Abstract
High levels of liver enzymes GGT, ALT and AST are predictive of disease and all-cause mortality and can reflect liver injury, fatty liver and/or oxidative stress. Variation in GGT, ALT and AST levels is heritable. Moderation of the heritability of these liver enzymes by age and sex has not often been explored, and it is not clear to what extent non-additive genetic and shared environmental factors may play a role. To examine the genetic architecture of GGT, ALT and AST, plasma levels were assessed in a large sample of twins, their siblings, parents and spouses (N = 8,371; age range 18–90). For GGT and ALT, but not for AST, genetic structural equation modeling showed evidence for quantitative sex differences in the genetic architecture. There was no evidence for qualitative sex differences, i.e. the same genes were expressed in males and females. Both additive and non-additive genetic factors were important for GGT in females (total heritability h2 60 %) and AST in both sexes (total h2 43 %). The heritability of GGT in males and ALT for both sexes was due to additive effects only (GGT males 30 %; ALT males 40 %, females 22 %). Evidence emerged for shared environmental factors influencing GGT in the male offspring generation (variance explained 28 %). Thus, the same genes influence liver enzyme levels across sex and age, but their relative contribution to the variation in GGT and ALT differs in males and females and for GGT across age. Given adequate sample sizes these results suggest that genome-wide association studies may result in the detection of new susceptibility loci for liver enzyme levels when pooling results over sex and age.
Keywords: Liver enzymes, Heritability, Gamma-glutamyl transferase (GGT), Alanine aminotransferase (ALT), Aspartate aminotransferase (AST)
Introduction
High levels of liver enzymes γ-glutamyl transferase (GGT), alanine and aspartate aminotransferase (ALT, AST) increase risk of disease and all-cause mortality. For GGT, high correlations are found with liver disease (Kazemi-Shirazi et al. 2007; Ruhl and Everhart 2009), type 2 diabetes (Fraser et al. 2009) and cardiovascular disease (Targher 2009; Fraser et al. 2009). There is also evidence for a relationship of GGT with cancer and chronic kidney disease (Targher 2009). ALT and AST levels are predictive of liver disease (Kim et al. 2004; Lee et al. 2008) and liver-related mortality (Ruhl and Everhart 2009). In addition, ALT is associated with type 2 diabetes (Fraser et al. 2009).
In clinical practice, blood levels of GGT, ALT and AST are used to index liver injury. GGT is found in liver and biliary epithelial cells, and is a sensitive marker of hepatobiliary disease, although non-specific to its cause. AST and ALT blood levels increase when the liver cell membrane is damaged and thus mark hepatocellular injury (Pratt and Kaplan 2000). To explain their role in other diseases, GGT and ALT have been proposed as surrogate markers of fatty liver (Targher 2009; Schindhelm et al. 2006; Vernon et al. 2011), while GGT is also seen as a marker of oxidative stress (Lee et al. 2004). Variation in liver enzyme levels within the normal reference range predicts disease (Targher 2009) and it is therefore important to investigate the underlying sources of variation that explain individual differences in liver enzyme levels.
Genetic factors influence variation in liver enzyme levels. For adults, heritability estimates for GGT, ALT and AST range between 32–69 %, 22–44 % and 21–40 % respectively (Rahmioglu et al. 2009; Whitfield et al. 2002; Pilia et al. 2006) (studies with N ≥ 3,000). For ALT and AST these estimates increase to 64 and 61 % if smaller studies are also included (Whitfield and Martin 1985; Makkonen et al. 2009; Sung et al. 2011; Lin et al. 2009; Loomba et al. 2010; Nilsson et al. 2009; Bathum et al. 2001).
The contributions of additive, non-additive genetic factors and shared environmental influences in explaining differences in liver enzyme levels thus vary widely. This may be due to the fact that most studies are based on a comparison of resemblance in monozygotic and dizygotic twins, which gives an overall estimate of heritability (h2), but cannot distinguish well between additive and non-additive genetic effects (Keller et al. 2010) and in smaller twin studies it is also difficult to estimate the contribution of shared environmental factors (Posthuma and Boomsma 2000). Evidence for non-additive genetic effects that can be attributed to intra-locus (dominance) or inter-locus interaction effects (epistasis), was reported in two studies (Bathum et al. 2001; Makkonen et al. 2009) but not in two others (Whitfield et al. 2002; Rahmioglu et al. 2009). Shared environmental influences on liver enzymes can consist for example of effects of stress or dietary similarity that some families may have been exposed to while others have not (Whitfield and Martin 1985; Rahmioglu et al. 2009). Such factors may be shared within generations e.g. by the offspring or between spouses, or by all family members. Spousal resemblance is present when the similarity between spouses for a particular trait is higher than expected by chance. If individuals choose a spouse who is similar for a particular trait (phenotypic assortment), the genetic factors influencing the spousal phenotypes become correlated which results in a higher genetic similarity between first-degree relatives. If spousal resemblance is not modeled, estimates of heritability and shared environmental influences may be biased (Van Grootheest et al. 2008; Keller et al. 2010). One form of shared environment is represented by the influence of the parental phenotype on their offspring's phenotype after genetic transmission has been taken into account (sometimes referred to as cultural transmission). Effects of the shared environment on GGT, ALT and AST have been detected in a combined sample of adolescents and adults (estimates 9–14 %) (Herbeth et al. 2010), and by two studies in adults (Rahmioglu et al. 2009; Whitfield and Martin 1985), but not by six other studies (Pilia et al. 2006; Nilsson et al. 2009; Makkonen et al. 2009; Bathum et al. 2001; Whitfield et al. 2002; Sung et al. 2011). The estimation of non-additive genetic and shared environmental effects requires additional data from non-twin family members and large sample sizes (Posthuma and Boomsma 2000; Keller et al. 2010).
Few studies have examined whether the genetic architecture of liver enzymes differed in males and females or across age. Results have been inconclusive. A study among >6,000 Sardinians detected a larger heritability of GGT in females than males, although there was no evidence for qualitative sex differences in the heritability (Pilia et al. 2006). Results of two studies on quantitative sex differences in the heritability of GGT among the elderly were not consistent (Bathum et al. 2001; Nilsson et al. 2009). For ALT, although quantitative sex differences in the herita-bility were not observed (Bathum et al. 2001; Nilsson et al. 2009; Pilia et al. 2006), a linkage study suggested qualitative sex differences in the association of ALT with quantitative trait loci (QTLs) at the chromosomal region 7p12–15. ALT levels in males, but not in females, were associated with QTLs in this area, suggesting differential gene expression in this region over sex (Brouwers et al. 2006). Results on sex differences in the heritability of AST have not been reported. A recent genome wide association study demonstrated that the effect of genetic loci at the GGT1 locus (chromosome 22) interact with age (Middelberg et al. 2012). Several SNPs (including one that affects expression of GGT1, GGT2 and GGTLA4 in the human liver) decreased GGT levels in adults, whereas it increased GGT levels in adolescents. Other SNPs (located between GGT1 and PIWIL3) increased GGT levels in adolescents, but not in adults. Additional age heterogeneity was observed for SNPs in the CELF2 gene at chromosome 10 that may affect GGT expression through the coding for specific transcription factors (Middelberg et al. 2012). Evidence for age by genotype interactions on GGT and AST also comes from the rather low genetic resemblance between parents and their offspring in a study by Herbeth et al. (2010), although Pilia et al. (2006) did not detect differences across age in the (standardized estimates of the) heritability of either GGT, ALT or AST.
