnosis of hyperlipidemia was defined as an active ICD-9 code from 272.xx on at least one visit, hypertension as a code from 401 – 405.xx or 437.2 on at least one visit, diabetes mellitus as codes from 250.xx on at least two visits or ≥ 1 antidiabetic medication, and chronic kidney disease (CKD) as a code from 403 – 404.xx, 581 – 582.x, 585 – 586.x, V45.11, V45.12, or V56.x on at least one visit or a calculated glomerular filtration rate16 (eGFR) of >60 ml/min/1.73 m2. Patients were assumed to be white for purposes of eGFR calculations. Finally, therapeutic goals were defined as per JNC7 (<130/80 mm Hg for patients with CKD or diabetes mellitus and <140/90 for all others)5.
Antihypertensive agents were categorized into the following therapeutic classes: angiotensin-converting-enzyme inhibitor (ACEI), angiotensin II receptor blockers (ARB), cardio-selective beta blockers (β-blocker), calcium channel blockers (CCB), and thiazides and thiazide-like diuretics (thiazide) using the Medi-Span® Master Drug Data Base (MDDB®) Version 2.5. FDCs and FECs were defined as being composed of two of the five monotherapy therapeutic classes (vide infra). All other antihypertensive therapeutic classes were excluded due to insufficient numbers for adequate analyses.
Patient Inclusion
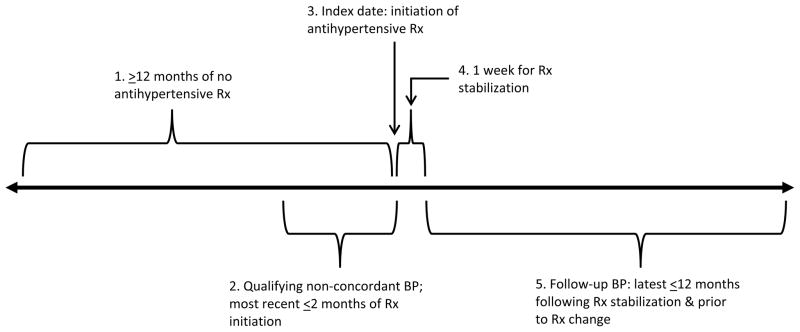
Figure 1 defines the inclusion criteria for this study. The index date was defined as the date of the first prescription for an antihypertensive agent, prior to which the patient had been followed with no antihypertensive prescriptions for ≥12 months. The index blood pressure was the closest to and falling within the two-month interval prior to the initiation of antihypertensive therapy. If the patient’s treatment was classified as FEC, the second drug must have been started within three days of the first. The blood pressures used to assess reduction and goal attainment from the index pressure was the one with the latest date-time stamp within the time period of one week to one year following the index time point during which no change had been made in the antihypertensive drug regime.
Figure 1.
Time-line defining key events in study: for initial use antihypertensive (anti-HTN) patients.
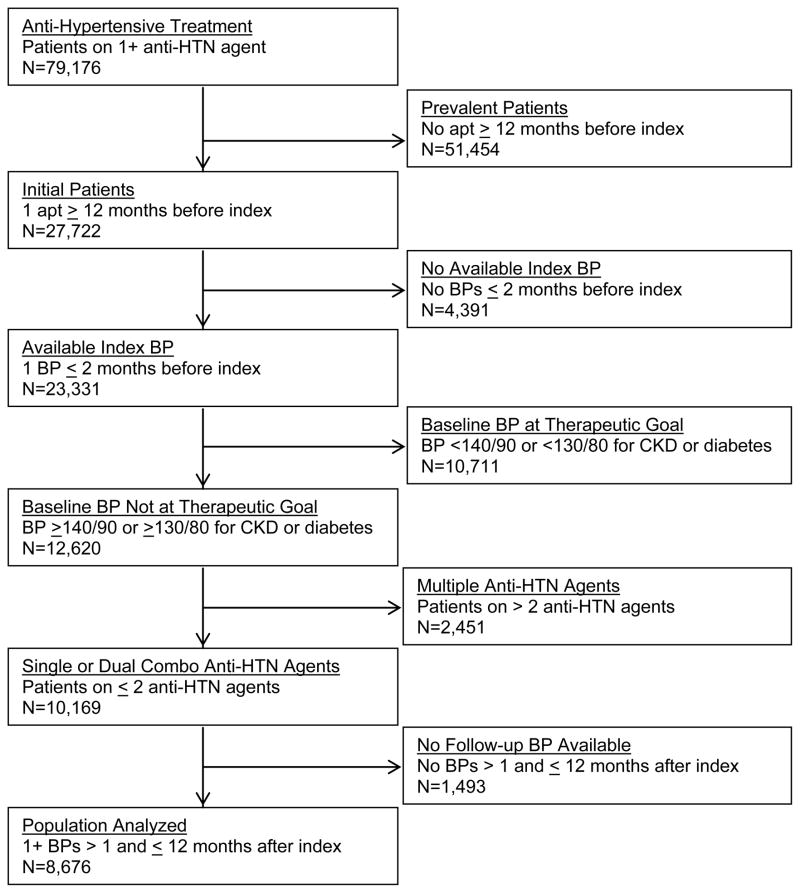
Figure 2 shows the STROBE diagram for inclusion of patients in our analyses. There were 79,176 patients ≥18 years and receiving at least one antihypertensive agent. Exclusions were as follows: 1) 51,454 patients classified as prevalent users of antihypertensive agents because there was no period ≥12 months in duration when they received no antihypertensive agents, 2) 4,391 patients who had no BP measured in the two months prior to initiation of antihypertensive therapy; 3) 10,711 patients whose blood pressure was at goal in the two months prior to initiating antihypertensive therapy; 4) 2,451 patients on three or more antihypertensive agents; and 5) 1,493 patients with no blood pressure measurements available following initiation of antihypertensive therapy. The final analytic subset consists of 8,676 patients or 11.0% of the 79,176 patients ≥18 years and having received at least one prescription for an antihypertensive agent and had elevated blood pressures as defined by JNC7 prior to or on the index date.