Our study examines to what extent variation in liver enzyme levels can be attributed to genetic factors (additive and non-additive) and environmental factors (shared and non-shared among family members) and if there is evidence for sex and age by genotype interaction. These analyses use data on plasma GGT, ALT and AST levels that were collected in a large sample of twins, their siblings, parents and spouses (N = 8,371) as part of the Netherlands Twin Register (NTR) biobank project (Willemsen et al. 2010, 2013) .
Methods
Participants
Data originate from twins and their family members who participated in the NTR biobank study that was conducted from 2004 to 2008. Details can be found in Willemsen et al. (2010). The study protocol was approved by the Central Ethics Committee on Research Involving Human Subjects of the VU University Medical Center, Amsterdam.
Data on GGT, ALT and AST plasma levels were assessed in 8,758 participants. We excluded data for 387 family members, who were (a) not biological parents, or full siblings of twins (N = 37), (b) twins from pairs for whom information on zygosity was not yet known (one twin was randomly selected to be included, N = 4), (c) triplets if data from their two brothers or sisters were already included (N = 6), (c) siblings from families with more than two same-sex siblings (a maximum of two siblings were included per family; N = 135), or (d) relatives other than twins, siblings or parents, such as children of twins or siblings, grandparents, aunts, uncles, cousins (N = 205).
Families were categorized into five groups, based on sex and zygosity (monozygotic, MZ; dizygotic, DZ) of the twin pair. Data of 257 spouses of twins were included in analyses of spouses resemblance. Analyses were performed on 8,371 individuals (8,114 individuals for the genetic analyses and 257 spouses of twins; 62.8 % female; age range 18–90) (see Table 1).
Table 1. Number of participants as a function of zygosity by sex of twins within the family.
| Zygosity by sex group | Twinsa | Siblings | Parents | Spousesb |
|---|---|---|---|---|
| MZM | 603 (78.8 %) | 131 | 298 | 53 |
| DZM | 329 (68.1 %) | 81 | 213 | 24 |
| MZF | 1,391 (81.5 %) | 268 | 536 | 102 |
| DZF | 697 (78.3 %) | 188 | 352 | 40 |
| DOS | 767 (66.8 %) | 210 | 455 | 38 |
| Families without twins | 835 | 760 | ||
| Total | 3,787 | 1,713 | 2,614 | 257 |
MZM monozygotic males, DZM dizygotic males, MZF monozygotic females, DZF dizygotic females, DOS dizygotic opposite-sex
Percentage reflects what proportion of twins is part of a complete twin pair
Data of spouses were included in the analyses on the mechanism underlying spousal resemblance, not in the genetic analyses
Assessment of liver enzymes
Liver enzymes were determined in plasma that was collected in heparin plasma tubes. Vitros assays (Vitros 250, Ortho-Clinical Diagnostics; Johnson & Johnson, Rochester, USA) were used to determine GGT, ALT and AST levels in units per liter (U/L) (Willemsen et al. 2010). Reference values provided by the laboratory were as follows: for GGT < 50 U/L (males) and <35 U/L (females); for ALT<45 U/L (males) and <40 U/L (females); for AST <40 U/L (both sexes). These reference values were based on the guidelines of the Dutch Association of Clinical Chemistry and Laboratory Medicine (NVKC). Enzyme levels were compared to the reference values.
Statistical analyses
Liver enzyme values were ln-transformed to approximate normality. Age, transformed to z-scores, was used as a covariate (z-score = 0 corresponding to mean age 45.5, SD = 14.7; range z-scores −1.86 to 3.07). Seasonal effects on liver enzyme levels were first explored with ANOVA in SPSS. The decrease in liver enzyme levels for individuals whose blood was sampled in spring, was tested for significance by including it as an (additional) fixed effect (dummy coded as 0/1) in the models fit with Mx v3.2 (Neale et al. 2006). Model comparisons were based on the likelihood ratio test (Bentler and Bonett 1980) with a significance level of 0.01.
A saturated model served to obtain familial correlations, which were computed while correcting liver enzyme values for sex and age. Sixteen sex-specific correlations were estimated: five twin correlations (MZM, DZM, MZF, DZF, DOS), three twin-sibling and three sibling–sibling correlations (male–male, male–female, female–female), four parent-offspring correlations (father–son, mother–son, father–daughter, mother–daughter) and one spouse correlation (father–mother). Next, the effects of age and sex were examined on enzyme levels and on the familial correlations. To explore age by genotype effects, we tested whether correlations are lower for pairs of relatives who show a larger age difference, i.e. we compared correlations for DZ twin pairs, sibling pairs, and parent-offspring pairs.
Quantitative sex differences in the correlation structure were tested by equating correlations over sex between all first-degree relatives and for MZ pairs. Qualitative sex differences were examined by equating the opposite-sex offspring correlation to the same-sex offspring correlation. Qualitative sex differences in the parent-offspring correlations were tested by comparing the correlation for opposite-sex parent-offspring pairs to same-sex parent-offspring pairs.
Finally, spousal resemblance was examined by testing if the correlation between liver enzyme values of spouses was larger than zero. If this was the case, we explored whether correlations could be explained by cohabitation effects by estimating the correlation between the absolute difference in enzyme levels for spouses and the duration of their relationship. If living together influences resemblance between spouses, we expect this correlation to be negative, i.e. smaller differences between spouses the longer they are together. Data for these analyses were available for 984 spouse pairs with information on the duration of their relationship (727 parents of twins and 257 twin-spouse pairs). If no strong evidence was detected for cohabitation effects, it was assumed that phenotypic assortment was the mechanism underlying the spousal correlations, and hence was implemented as such in the genetic model.
To estimate the sources of variance of liver enzyme levels, variance decomposition models were fit to the data (depicted in Fig. 1, see the supplementary materials online methods for an explanation and identification of these models). The variance in liver enzyme levels due to additive genetic influences (A), common environmental influences shared within the offspring generation (C), and non-shared individual-specific environmental influences (E) was estimated in two models. In the ACE model, shared environmental transmission from parents to offspring was taken into account (also known as cultural transmission), whereas in the ACDE model parent-offspring these transmission paths were set to zero (for reasons of model identification) and variance due to non-additive genetic influences was estimated (see Neale and Cardon (1992), Posthuma et al. (2003) for details on twin-family variance decomposition models). Constraints in the genetic model were based on the results from saturated models (including those on spousal resemblance and sex differences). A detailed description of all tests performed is given in the supplementary materials online methods.