Figure 2.
STROBE diagram of initial use antihypertensive (anti-HTN) patient inclusion.
The outcomes evaluated in the present study were changes in systolic and diastolic blood pressure at follow-up from index blood pressures and JNC7 therapeutic goal attainment rates at follow-up.
Statistical Analyses
To characterize the study population, we calculated descriptive statistics using means and standard deviations for continuous variables and compared the different antihypertensive therapeutic classes using analysis of variance. For categorical variables, frequencies and percents were calculated and logistic regression was used to compare patient characteristics between the groups.
We used analysis of covariance to model mean reductions in blood pressures and logistic regression for comparing the proportion of patients in each group achieving blood pressure control. To account for patient differences, we constructed multivariable prediction models using the covariates listed in Table 1, which were chosen using clinical judgment and prior research. All covariates were included in each risk-adjusted model except in the case of mean reductions in blood pressures where baseline diastolic was not included in the model of mean reductions in systolic and vice versa. Hierarchical linear models including clinic and organization were also evaluated, which gave similar results (not shown).
Table 1.
Population Characteristics
| Selected risk-adjustment variables | Study cohort (n=8,676) |
|---|---|
| Gender (female), n (%) | 4,693 (54.1) |
| Age at index (y), mean (SD) | 54.4 (13.4) |
| Body mass index (kg/m2), mean (SD) | 30.7 (6.9) |
| Index systolic (mm Hg), mean (SD) | 148.7 (15.1) |
| Index diastolic (mm Hg), mean (SD) | 90.4 (10.9) |
| Chronic kidney disease (CKD), n (%) | 854 (9.8) |
| Diabetes mellitus, n (%) | 1,325 (15.3) |
| Hyperlipidemia diagnosis, n (%) | 2,841 (32.8) |
| Hypertension diagnosis, n (%) | 5,330 (61.4) |
| Follow-up duration in months, mean (SD) | 6.3 (3.9) |
| Follow-up duration in months, median (IQR) | 6.5 (2.5–10.5) |
| Number of clinic visits within 1 year prior to index, mean (SD) | 3.9 (3.4) |
| Number of clinic visits within 1 year prior to index, median (IQR) | 3.0 (2.0–5.0) |
Risk-adjusted outcomes for each antihypertensive therapeutic class were calculated from predicted values obtained from models fitted with covariates only. Means and standard deviations of the predicted values were calculated to obtain risk-adjusted average reductions in blood pressures. For risk-adjusted control rates, expected (E) rates of control for each antihypertensive therapeutic class were compared to the observed (O) rates of control as an O/E ratio which was converted to standardized rates by multiplying each O/E ratio by the observed rate of control for all patients across all antihypertensive therapeutic classes.
For all outcomes, p values were adjusted for multiple tests by the Bonferroni method. All statistical tests were considered to be significant at a two-sided p <0.05. All analyses were performed using SAS®, version 9.3.
RESULTS
Population Characteristics
The population characteristics for all 8,676 initial use antihypertensive in primary care are shown in Table 1. They tended to be middle-aged and overweight, and slightly less than a quarter had either diabetes or chronic kidney disease requiring lower blood pressure goals. Only 61% had a diagnosis of hypertension. The median follow-up duration was a little more than six months and, in general, the patients had approximately three clinic visits in the year prior to index date.
Population characteristics stratified by therapeutic class are presented in Appendix Table 1 and by treatment strategy in Appendix Table 2. The proportions and mean values for essentially all risk factors differed significantly across therapeutic classes and treatment strategies. The prevalence of CKD was greater in those initiated on a β-blocker (11.5%), ARB (9.8%), and CCB (17.8%) compared to ACEI (7.1%) or a thiazide (6.5%) and patients with diabetes and hyperlipidemia tended to receive an ACEI (25.2% and 37.6%, respectively) or an ARB (20.5% and 36.8%) more than the other therapeutic classes. For the groups defined by treatment strategy, females, older patients, and patients with CKD or diabetes were initiated on a FEC more often than a FDC or monotherapy; and patients with a hypertension diagnosis were initiated on a FDC or a FEC more often than monotherapy. Also of note is the index systolic and diastolic blood pressures were substantially higher in those initiated on a FDC (154 and 94 mm Hg, respectively) compared to those initiated on a FEC (148 and 86 mm Hg) or monotherapy (148 and 90 mm Hg).
Appendix Table 1.
Unadjusted Population Characteristics for Initial Use Antihypertensive Primary Care Patients Initiated on a Monotherapy by Therapeutic Class
| Characteristic | ACEI* (n=3,131) | Thiazide* (n=1,947) | β-blocker* (n=1,029) | ARB* (n=533) | CCB* (n=529) | p values† |
|---|---|---|---|---|---|---|
| Gender (female), n (%) | 1,442 (46.1) | 1,248 (64.1) | 601 (58.4) | 275 (51.6) | 312 (59.0) | <0.001 |
| Age at index (y), mean (SD) | 53.6 (13.0) | 52.6 (13.0) | 54.1 (14.4) | 55.2 (12.4) | 57.8 (14.7) | <0.001 |
| BMI (kg/m2), mean (SD) | 30.9 (6.8) | 31.1 (7.3) | 28.9 (6.2) | 30.4 (6.1) | 29.7 (6.7) | <0.001 |
| Index systolic (mm Hg), mean (SD) | 147.6 (15.0) | 149.3 (13.4) | 147.7 (15.4) | 147.8 (14.9) | 149.5 (15.7) | <0.001 |
| Index diastolic (mm Hg), mean (SD) | 90.1 (10.7) | 91.5 (10.1) | 90.1 (10.9) | 89.7 (10.4) | 89.4 (11.4) | <0.001 |
| CKD, n (%) | 221 (7.1) | 127 (6.5) | 118 (11.5) | 52 (9.8) | 94 (17.8) | <0.001 |
| Diabetes, n (%) | 789 (25.2) | 89 (4.6) | 75 (7.3) | 109 (20.5) | 29 (5.5) | <0.001 |
| Hyperlipidemia, n (%) | 1,177 (37.6) | 557 (28.6) | 296 (28.8) | 196 (36.8) | 155 (29.3) | <0.001 |
| Hypertension, n (%) | 1,902 (60.8) | 1,335 (68.6) | 506 (49.2) | 258 (48.4) | 279 (52.7) | <0.001 |
| Follow-up duration (months), mean (SD) | 6.5 (3.8) | 5.9 (3.9) | 6.3 (3.9) | 6.2 (3.8) | 6.4 (4.0) | <0.001 |
| Number of clinic visits, mean (SD) | 3.8 (3.2) | 3.9 (3.2) | 4.2 (4.0) | 3.6 (3.4) | 4.3 (3.8) | <0.001 |
Abbrev: ACEI, angiotensin-converting-enzyme inhibitor; Thiazide, thiazide and thiazide-like diuretics; β-blocker, beta blockers cardio-selective; ARB, angiotensin II receptor antagonist; CCB, calcium channel blockers.
P values were from analysis of variance (ANOVA) or logistic regression and test the overall effect across the five therapeutic classes.
Appendix Table 2.