Fig. 1.
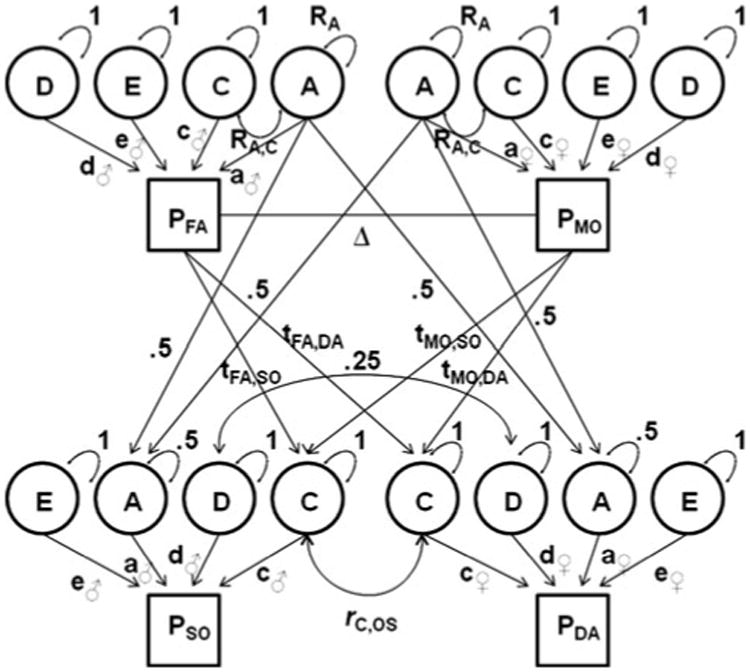
Path diagram for variance decomposition (shown for DOS twin pair with parents). Pfa, Pmo, Pso, Pda phenotype of father, mother, son, daughter, A additive genetic factor with variance Ra (differs from 1 in the case of phenotypic assortment), D non-additive genetic factor, C shared environmental factor with variance Rc (differs from 1 in the case of shared environmental transmission), E individual-specific environmental factor, a, c, d, e path loadings (d-paths estimated in ACDE model; set to zero in ACE model with shared environmental transmission), tfa,so, tfa,da, tmo,so, tmo,da shared environmental transmission paths (estimated in ACE model with shared environmental transmission; set to zero in ACDE model), Ra,c covariation between A and C (differs from 1 in the case of shared environmental transmission), rc,os correlation between shared environmental factors for opposite-sex, Δ path representing correlations between the latent factors induced by phenotypic assortment, ♂ male-specific path loadings, ♀ female-specific path loadings
Results
For 1,318 (15.7 %), 40 (0.5 %) and 165 (2.0 %) individuals, GGT, ALT or AST levels (respectively) were elevated compared to the reference values. Age and sex affected liver enzyme levels. Table 2 shows the means and medians of GGT, ALT and AST, with SDs, ranges and the correlations with age. The association with age was significant for all three liver enzymes (GGT: χ2(6) = 183.29, p <0.001; ALT: χ2(6) = 68.32, p <0.001; AST: χ2(6) = 143.86, p <0.001). Mean GGT levels were higher among parents than offspring (males: χ2(1) = 12.74, p < 0.001; females: χ2(1) = 15.72, p <0.001), although ALT and AST levels did not differ for the parental and offspring generation (ALT males: χ2(2) = 3.53, p = 0.172; ALT females: χ2(2) = 9.01, p = 0.011; AST males: χ2(2) = 0.41, p = 0.816; AST females: χ2(2) = 3.95, p = 0.139). Means were higher for men than women (GGT parents: χ2(1) = 66.03, p <0.001; GGT offspring: χ2(1) = 401.47, p <0.001; ALT: χ2(1) = 265.96, p <0.001; AST: χ2(1) = 316.38, p <0.001).
Table 2. Descriptive statistics for males and females, separately for twins, siblings and parents.
| Males | Females | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| Meana (SD) | Mediana (range) | Correlations withb | Meana (SD) | Mediana (range) | Correlations withb | |||||
|
|
|
|||||||||
| ALT | AST | Age | ALT | AST | Age | |||||
| Twins | ||||||||||
| GGT | 36.1 (34.6) | 28 (9–820) | 0.31 | 0.38 | 0.22c | 24.3 (24.9) | 20 (8–819) | 0.25 | 0.36 | 0.21c |
| ALT | 12.9 (7.06) | 11 (3–75) | 0.63 | −0.10d | 9.1 (5.1) | 8 (3–97) | 0.53 | 0.06d | ||
| AST | 23.7 (8.09) | 22 (11–122) | 0.05e | 19.4 (6.7) | 18 (9–107) | 0.20e | ||||
| Age | 35.4 (11.0) | 32 (18–77) | 36.5 (11.0) | 33 (18–90) | ||||||
| Siblings | ||||||||||
| GGT | 36.9 (34.5) | 29 (13–564) | 0.34 | 0.34 | 0.20c | 25.2 (22.1) | 20 (8–439) | 0.31 | 0.38 | 0.18c |
| ALT | 12.3 (6.8) | 11 (3–54) | 0.55 | −0.11d | 9.6 (6.2) | 8 (3–100) | 0.52 | 0.07d | ||
| AST | 23.8 (12.1) | 22 (11–220) | 0.08e | 19.6 (7.1) | 18 (9–142) | 0.20e | ||||
| Age | 40.2 (13.3) | 36 (18–82) | 42.2 (10.3) | 42 (18–84) | ||||||
| Parents | ||||||||||
| GGT | 45.4 (40.5) | 34 (14–456) | 0.27 | 0.40 | −0.04c | 33.9 (40.4) | 25 (10–867) | 0.30 | 0.37 | 0.06c |
| ALT | 10.8 (6.4) | 10 (3–107) | 0.52 | −0.16d | 9.4 (5.5) | 8 (3–89) | 0.51 | −0.07d | ||
| AST | 23.5 (8.3) | 22 (7–111) | −0.06e | 21.3 (7.3) | 20 (7–128) | 0.09e | ||||
| Age | 61.4 (7.0) | 60 (33–89)f | 60.1 (7.8) | 59 (19–90)f | ||||||
| Spousesg | ||||||||||
| GGT | 41.2 (75.4) | 27 (14–917) | 0.49 | 0.57 | 0.22c | 24.2 (18.9) | 20 (9–181) | 0.21 | 0.20 | 0.31c |
| ALT | 12.5 (7.2) | 11 (3–54) | 0.65 | −0.05d | 8.9 (4.1) | 8 (3–28) | 0.52 | 0.06d | ||
| AST | 24.4 (9.3) | 22 (14–92) | 0.19e | 19.2 (5.2) | 19 (12–41) | 0.16e | ||||
| Age | 42.5 (10.7) | 39 (25–71) | 38.7 (12.4) | 34 (22–76) | ||||||
Untransformed liver enzyme levels
ln-transformed liver enzyme levels
In the genetic models for GGT, two betas for the age regression were estimated: fathers −0.32, others 1.16, and four means (male/female offspring, fathers, mothers)
In the genetic models for ALT, four betas for the age regression were estimated: male offspring −0.52, fathers −1.29, female offspring 0.56, mothers −0.25, and two sex-specific means
In the genetic models for AST, two betas for the age regression were estimated: males 0.02, females 0.63, and two sex-specific means
Minimum age for parents who participated with their offspring (aged 18+) was 45 for mothers and 48 for fathers; lower ages in the parental group reflect those of parents of twins who were younger than 18 at the time of the assessment
Data of spouses were included in the analyses on the mechanism underlying spousal resemblance, not in the genetic analyses
Seasonal effects were present for ALT and AST levels, but not for GGT. Individuals who were visited in spring (April–June) had significantly lower ALT and AST levels than individuals who were visited in other seasons (ALT: χ2(1) = 15.17, p <0.001; AST: χ2(1) = 341.66, p <0.001; GGT: χ2(1) = 5.66, p = 0.017). Correcting for the effect of season when individuals were visited, did not change the familial correlations (data available upon request).