Unadjusted Population Characteristics for Initial Use Antihypertensive Primary Care Patients by Treatment Strategy
| Characteristic | FDC* (n=795) | FEC* (n=712) | Mono* (n=7,169) | p values† |
|---|---|---|---|---|
| Gender (female), n (%) | 373 (46.9) | 442 (62.1) | 3,878 (44.7) | <0.001 |
| Age at index (y), mean (SD) | 52.9 (12.6) | 61.9 (12.9) | 53.8 (13.4) | <0.001 |
| BMI (kg/m2), mean (SD) | 31.9 (7.2) | 30.4 (6.9) | 30.6 (6.8) | <0.001 |
| Index systolic (mm Hg), mean (SD) | 153.8 (16.5) | 147.8 (16.4) | 148.2 (14.7) | <0.001 |
| Index diastolic (mm Hg), mean (SD) | 94.3 (11.1) | 85.9 (12.2) | 90.4 (10.6) | <0.001 |
| CKD, n (%) | 54 (6.8) | 188 (26.4) | 612 (7.1) | <0.001 |
| Diabetes, n (%) | 66 (8.3) | 168 (23.6) | 1,091 (12.6) | <0.001 |
| Hyperlipidemia, n (%) | 251 (31.6) | 209 (29.4) | 2,381 (27.4) | 0.09 |
| Hypertension, n (%) | 594 (74.7) | 456 (64.0) | 4,280 (49.3) | <0.001 |
| Follow-up duration (months), mean (SD) | 6.2 (3.8) | 6.7 (3.9) | 6.3 (3.9) | 0.02 |
| Number of clinic visits, mean (SD) | 3.0 (2.8) | 4.2 (3.8) | 3.9 (3.4) | <0.001 |
Abbrev: FDC, fixed-dose combinations; FEC, free-equivalent combinations; Mono, monotherapy.
P values were from analysis of variance (ANOVA) or logistic regression and test the overall effect across the three treatment strategies.
Appendix table 3 presents the frequencies and proportion of patients initiated on FDC or FEC treatment strategies by different therapeutic class combinations. Although similar numbers of patients were initiated on a FDC (795) as on our definition of a FEC (712), some combination of an ACEI plus a thiazide or ARB plus a thiazide accounted for 81% of those initiated on a FDC, while the distribution among two-drug combinations was much more heterogeneous for those started on a FEC.
Appendix Table 3.
Therapeutic Classes for Initial Use Patients Initiated on a Fixed-Dose or Free-Equivalent Combination
| Therapeutic class* | FDC* (n=795) | FEC* (n=712) |
|---|---|---|
| ACEI & Thiazide, n (%) | 397 (49.9) | 187 (26.3) |
| ARB & Thiazide, n (%) | 250 (31.5) | 39 (5.5) |
| ACEI & CCB, n (%) | 83 (10.4) | 80 (11.2) |
| ACEI & β-blocker, n (%) | 0 (0) | 140 (19.7) |
| β-blocker & Thiazide, n (%) | 34 (4.3) | 81 (11.4) |
| CCB & Thiazide, n (%) | 0 (0) | 73 (10.3) |
| ARB & CCB, n (%) | 31 (3.9) | 28 (3.9) |
| β-blocker & CCB, n (%) | 0 (0) | 38 (5.3) |
| ARB & β-blocker, n (%) | 0 (0) | 34 (4.8) |
| ACEI & ARB, n (%) | 0 (0) | 12 (1.7) |
Abbrev: FDC, fixed-dose combinations; FEC, free-equivalent combinations; ACEI, angiotensin-converting-enzyme inhibitor; Thiazide, thiazide and thiazide-like diuretics; β-blocker, beta blockers cardio-selective; ARB, angiotensin II receptor antagonist; CCB, calcium channel blockers.
Antihypertensive Therapeutic Class Outcomes
Table 2 presents the unadjusted and risk-adjusted change in blood pressure and goal attainment rates for patients initiated on a monotherapy. All five therapeutic classes were efficacious in reducing blood pressure with remarkably similar average reductions. There were no significant differences in the unadjusted average systolic blood pressure reductions and only two statistically but of questionable clinical significance (both < 1.0 mm Hg) differences after risk-adjustment. The unadjusted average diastolic blood pressure reductions were also similar with only a single significant difference (between ACEI and thiazide), albeit in reverse, after risk-adjustment. However, while statistically significant, these differences were small (unadjusted 1.5 mm Hg; risk-adjusted 0.3 mm Hg).
Table 2.
Unadjusted and Risk-Adjusted Change in Blood Pressure (BP) and Goal Attainment Rates by Antihypertensive Therapeutic Classes Initiated as Monotherapy
| Outcomes | ACEI* (n=3,131) | Thiazide* (n=1,947) | β-blocker* (n=1,029) | ARB* (n=533) | CCB* (n=529) |
|---|---|---|---|---|---|
| Systolic, mm Hg† | |||||
| BP at index, mean (SD) | 147.6 (15.0) | 149.3 (13.4) | 147.7 (15.4) | 147.8 (14.9) | 149.5 (15.7) |
| BP at follow-up, mean (SD) | 133.6 (16.2) | 136.6 (14.9) | 134.9 (16.9) | 135.1 (15.8) | 136.7 (17.1) |
| Unadjusted change, mean (SD) | −14.0 (18.1) | −12.7 (17.1) | −12.8 (18.2) | −12.7 (17.7) | −12.8 (19.5) |
| Risk-adjusted change, mean (SD) | −13.4 (10.3) | −14.1 (9.3)‡ | −13.4 (10.8) | −13.3 (10.4) | −14.0 (10.9)§ |
| Diastolic, mm Hg† | |||||
| BP at index, mean (SD) | 90.1 (10.7) | 91.5 (10.1) | 90.1 (10.9) | 89.7 (10.4) | 89.4 (11.4) |
| BP at follow-up, mean (SD) | 81.8 (10.1) | 84.6 (10.5) | 82.8 (10.8) | 82.5 (10.5) | 82.0 (11.1) |
| Unadjusted change, mean (SD) | −8.3 (11.4)‡ | −6.8 (11.3) | −7.4 (11.3) | −7.1 (11.3) | −7.3 (11.9) |
| Risk-adjusted change, mean (SD) | −7.9 (6.6) | −8.2 (6.3)‡ | −7.7 (6.8) | −7.6 (6.4) | −7.5 (7.0) |
| Goal attainment† | |||||
| Unadjusted at goal, n (%) | 1,465 (46.8) | 826 (42.4) | 508 (49.4)¶ | 229 (43.0) | 239 (45.2) |
| Risk-adjusted at goal, n (%) | 1,495 (47.8)‡ | 776 (39.9) | 472 (45.9)# | 230 (43.1) | 234 (44.2) |
Abbrev: ACEI, angiotensin-converting-enzyme inhibitor; Thiazide, thiazide and thiazide-like diuretics; β-blocker, beta blockers cardio-selective; ARB, angiotensin II receptor antagonist; CCB, calcium channel blockers.
Unadjusted p values were from analysis of variance (ANOVA) or logistic regression while risk-adjusted p values were from analysis of covariance (ANCOVA) or logistic regression.
ACEI vs. Thiazide p <0.001.
ACEI vs. CCB p=0.04.
ACEI vs. Thiazide p=0.02.
β-blocker vs. Thiazide p=0.003.
β-blocker vs. Thiazide p=0.01.
Patients initiated on either an ACEI or β-blocker had significantly higher rates of unadjusted and adjusted goal attainment than patients initiated on a thiazide.
Antihypertensive Treatment Strategy Outcomes
Table 3 presents the unadjusted and risk-adjusted change in blood pressure and goal attainment rates for patients initiated on FDC, FEC or monotherapy treatment strategies. Patients initiated on FDC had significantly larger average reductions in systolic and diastolic blood pressure and goal attainment rates than patients initiated on FEC or monotherapy for both the unadjusted and risk-adjusted outcomes. Patients whose combination therapy was a FEC had significantly smaller unadjusted reductions in systolic and diastolic blood pressure and unadjusted goal attainment rates when compared to patients initiated on monotherapy. After risk-adjustment, patients initiated on monotherapy demonstrated a significantly larger average reduction in systolic blood pressure only, over patients initiated on FEC, although this difference was less than 2 mm Hg.
Table 3.