Familial correlations for GGT, ALT and AST are displayed in Fig. 2a–c respectively. Supplementary materials Table S1 presents the model fit statistics for all submodels, described below, that tested age and sex effects on the correlations. Furthermore, supplementary materials Tables 2a–c give an overview of the parameter constraints that are tested in each submodel, and thus provide an explanation of the number of degrees of freedom that are associated with these tests. The amount of variance explained by genetic and environmental factors as estimated in the best fittinge genetic models, is presented in Table 3.
Fig. 2. a Familial correlations for GGT (with 95 % confidence intervals). b Familial correlations for ALT (with 95 % confidence intervals). c Familial correlations for AST (with 95 % confidence intervals).
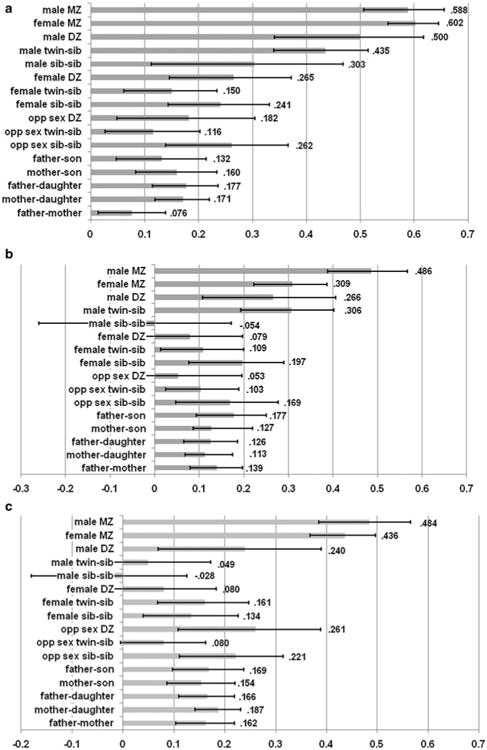
Table 3. Percentage of phenotypic variance in GGT, ALT and AST levels explained by additive (A) and non-additive genetic effects (D), and shared (C) and individual-specific (E) environmental effects as estimated in the final genetic models.
| SDa | Spouse Corr | Additive genetic variance | Proportion of phenotypic variance explained by | ||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| A (%) | D (%) | C (%) | E (%) | ||||
| GGT | |||||||
| Males | 5.46 | 0.00 | 1.00 | 29.6 | 28.5 | 41.9 | |
| 95 % C.I. | 19.8–40.2 | 19.2–37.5 | 35.6–49.1 | ||||
| Females | 5.46 | 0.00 | 1.00 | 32.2 | 27.6 | 40.2 | |
| 95 % C.I. | 23.4–41.4 | 17.8–37.5 | 35.3–45.7 | ||||
| ALT | |||||||
| Males | 4.54 | 0.15 | 1.42 | 40.4 | 59.6 | ||
| 95 % C.I. | 31.9–51.8 | 51.8–68.0 | |||||
| Females | 4.54 | 0.15 | 1.42 | 22.4 | 77.6 | ||
| 95 % C.I. | 16.6–28.5 | 71.6–83.7 | |||||
| AST | |||||||
| Males | 2.86 | 0.15 | 1.47 | 28.0 | 14.7 | 57.3 | |
| 95 % C.I. | 22.6–33.5 | 7.1–22.3 | 51.8–63.5 | ||||
| Females | 2.62 | 0.15 | 1.47 | 28.0 | 14.7 | 57.3 | |
| 95 % C.I. | 22.6–33.5 | 7.1–22.3 | 51.8–63.5 | ||||
SDs for ln-transformed liver enzyme values × 10. For GGT, SDs in the offspring generation were smaller than those in the parental generation and differed over sex (male offspring: 4.94; female offspring 4.25; parental generation 5.46)
GGT
For GGT, there were quantitative sex differences in the offspring correlations (rDZM/SIB 0.43; rDZF/SIB 0.21), but not in the MZ correlations (rMZ 0.60). The correlations among opposite-sex family members indicated no qualitative sex differences. The correlations among family members differed as a function of age, especially among men (χ2(3) = 32.06, p < 0.001; rDZ 0.50; rSIBS 0.41; rPAR-OFF 0.13). Spousal resemblance was not present.
The ACDE model provided a better fit (χ2(8) = 16.98, p = 0.030) than the ACE model with shared environmental transmission (χ2(4) = 46.83, p < 0.001), compared to the saturated model. For females, both additive and non-additive genetic influences were important (drop D: χ2(1) = 25.68, p < 0.001). Shared environmental effects were not present (χ2(1) = 0.000, p = 0.999). For men, additive genetic influences explained a significant amount of variance (drop A + D: χ2(2) = 74.31, p <0.001), whereas non-additive genetic influences did not (drop D: χ2(1) = 0.33, p = 0.565). In men, shared environmental factors played a role (χ2(1) = 26.82, p <0.001), which explains the age differences and quantitative sex differences in the correlation structure noted above. The absence of qualitative sex differences in the correlations (see supplementary materials Table S1) indicates that the same genes are expressed in men and women. Additional analyses showed that the age differences in the correlations for GGT among males were quantitative, due to additional environmental effects shared within the offspring generation (C), rather than being due to a different expression of genes across age (qualitative age differences). This was tested by estimating the genetic correlation between fathers and their male offspring and testing whether this correlation was significantly lower than 0.5. Taking the ACE (males)–ADE (females) model as a reference, allowing the genetic correlation between father and their male offspring to be lower than 0.5 did not significantly improve model fit, whereas dropping shared environmental effects among males from the model deteriorated model fit (still allowing for a genetic father–son correlation <0.5) (χ2(1) = 35.44, p < 0.001).
In the final model, for males, 30 % of the variance was explained by additive genetic, 28 % by shared environmental and 42 % by non-shared individual-specific environmental factors. For females, this was 32 % by additive genetic, 28 % by non-additive genetic, and 40 % by individual-specific environmental factors.
ALT
For ALT, the correlation structure was similar over age, thus genotype by age interactions were not present. There was an indication for sex by genotype interactions, however. There were quantitative sex differences in the MZ correlations (rMZM 0.43; rMZF 0.31), but not in the correlations among first-degree relatives (r 0.14). Spouses resembled each other in ALT levels (r 0.14) which was not explained by cohabitation effects, as the correlation between the difference in liver enzyme levels within spouse pairs and the duration of their relationship was not significant (r −0.02, 95 % CI: −0.08, 0.04).
The ACDE model fit the data better (χ2(6) = 12.92, p = 0.044) than the ACE model with shared environmental transmission (χ2(6) = 25.50, p < 0.001), compared to the saturated model. Analyses of submodels showed that additive genetic factors influenced variation in enzyme levels (drop A + D: χ2(5) = 87.38, p < 0.001), but non-additive genetic factors (drop D: χ2(2) = 5.72, p = 0.057), or shared environmental influences (χ2(2) = 1.32, p = 0.518) did not. This resulted in the AE model as the final model. The amount of variance explained by additive genetic factors and non-shared individual-specific environmental factors was 40 and 60 % for males, and 22 and 78 % for females respectively.
AST
For AST, the correlation structure was similar over age and sex, thus genotype by age or sex interactions were not present. Spousal resemblance was significant (r 0.16). The correlation between the difference in liver enzyme levels within spouse pairs and the duration of their relationship was significant (χ2(1) = 6.92, p = 0.009) but the effect was small (r −0.09; 95 % CI: −0.15, −0.02) suggesting that cohabitation effects play a minor role. Familial correlations were 0.45 for MZ pairs and 0.16 for first-degree relatives, suggesting the presence of non-additive genetic effects rather than shared environmental effects.
The ACE model with shared environmental transmission did not fit the data as well (χ2(9) = 42.55, p < 0.001) as the ACDE model (χ2(10) = 28.24, p = 0.002), compared to the saturated model. Submodels were evaluated for the ACDE model and showed evidence for both additive and non-additive genetic effects (drop D: χ2(1) = 14.32, p < 0.001). Shared environmental influences did not explain any variation (χ2(1) = 0.00, p = 1). According to the final ADE model, 28 % of the variance could be explained by additive genetic factors, 15 % by non-additive genetic factors and 57 % by non-shared (individual-specific) environmental factors (for both men and women).
Discussion
Variation in liver enzyme levels is moderately heritable. For GGT and ALT, quantitative sex differences in the genetic architecture were present, but there was no evidence that different genes were expressed in men and women. In addition, there were quantitative age differences in the relative importance of genetic and environmental influences on GGT that were due to environmental effects that were shared within the male offspring generation (c2 28 %), but not among parents and offspring. The results from our sex-specific analyses in which additive and non-additive genetic and shared and non-shared environmental influences could be estimated simultaneously may explain variability in previous heritability estimates (Rahmioglu et al. 2009; Whitfield et al. 2002; Pilia et al. 2006) (studies N > 3,000). For GGT in males as well as for ALT (both sexes), only additive genetic influences were important (GGT h2 males 30 %, ALT h2 males 40 %, females 22 %). For GGT in females and AST (both sexes), genetic influences consisted of both additive and non-additive effects (h2 60 and 43 % respectively). The different heritability estimates over sex for GGT and ALT correspond to the lower and upper bounds of previous heritability estimates (32–69 % and 22–44 % respectively). Likewise, the narrow-sense heritability estimates for GGT in females and AST (both sexes) (32 and 28 % respectively) reflect the lower bound of previous heritability estimates (32 and 21 %), whereas the broad-sense heritability estimates (60 and 43 %) correspond to the upper bound of these estimates (69 and 40 %).
The genetic and environmental influences on liver enzyme levels may derive from the biological effects of factors that are in turn partly genetically and partly environmentally mediated. These include metabolic risk factors (Whitfield et al. 2002; Loomba et al. 2010; Makkonen et al. 2009; Skurtveit and Tverdal 2002), inflammation parameters (Rahman et al. 2009), alcohol use (Whitfield and Martin 1985; Sung et al. 2011; Skurtveit and Tverdal 2002), smoking (Honjo et al. 2001; Skurtveit and Tverdal 2002; Vink et al. 2004), and coffee consumption (Honjo et al. 2001; Vink et al. 2009; Skurtveit and Tverdal 2002). Shared environmental effects for GGT (28 % for males in our sample) have been detected before for both males and females (aged 18–34) (Whitfield and Martin 1985). These effects may reflect shared environmental influences on the above mentioned metabolic and lifestyle factors. In studies that examined the association between liver enzyme levels and metabolic risk factors (Whitfield et al. 2002; Makkonen et al. 2009) or alcohol use (Whitfield and Martin 1985; Sung et al. 2011), shared environmental factors could not explain the covariance among these traits however.
Genome-wide association (GWA) studies have started to elucidate the genetic pathways underlying variation in liver enzyme levels. For GGT, genome-wide significant associations have been found for loci in or near the genes involved in glutathione metabolism (GSTT2B, GGT1) (Chambers et al. 2011; Middelberg et al. 2012; Yuan et al. 2008; Kamatani et al. 2010), biliary transport (ATP8B1) (Chambers et al. 2011), alcohol metabolism (ALDH2) (Kamatani et al. 2010), lipid metabolism (HNF1A, CEPT1), carbohydrate metabolism and insulin signalling (GCKR, MLXIPL, SLC2A2) (Chambers et al. 2011; Middelberg et al. 2012; Yuan et al. 2008), inflammation and immunity (GCKR, STAT4, CDH6, ITGA1, HNF1A, RORA, CD276 (Chambers et al. 2011; Middelberg et al. 2012), glycoprotein biology (FUT2) (Chambers et al. 2011), as well as for other genes, including the C14orf73 gene (Chambers et al. 2011; Middelberg et al. 2012) that is strongly expressed in the liver but of which the function is uncertain (Chambers et al. 2011). Variants in the PNPLA3 gene, involved in energy utilisation and storage by adipocytes, has been associated with both AST and ALT levels (Chambers et al. 2011; Kollerits et al. 2010; Sookoian and Pirola 2011; Yuan et al. 2008; Kamatani et al. 2010). For AST, a significant variant was detected in a gene implicated in inflammation and immunity (MRC1) (Kamatani et al. 2010). Additional loci have been detected for ALT in or near genes involved in glucose and lipid metabolism (TRIB1, CHUK) (Chambers et al. 2011; Yuan et al. 2008), inflammation and immunity (CPN1) (Chambers et al. 2011; Yuan et al. 2008) and the biogenesis of mitochondria (SAMM50) (Yuan et al. 2008).