Unadjusted and Risk-Adjusted Change in Blood Pressure (BP) and Goal Attainment Rates by Initial Antihypertensive Treatment Strategy
| Outcomes | FDC* | FEC* | Mono* | p values† | ||
|---|---|---|---|---|---|---|
| (n=795) | (n=712) | (n=7,169) | FDC vs. FEC | FDC vs. Mono | FEC vs. Mono | |
| Systolic, mm Hg | ||||||
| BP at index, mean (SD) | 153.8 (16.5) | 147.8 (16.4) | 148.2 (14.7) | <0.001 | <0.001 | 0.47 |
| BP at follow-up, mean (SD) | 132.3 (16.1) | 137.5 (18.6) | 134.9 (16.1) | <0.001 | <0.001 | <0.001 |
| Unadjusted change, mean (SD) | −21.5 (20.5) | −10.3 (20.7) | −13.3 (17.9) | <0.001 | <0.001 | <0.001 |
| Risk-adjusted change, mean (SD) | −17.3 (11.6) | −12.0 (11.5) | −13.6 (10.2) | <0.001 | <0.001 | 0.04 |
| Diastolic, mm Hg | ||||||
| BP at index, mean (SD) | 94.3 (11.1) | 85.9 (12.2) | 90.4 (10.6) | <0.001 | <0.001 | <0.001 |
| BP at follow-up, mean (SD) | 82.0 (10.6) | 79.8 (11.7) | 82.8 (10.5) | <0.001 | 0.06 | <0.001 |
| Unadjusted change, mean (SD) | −12.3 (12.3) | −6.1 (12.5) | −7.6 (11.4) | <0.001 | <0.001 | 0.003 |
| Risk-adjusted change, mean (SD) | −10.1 (6.8) | −6.0 (7.5) | −7.9 (6.6) | <0.001 | <0.001 | 0.34 |
| Goal attainment | ||||||
| Unadjusted at goal, n (%) | 440 (55.4) | 226 (37.4) | 3,267 (45.6) | <0.001 | <0.001 | <0.001 |
| Risk-adjusted at goal, n (%) | 455 (57.2) | 303 (42.5) | 3,216 (44.9) | <0.001 | <0.001 | 0.69 |
Abbrev: FDC, fixed-dose combinations; FEC, free-equivalent combinations; Mono, monotherapy.
Unadjusted p values were from analysis of variance (ANOVA) or logistic regression while risk-adjusted p values were from analysis of covariance (ANCOVA) or logistic regression.
DISCUSSION
This study found that patients receiving care from 33 primary care clinics initiated on one of the five antihypertensive therapeutic classes as monotherapy had remarkably similar average reductions in blood pressure at follow up (Table 2). These results are consistent with those found in a meta-analysis of 354 randomized, double-blind trials, which reported little difference in mean placebo-corrected reduction in blood pressure across the same five therapeutic classes16. Our results extend this meta-analysis of efficacy studies done under strictly controlled conditions to the real world of effectiveness in daily practice.
Despite similar reductions in blood pressure across therapeutic classes, patients initiated on ACEI and β-blocker had higher rates of JNC7 goal attainment than patients initiated on thiazide even after adjusting for patient risk factors. Our study results cannot be directly compared with the Treatment of Mild Hypertension Study17, ALLHAT18, or that of Matterson19, which evaluated efficacy by individual agents, while we evaluated the effectiveness by therapeutic class. Also, response to therapeutic classes has been shown to vary by race (19), and racial distributions are likely to be different between the studies. Unfortunately, we did not have adequate racial data to adjust for this factor.
We also observed that primary care patients initiated on FDC had considerably larger reductions in blood pressure and higher goal attainment rates at follow up than patients initiated on monotherapy (Table 3). These results in primary care patients confirm those observed in short term, randomized, clinical efficacy studies and two recent observational studies – all showing that patients initiated on FDC obtain better control of their blood pressure than patients initiated on monotherapy alone7–9, 20–21. However, both of these observational studies also demonstrated superior effectiveness in blood pressure control in patients initiated on FEC when compared to patients on monotherapy, while there was very little difference between these two treatment strategies observed in the present study. The lack of improved blood pressure control in patients initiated on FEC could be due to the inclusion of combinations that have been identified by the American Society of Hypertension as being less effective (e.g., ACEI and β-blocker, ARB and β-blocker, and ACEI and ARB)22. Another difference between the present study and the two observational studies is that the latter allowed for adding therapeutic classes and changes in dosage while the present study did not. In addition, several studies have shown that patients taking FDC have improved adherence over patients on FEC which might further explain the differences seen in our study10–11.
Finally, this study found that patients initiated on a FDC had larger reductions in blood pressure and superior rates of blood pressure control when compared to patients initiated on a FEC, which is similar to that reported by Egan et al21.