Spousal resemblance was detected for ALT and AST. It can thus be hypothesized that the seasonal effects that were observed for ALT and AST may underlie this spousal resemblance. If family members are measured closer in time, seasonal effects, if present, will come up as an effect of the shared environment and/or spousal resemblance. Correcting for the effect of season, did not change the familial correlations, rendering this interpretation less likely (data available upon request). The seasonal effects were similar to those found in a large Japanese study (based on almost 300,000 liver function test results). While ALT and AST levels showed seasonal variation, showing peak values in the winter and a nadir in the summer, GGT did not (Miyake et al. 2009). In future research, it will be of interest to further examine the mechanism that underlies this annual variation in liver enzyme levels.
There was some evidence for cohabitation effects (Van Grootheest et al. 2008) on the spousal resemblance for AST. That is, spouses are more similar in their AST levels the longer they are together. The effects were small however. Spousal resemblance for AST and ALT was therefore modelled as resulting from phenotypic assortment, assuming that the largest part of spousal resemblance is due to phenotypic assortment for factors correlated with liver enzyme levels, such as alcohol, cigarette and coffee consumption, metabolic risk factors and physical exercise (Conigrave et al. 2003; Skurtveit and Tverdal 2002). This will not have affected the results for AST to a great extent. Assuming spousal resemblance was absent changed the heritability with only 1.3 %, indicating that if spousal resemblance for AST would be due to cohabitation effects rather than to phenotypic assortment, by assuming it was fully explained by phenotypic assortment, did not drastically change the results.
The effects of sex and age effects on mean liver enzyme levels are in accordance with the literature. A different distribution of levels of GGT, ALT and AST over sex and age has been previously reported (Pilia et al. 2006; Stromme et al. 2004), but the exact mechanisms for these effects are not clear. We detected a positive correlation for GGT with age for females (rDZF/SIB/MOTHER = 0.27) and males in the offspring generation (rDZM/SIB = 0.21), but little change with age for older men (rFATHERS = −0.04) in agreement with Middelberg et al. (2012). Lower ALT levels in older men (age >60) have been detected before (Pilia et al. 2006; Stromme et al. 2004), supporting our finding that ALT levels among men were negatively associated with age. For females, lower ALT levels among older women were not reported by earlier studies (Pilia et al. 2006; Stromme et al. 2004). AST levels in females increased with age, whereas those for males remained constant, in accordance with Pilia et al. (2006).
The sex differences in the genetic architecture of GGT and ALT might reflect genetic effects on alcohol use and/or BMI that show different relationships with liver enzyme levels in men and women (Skurtveit and Tverdal 2002), which may partly result from the effect of sex hormones. GGT levels are known to vary with differences in the hormonal balance in women (Sillanaukee et al. 2000) and some animal studies suggests that estrogens may be important in the development of alcohol-related liver injury. Chronic alcohol consumption results in a different gene expression in female rat livers, although it is not clear whether this is directly caused by hormonal influences (Eagon 2010).
The current study allowed to disentangle additive genetic, non-additive genetic effects, shared environmental and non-shared environmental effects on variation in liver enzyme levels that mark liver injury and other disease. By the inclusion of data on non-twin family members, power has improved (Posthuma and Boomsma 2000) allowing us to test for genotype by sex and genotype by age interactions. Results from these tests show that overall, there was no evidence for qualitative sex or age differences in the heritability, although quantitative sex and age differences were detected for GGT and ALT. It should be noted however that the current study does not have the power to detect the small gene by age (and gene by sex) effects that may be present at the level of individual SNPs. Our results therefore do not imply that these do not exist, as they evidently are present, as demonstrated by Middelberg et al. (2012). It will be interesting to explore whether a possible implication of our results, namely that gene finding studies on liver enzyme levels may benefit from combining data across sex and age, holds true. Although pooling data over sex and age in gene finding studies will introduce noise in the prediction model, because of the sex- or age-dependent effects of some SNPs, it is hypothesized that, overall, combining results over sex and age will increase the true association signal to a greater extent because of the increase in power and the fact that for these twin-family based estimates of the heritability, qualitative sex or age differences were not important.
In summary, genes play a substantial role in explaining differences in plasma levels of GGT, ALT and AST (h2 22–60 %), which are important markers of liver injury and other disease. Genetic influences on liver enzyme levels include additive and non-additive genetic effects. Genotype by sex interactions (for GGT and ALT) and genotype by age interactions (for GGT in males) were present, but there was no evidence that different genes are expressed across sex or age. This suggests a positive message for genome-wide association studies. Pooling data over sex and age (with appropriate covariates to adjust sex and age mean level differences) may increase the signal-to-noise ratio to detect susceptibility loci because of increased power, without introducing too much heterogeneity that will dilute the association signal.
Supplementary Material
Acknowledgments
This work has been executed in the Mental Health research program of the EMGO Institute for Health and Care Research and was supported by grants from the Netherlands Organization for Scientific Research (NWO) (ZonMW Addiction 31160008; NWO/SPI 56-464-14192; NWO 016-115-035; NWO-MW 904-61-193) and the European Research Council (Genetics of Mental Illness: ERC-230374; ERC starting grant 284167). We thank Professor J.B. Whitfield for useful discussions and the twin families for their participation in the NTR research.
Footnotes
Electronic supplementary material The online version of this article (doi: 10.1007/s10519-013-9593-y) contains supplementary material, which is available to authorized users.
Contributor Information
Jenny H. D. A. van Beek, Email: J.H.D.A.van.Beek@vu.nl, Department of Biological Psychology, VU University Amsterdam, Van der Boechorststraat 1, 1081 BT Amsterdam, The Netherlands; EMGO Institute for Health and Care Research, VU University Medical Center, Amsterdam, The Netherlands.
Marleen H. M. de Moor, Department of Biological Psychology, VU University Amsterdam, Van der Boechorststraat 1, 1081 BT Amsterdam, The Netherlands; EMGO Institute for Health and Care Research, VU University Medical Center, Amsterdam, The Netherlands
Eco J. C. de Geus, Department of Biological Psychology, VU University Amsterdam, Van der Boechorststraat 1, 1081 BT Amsterdam, The Netherlands; EMGO Institute for Health and Care Research, VU University Medical Center, Amsterdam, The Netherlands; Neuroscience Campus Amsterdam, Amsterdam, The Netherlands
Gitta H. Lubke, Department of Biological Psychology, VU University Amsterdam, Van der Boechorststraat 1, 1081 BT Amsterdam, The Netherlands; Department of Psychology, University of Notre Dame, Notre Dame, IN, USA
Jacqueline M. Vink, Department of Biological Psychology, VU University Amsterdam, Van der Boechorststraat 1, 1081 BT Amsterdam, The Netherlands; Neuroscience Campus Amsterdam, Amsterdam, The Netherlands
Gonneke Willemsen, Department of Biological Psychology, VU University Amsterdam, Van der Boechorststraat 1, 1081 BT Amsterdam, The Netherlands; EMGO Institute for Health and Care Research, VU University Medical Center, Amsterdam, The Netherlands.
Dorret I. Boomsma, Department of Biological Psychology, VU University Amsterdam, Van der Boechorststraat 1, 1081 BT Amsterdam, The Netherlands; EMGO Institute for Health and Care Research, VU University Medical Center, Amsterdam, The Netherlands; Neuroscience Campus Amsterdam, Amsterdam, The Netherlands
References
- Bathum L, Petersen HC, Rosholm JU, Hyltoft Petersen P, Vaupel J, Christensen K. Evidence for a substantial genetic influence on biochemical liver function tests: results from a population-based Danish twin study. Clin Chem. 2001;47:81–87. [PubMed] [Google Scholar]
- Bentler PM, Bonett DG. Significance tests and goodness of fit in the analysis of covariance structures. Psychol Bull. 1980;88:588–606. [Google Scholar]
- Brouwers MCGJ, Cantor RM, Kono N. Heritability and genetic loci of fatty liver in familial combined hyperlipidemia. J Lipid Res. 2006;47:2799–2807. doi: 10.1194/jlr.M600312-JLR200. [DOI] [PubMed] [Google Scholar]
- Chambers JC, Zhang W, Sehmi J, Li X, Wass MN, Van der Harst P, et al. Genome-wide association study identifies loci influencing concentrations of liver enzymes in plasma. Nat Genet. 2011;43:1131–1138. doi: 10.1038/ng.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conigrave KM, Davies P, Haber P, Whitfield JB. Traditional markers of excessive alcohol use. Addiction. 2003;98:31–43. doi: 10.1046/j.1359-6357.2003.00581.x. [DOI] [PubMed] [Google Scholar]
- Eagon PK. Alcoholic liver injury: influence of gender and hormones. World J Gastroenterol. 2010;16:1377–1384. doi: 10.3748/wjg.v16.i11.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser A, Harris R, Sattar N, Ebrahim S, Davey Smith G, Lawlor DA. Alanine aminotransferase, gamma-glutamyltransferase, and incident diabetes. Diabetes Care. 2009;32:741–750. doi: 10.2337/dc08-1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbeth B, Samara A, Ndiaye C, Marteau JB, Berrahmoune H, Siest G, et al. Metabolic syndrome-related composite factors over 5 years in the STANISLAS family study: genetic heritability and common environmental influences. Clin Chim Acta. 2010;411:833–839. doi: 10.1016/j.cca.2010.02.070. [DOI] [PubMed] [Google Scholar]
- Honjo S, Kono S, Coleman MP, Shinchi K, Sakurai Y, Todoroki I, et al. Coffee consumption and serum aminotransferases in middle-aged Japanese men. J Clin Epidemiol. 2001;54:823–829. doi: 10.1016/s0895-4356(01)00344-4. [DOI] [PubMed] [Google Scholar]
- Kamatani Y, Matsuda K, Okada Y, Kubo M, Hosono N, Daigo Y, et al. Genome-wide association study of hematological and biochemical traits in a Japanese population. Nat Genet. 2010;42:210–215. doi: 10.1038/ng.531. [DOI] [PubMed] [Google Scholar]
- Kazemi-Shirazi L, Endler G, Winkler S, Schickbauer T, Wagner O, Marsik C. Gamma glutamyltransferase and long-term survival: is it just the liver? Clin Chem. 2007;53:940–946. doi: 10.1373/clinchem.2006.081620. [DOI] [PubMed] [Google Scholar]
- Keller MC, Medland SE, Duncan LE. Are extended twin family designs worth the trouble? A comparison of the bias, precision, and accuracy of parameters estimated in four twin family models. Behav Genet. 2010;40:377–393. doi: 10.1007/s10519-009-9320-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HC, Nam CM, Jee SH, Kan KH, Oh DK, Suh I. Normal serum aminotransferase concentration and risk of mortality from liver diseases: prospective cohort study. BMJ. 2004;328:983. doi: 10.1136/bmj.38050.593634.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollerits B, Coassin S, Kiechl S, Hunt SC, Paulweber B, Willeit J, et al. A common variant in the adiponutrin gene influences liver enzyme values. J Med Genet. 2010;47:116–119. doi: 10.1136/jmg.2009.066597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DH, Blomhoff R, Jacobs DR. Review: is serum gamma glutamyltransferase a marker of oxidative stress? Free Radic Res. 2004;38:535–539. doi: 10.1080/10715760410001694026. [DOI] [PubMed] [Google Scholar]
- Lee TH, Kim W, Benson JT, Therneau TM, Melton LJ., III Serum aminotransferase activity and mortality risk in a United States community. Hepatology. 2008;47:880–887. doi: 10.1002/hep.22090. [DOI] [PubMed] [Google Scholar]
- Lin JP, O’Donnell CJ, Fox CS, Cupples LA. Heritability of serum glutamyltransferase level: genetic analysis from the Framingham Offspring Study. Liver Int. 2009;29:776–777. doi: 10.1111/j.1478-3231.2008.01965.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loomba R, Rao F, Zhang L, Khandrika S, Ziegler MG, Brenner DA, et al. Genetic covariance between gamma-glutamyl transpeptidase and fatty liver risk factors: role of beta 2-adrenergic receptor genetic variation in twins. Gastroenterology. 2010;139:836–845. doi: 10.1053/j.gastro.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makkonen J, Pietiläinen KH, Rissanen A, Kaprio J, Yki-Järvinen H. Genetic factors contribute to variation in serum alanine aminotransferase activity independent of obesity and alcohol: a study in monozygotic and dizygotic twins. J Hepatol. 2009;50:1035–1042. doi: 10.1016/j.jhep.2008.12.025. [DOI] [PubMed] [Google Scholar]
- Middelberg RP, Benyamin B, De Moor MHM, Warrington NM, Gordon S, Henders AK, et al. Loci affecting gamma-glutamyl transferase in adults and adolescents show age × SNP interaction and cardiometabolic disease associations. Hum Mol Genet. 2012;21:446–455. doi: 10.1093/hmg/ddr478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake K, Miyake N, Kondo S, Tabe Y, Ohsaka A, Miida T. Seasonal variation in liver function tests: a time-series analysis of outpatient data. Ann Clin Biochem. 2009;46:377–384. doi: 10.1258/acb.2009.008203. [DOI] [PubMed] [Google Scholar]
- Neale MC, Cardon LR. Methodology for genetic studies of twins and families. Kluwer; Dordrecht: 1992. [Google Scholar]
- Neale MC, Boker SM, Xie G, Maes HH. Mx: Statistical modeling (7 ed) 7th. VCU; Richmond: 2006. [Google Scholar]
- Nilsson SE, Read S, Berg S, Johansson B. Heritabilities for fifteen routine biochemical values: findings in 215 Swedish twin pairs 82 years of age or older. Scand J Clin Lab Invest. 2009;69:562–569. doi: 10.1080/00365510902814646. [DOI] [PubMed] [Google Scholar]