Effective treatment of high blood pressure is the key therapeutic strategy shown to reduce hypertension-related major adverse cardiovascular events (MACE). However, hypertension frequently remains uncontrolled in the general population, and most patients require the combination of two or more drugs to reach recommended blood pressure goals6. Recently the American Society of Hypertension published a position paper recommending the use of combination therapy routinely to achieve blood pressure targets and classified combinations as preferred, acceptable, or less effective22. The use of combination of drugs from complementary classes at low doses has been shown to be more effective at lowering blood pressure than increasing the dose of a single agent23. The use of low dose combinations would potentially improve the overall tolerability since most side effects of antihypertensive agents are dose-dependent and often drug-specific. While these benefits should apply regardless of whether they were initiated as a FDC or FEC, several studies have shown that patients taking FDC have improved adherence over patients on a FEC since patient adherence is inversely related to the number of pills prescribed10–11. The oft-stated disadvantage of FDCs has been their inability to independently titrate component drug doses along with higher cost due to limited availability of generic FDCs. However, if goal attainment is better, the need for titration becomes less.
The strengths of the present study are in the use of patients from clinical practices which allows for comparisons of initial hypertension treatment strategies and therapeutic classes outside of a research intensive setting such as is seen in clinical trials; a geographically diverse sample of primary care clinics; relatively large sample size; and the ability to adjust for differences in patient characteristics. However, there were several limitations to the study which include: (1) the observational nature of the study as compared to a randomized clinical trial, which could lead to selection biases in the choice of treatments for the patients and residual confounding even after statistical adjustment for some important variables; (2) the inability to adjust for patient race/ethnicity, which had been shown to be associated with hypertension treatment; (3) the lack of prescription fulfillment data and pill counts or use of other techniques to monitor pills consumed by the patient; and (4) the lack of data on dose and dosing frequency.
CONCLUSIONs
Patients initiated on any of the five antihypertensive therapeutic classes as a monotherapy had similar reductions in blood pressure, while patients initiated on ACEIs and Beta-blockers had slightly higher rates of BP control than patients initiate on a thiazide. The use of FDCs as initial therapy is more effective in the control of hypertension than monotherapy or FECs.
Acknowledgments
This work was supported by The National Institutes of Health, National Heart, Lung, and Blood Institute (NHLBI) Grant 1RC1HL101071-01
The authors would like to thank Dr. Wilson Pace for his contribution to the design of the study.
Footnotes
The authors have no conflicting or competing interests.
References
- 1.Roger VL, Go AS, Lloyd-Jones DM, et al. Executive Summary: Heart Disease and Stroke Statistics-2012 Update: A Report from the American Heart Association. Circulation. 2012;125:188–197. doi: 10.1161/CIR.0b013e3182456d46. [DOI] [PubMed] [Google Scholar]
- 2.Lewington S, Clarke R, Qizibash N, Peto R, Collins R. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies: prospective studies collaboration. Lancet. 202;360:1903–1913. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 3.Collins R, Peto R, MachMahon S, et al. Blood pressure, stroke, and coronary heart disease. Part 2, Short-term reductions in blood pressure: overview of randomized drug trials in their epidemiological context. Lancet. 1990;335:827–838. doi: 10.1016/0140-6736(90)90944-z. [DOI] [PubMed] [Google Scholar]
- 4.Turnbull F. Blood Pressure Lowering Treatment Trialists’ Collaboration: Effects of different blood-pressure-lowering regimens on major cardiovascular events: results of prospectively-designed overviews of randomized trials. Lancet. 2003;362:1527–1535. doi: 10.1016/s0140-6736(03)14739-3. [DOI] [PubMed] [Google Scholar]
- 5.Chobanian AV, Bakris GL, Black HR, et al. the National High Blood Pressure Education Program Coordinating Committee. Seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 6.Cushman WC, Ford CE, Cutler JA, et al. ALLHAT Collaborative Research Group. Success and predictors of blood pressure control in diverse North American settings: the antihypertensive and lipid-lowering treatment to prevent heart attack trial (ALLHAT) J Clin Hypertens (Greenwich) 2002;4:393–404. doi: 10.1111/j.1524-6175.2002.02045.x. [DOI] [PubMed] [Google Scholar]