- Pilia G, Chen WM, Scuteri A, Orru M, Albai G, Dei M, et al. Heritability of cardiovascular and personality traits in 6,148 Sardinians. PLoS Genet. 2006;2:e132. doi: 10.1371/journal.pgen.0020132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posthuma D, Boomsma DI. A note on the statistical power in extended twin designs. Behav Genet. 2000;30:147–158. doi: 10.1023/a:1001959306025. [DOI] [PubMed] [Google Scholar]
- Posthuma D, Beem AL, de Geus EJC, van Baal GCM, von Hjelmborg JB, Iachine I, et al. Theory and practice in quantitative genetics. Twin Res Hum Genet. 2003;6:361–376. doi: 10.1375/136905203770326367. [DOI] [PubMed] [Google Scholar]
- Pratt DS, Kaplan MM. Evaluation of abnormal liver-enzyme results in asymptomatic patients. N Eng J Med. 2000;342:1266–1271. doi: 10.1056/NEJM200004273421707. [DOI] [PubMed] [Google Scholar]
- Rahman I, Bennet AM, Pedersen NL, de Faire U, Svensson P, Magnusson PKE. Genetic dominance influences blood biomarker levels in a sample of 12,000 Swedish elderly twins. Twin Res Hum Genet. 2009;12:286–294. doi: 10.1375/twin.12.3.286. [DOI] [PubMed] [Google Scholar]
- Rahmioglu N, Andrew T, Cherkas L, Surdulescu G, Swaminathan R, Spector TD, et al. Epidemiology and genetic epidemiology of the liver function test proteins. PLoS One. 2009;4:e4435. doi: 10.1371/journal.pone.0004435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruhl CE, Everhart JE. Elevated serum alanine aminotransferase and gamma-glutamyltransferase and mortality in the United States population. Gastroenterology. 2009;136:477–485. doi: 10.1053/j.gastro.2008.10.052. [DOI] [PubMed] [Google Scholar]
- Schindhelm RK, Diamant M, Dekker JM, Tushuizen ME, Teerlink T, Heine RJ. Alanine aminotransferase as a marker of nonalcoholic fatty liver disease in relation to type 2 diabetes mellitus and cardiovascular disease. Diabetes Metab Res Rev. 2006;22:437–443. doi: 10.1002/dmrr.666. [DOI] [PubMed] [Google Scholar]
- Sillanaukee P, Alho H, Strid N, Jousilahti P, Vartiainen E, Olsson U, et al. Effect of hormone balance on carbohydratedeficient transferrin and gammaglutamyltransferase in female social drinkers. Alcohol Clin Exp Res. 2000;24:1505–1509. [PubMed] [Google Scholar]
- Skurtveit S, Tverdal A. Sex differences in gamma-glutamyl-transferase in people aged 40–42 years in two Norwegian counties. Drug Alcohol Depend. 2002;67:95–98. doi: 10.1016/s0376-8716(02)00015-7. [DOI] [PubMed] [Google Scholar]
- Sookoian S, Pirola CJ. Meta-analysis of the influence of I148 M variant of patatin-like phospholipase domain containing 3 gene (PNPLA3) on the susceptibility and histological severity of nonalcoholic fatty liver disease. Hepatology. 2011;53:1883–1894. doi: 10.1002/hep.24283. [DOI] [PubMed] [Google Scholar]
- Stromme JH, Rustad P, Steensland H, Theodorsen L, Urdal P. Reference intervals for eight enzymes in blood of adult females and males measured in accordance with the International Federation of Clinical Chemistry reference system at 37 C: part of the Nordic Reference Interval Project. Scand J Clin Lab Invest. 2004;64:371–384. doi: 10.1080/00365510410002742. [DOI] [PubMed] [Google Scholar]
- Sung J, Lee K, Song YM. Heritabilities of Alcohol Use Disorders Identification Test (AUDIT) scores and alcohol biomarkers in Koreans: the KoGES (Korean Genome Epi Study) and Healthy Twin Study. Drug Alcohol Depend. 2011;113:104–109. doi: 10.1016/j.drugalcdep.2010.07.012. [DOI] [PubMed] [Google Scholar]
- Targher G. Elevated serum gamma-glutamyltransferase activity is associated with increased risk of mortality, incident type 2 diabetes, cardiovascular events, chronic kidney disease and cancer: a narrative review. Clin Chem Lab Med. 2009;48:147–157. doi: 10.1515/CCLM.2010.031. [DOI] [PubMed] [Google Scholar]
- Van Grootheest DS, van den Berg SM, Cath DC, Willemsen G, Boomsma DI. Marital resemblance for obsessive-compulsive, anxious and depressive symptoms in a population-based sample. Psychol Med. 2008;38:1731–1740. doi: 10.1017/S0033291708003024. [DOI] [PubMed] [Google Scholar]
- Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34:274–285. doi: 10.1111/j.1365-2036.2011.04724.x. [DOI] [PubMed] [Google Scholar]
- Vink JM, Beem AL, Posthuma D, Neale MC, Willemsen G, Kendler KS, et al. Linkage analysis of smoking initiation and quantity in Dutch sibling pairs. Pharmacogenomics J. 2004;4:274–282. doi: 10.1038/sj.tpj.6500255. [DOI] [PubMed] [Google Scholar]
- Vink JM, Staphorsius AS, Boomsma DI. A genetic analysis of coffee consumption in a sample of Dutch twins. Twin Res Hum Genet. 2009;12:127–131. doi: 10.1375/twin.12.2.127. [DOI] [PubMed] [Google Scholar]
- Whitfield JB, Martin NG. Individual differences in plasma ALT, AST and GGT: contributions of genetic and environmental factors, including alcohol consumption. Enzyme. 1985;33:61–69. doi: 10.1159/000469408. [DOI] [PubMed] [Google Scholar]
- Whitfield JB, Zhu G, Nestler JE, Heath AC, Martin NG. Genetic covariation between serum gamma-glutamyltransferase activity and cardiovascular risk factors. Clin Chem. 2002;48:1426–1431. [PubMed] [Google Scholar]
- Willemsen G, de Geus EJC, Bartels M, Van Beijsterveldt CEM, Brooks AI, Estourgie-van Burk GF, et al. The Netherlands Twin Register biobank: a resource for genetic epidemiological studies. Twin Res Hum Genet. 2010;13:231–245. doi: 10.1375/twin.13.3.231. [DOI] [PubMed] [Google Scholar]
- Willemsen G, Vink JM, Abdellaoui A, Den Braber A, Van Beek JHDA, Draisma HHM, et al. The Adult Netherlands Twin Register: 25 years of survey and biological data collection. Twin Res Hum Genet. 2013;16(1):271–281. doi: 10.1017/thg.2012.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan X, Waterworth D, Perry JRB, Lim N, Song K, Chambers JC, et al. Population-based genome-wide association studies reveal six loci influencing plasma levels of liver enzymes. Am J Hum Genet. 2008;83:520–528. doi: 10.1016/j.ajhg.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