- 7.Bakris GL, Weir MR on behalf of the study of hypertension and the efficacy of Lotrel in diabetes (SHIELD) investigators. Achieving goal blood pressure in patients with type 2 diabetes: Conventional versus fixed-dose combination approaches. J Clin Hypertens. 2003;5(3):202–209. doi: 10.1111/j.1524-6175.2002.2041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown MJ, McInnes GT, Papst CC, Zhang J, MacDonald TM. Aliskiren and the calcium channel blocker Amlodipine combination as an initial treatment strategy for hypertension control (ACCELERATE): a randomised, parallel-group trial. Lancet. 2011;377:312–320. doi: 10.1016/S0140-6736(10)62003-X. [DOI] [PubMed] [Google Scholar]
- 9.Neutel JM, Mancia G, Black HR, et al. behalf of the TEAMSTA severe HTN study investigators. Single-pill combination of Telmisartan/Amlodipine in patients with severe hypertension: Results from the TEAMSTA severe HTN study. J Clin Hypertens. 2012;14(4):206–215. doi: 10.1111/j.1751-7176.2012.00595.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baser O, Andrews LM, Wang L, Xie L. Comparison of real-world adherence, healthcare resource utilization and costs for newly initiated Valsartan/Amlodipine singe-pill combination versus angiotensin receptor blocker/calcium channel blocker free-combination therapy. J Med Econ. 2011;14(5):576–583. doi: 10.3111/13696998.2011.596873. [DOI] [PubMed] [Google Scholar]
- 11.Sherrill B, Halpern M, Khan S, Zhang J, Panjabi S. Single-pill vs. free-equivalent combination therapies for hypertension: A meta-analysis of health care costs and adherence. J Clin Hypertens. 2011;13(12):898–909. doi: 10.1111/j.1751-7176.2011.00550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Libby AM, Pace W, Bryan C, et al. Comparative effectiveness research in DARTNet primary care practices: point of care data collection on hypoglycemia and over-the -counter and herbal use among patients diagnosed with diabetes. Med Care. 2010;48(6):S39–S44. doi: 10.1097/MLR.0b013e3181ddc7b0. [DOI] [PubMed] [Google Scholar]
- 13.Pace WD, Cifuentes M, Valuck RJ, Staton EW, Brandt EC, West DR. An electronic practice-based network for observational comparative effectiveness research. Ann Intern Med. 2009;151(5):338–340. doi: 10.7326/0003-4819-151-5-200909010-00140. [DOI] [PubMed] [Google Scholar]
- 14.Pace WD, West DR, Valuck RJ, Cifuentes M, Staton EW. Distributed Ambulatory Research in Therapeutics Network (DARTNet): Summary Report. Rockville, MD: Agency for Healthcare Research and Quality; 2009. Jul 28, (Prepared by University of Colorado DEcIDE Center under Contract No. HHSA29020050037I TO2.) Report No.: 14. [Google Scholar]
- 15.Levely AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009 May 5;150(9):604–12. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Law MR, Wald NJ, Morris JK, Jordan RE. Value of low dose combination treatment with blood pressure lowering drugs: analysis of 354 randomised trials. BMJ. 2003;326:1427. doi: 10.1136/bmj.326.7404.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neaton JD, Grimm RH, Jr, Prineas RJ, et al. Treatment of Mild Hypertension Study. JAMA. 1993;270(6):713–724. [PubMed] [Google Scholar]
- 18.Cushman WC, Ford CE, Einhorn PT, et al. ALLHAT Collaborative Research Group. Blood pressure control by drug group in the antihypertensive and lipid-lowering treatment to prevent heart attack trial (ALLHAT) J Clin Hypertens (Greenwich) 2008;10(10):751–760. doi: 10.1111/j.1751-7176.2008.00015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Materson BJ, Reda DJ, Cushman WC, et al. Single-drug therapy for hypertension in men. N engl J Med. 1993;328:914–921. doi: 10.1056/NEJM199304013281303. [DOI] [PubMed] [Google Scholar]
- 20.Byrd JB, Zeng C, Tavel HM, et al. Combination therapy as initial treatment for newly diagnosed hypertension. Am Heart J. 2011;162:340–346. doi: 10.1016/j.ahj.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Egan BM, Bandyopadhyay D, Shaftman SR, Wagner CS, Zhao Y, Yu-Isenber KS. Initial monotherapy and combination therapy and hypertension control the first year. Hypertension. 2012;59:1124–1131. doi: 10.1161/HYPERTENSIONAHA.112.194167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gradman AH, Basile JN, Carter BL, Bakris GL. Combination therapy in hypertension. J Am Soc Hypertens. 2010;4:42–50. doi: 10.1016/j.jash.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 23.Wald DS, Law M, Morris JK, Bestwick JP, Wald NJ. Combination therapy versus monotherapy in reducing blood pressure: meta-analysis on 11,000 participants from 42 trials. Am J Med. 2009;122:243–262. doi: 10.1016/j.amjmed.2008.09.038. [DOI] [PubMed] [Google Scholar]