Abstract
Background and Purpose
Imaging advances allow investigation of white matter following stroke; a growing body of literature has shown links between diffusion-based measures of white matter microstructure and motor function. However, the relationship between these measures and motor skill learning has not been considered in individuals with stroke. The aim of this study was to investigate the relationships between post-training white matter microstructural status, as indexed by diffusion tensor imaging (DTI) within the ipsilesional posterior limb of the internal capsule (PLIC) and learning of a novel motor task in individuals with chronic stroke.
Methods
Thirteen participants with chronic stroke and nine healthy controls practiced a visuomotor pursuit task across five sessions. Change in motor behavior associated with learning was indexed by comparing baseline performance with a delayed retention test. Fractional anisotropy (FA) indexed at the retention test was the primary DTI-derived outcome measure.
Results
In individuals with chronic stroke, we discovered an association between post-training ipsilesional PLIC FA and the magnitude of change associated with motor learning; hierarchical multiple linear regression analyses revealed that the combination of age, time post stroke and ipsilesional PLIC FA post-training was associated with motor learning related change (R2=0.649, p=0.02). Baseline motor performance was not related to post-training ipsilesional PLIC FA.
Discussion and Conclusions
Diffusion characteristics of post-training ipsilesional PLIC were linked to magnitude of change in skilled motor behavior. These results imply that the microstructural properties of regional white matter indexed by diffusion behavior may be an important factor to consider when determining potential response to rehabilitation in persons with stroke. Video Abstract available (See Video, Supplemental Digital Content 1.) for more insights from the authors.
Keywords: Motor behavior, Diffusion tensor imaging, Cerebrovascular accident, Posterior limb of the internal capsule, Rehabilitation
Background
Past work robustly demonstrates that the learning of new motor skills is not abolished by stroke.1–3 However, the magnitude of motor learning related change associated with a fixed amount of practice is highly variable across individuals with stroke, making it difficult to predict response to treatment and potential for recovery. In part, the degree of change that may occur in response to skilled motor practice depends on stroke severity. Boyd et al3 demonstrated lower slopes of change in individuals with moderate to severe stroke as compared to those with more mild damage. However, this work examined motor learning when individuals employed their less-affected, non-hemiparetic arm and did not examine the relationship between residual white matter status and learning-related change.
Consideration of how residual brain tissue microstructure impacts motor learning related change is key in advancing the understanding of which individuals with stroke may benefit from certain forms of rehabilitation aimed at restoration of motor function. A large body of work highlights the relationship between motor learning and changes in gray matter; developments in imaging technology now allow us to examine the relationship between white matter and learning. Diffusion tensor imaging (DTI) can provide a noninvasive characterization of white matter tissue status in both healthy individuals and individuals after stroke.4 Specifically, DTI-derived metrics (e.g. fractional anisotropy (FA)) can be used to determine the magnitude and direction of water diffusion, which are influenced by the microstructural properties of brain tissue.5 In particular, FA is the most commonly reported DTI-derived measure of white matter integrity and has been associated with function in neurologically intact individuals and persons with neurologic injury.6–8
Past work examined white matter integrity in relation to training across different modalities. More specifically, training to improve knowledge of grammar rules, spelling, memory, or speech therapy in individuals with aphasia have all been linked to higher integrity (i.e., FA) of white matter in the tracts corresponding with cortical areas supporting these behaviors.9–12 However, limited work has linked motor training with change in diffusion behavior (i.e., increased FA) in regional white matter in healthy individuals. Past work has not considered whether DTI is sensitive to change in white matter microstructure associated with experience-dependent neuroplasticity in individuals with stroke or in older healthy adults. The few studies using DTI that show increased FA after training have employed very large doses of practice and been conducted in young healthy individuals. For example, after six weeks practicing juggling, healthy individuals demonstrate increased FA values in the right posterior intraparietalsulcus.13 Interestingly, these increases in FA values were not related to performance improvements, suggesting that the training time, rather than performance outcome is a key component to influencing white matter plasticity.13
A growing body of literature has examined white matter integrity using single time point diffusion scans in stroke populations. Hemispheric asymmetry of FA values within the posterior limb of the internal capsule (PLIC) and the corticospinal tract have been shown to predict change in Fugl-Meyer score following increased use of the hemiparetic arm to move a series of wooden blocks.14 Further, the degree of impairment in function of the upper limb is closely linked to FA values in the PLIC, even more so than the blood-oxygen-level-dependent signal in motor regions during training.15 The contralesional and ipsilesional corticospinal tracts have also been linked to motor function on measures such as grip strength, hand dexterity, pegboard, and index finger tapping.16,17 Similar trends have been shown with a varying array of functional measures and the corresponding white matter tracts.18,19 Short-term practice effects also appear to be related to baseline FA values in the PLIC bilaterally.20 However, it remains unclear if long-term practice effects are also mediated by white matter status in individuals with chronic stroke.
The purpose of the current study was to evaluate how post-training white matter status is linked to the magnitude of motor skill learning in individuals with chronic stroke. We hypothesized that ipsilesional white matter microstructural status at retention testing would be linked to motor learning related change such that higher ipsilesional FA values would be associated with larger change in motor performance after training. Evaluation of these relationships is of interest for both neuroscientists and clinicians alike, and should inform our collective understanding of how white matter tissue status within the stroke-affected brain may affect the capacity for motor learning. Further, a refined understanding of the relationships between microstructural status in the stroke-damaged brain and potential for motor learning related change may enable clinicians to better predict response to, and evaluation of, rehabilitation interventions.
Methods
Participants
Thirteen well-recovered individuals with a single, clinically diagnosed chronic (>6 months post-stroke)21 ischaemic stroke affecting the middle cerebral artery distribution (mean age 63.8 ± SD: 1.8) and nine healthy control participants (mean age 66.0 ± SD: 8.0) were recruited from the community (Table 1). The two groups had similar distributions of age, gender, and handedness. Informed consent was obtained from each participant in accordance with the Declaration of Helsinki. The University of British Columbia (UBC) research ethics boards approved all aspects of the study protocol.
Table 1.
Participant demographic information
| Subject ID | Age (y) | Gender | Affected/Trained Hand | Dominant Hand | Post-Stroke Duration (mo) | FA | RMSE | Lesion Location | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Contra | Ipsi | Day 1 | Day7 | |||||||
| S50 | 65 | M | L | R | 20 | 0.62 | 0.56 | 7.94 | 6.17 | Putamen, lentiform nucleus |
| S51 | 72 | M | L | R | 169 | 0.58 | 0.47 | 16.41 | 11.93 | Putamen, thalamus |
| S52 | 59 | F | L | R | 42 | 0.63 | 0.65 | 12.21 | 8.54 | Middle frontal gyrus, precentral gyrus |
| S55 | 72 | M | L | L | 101 | 0.63 | 0.45 | 22.76 | 18.57 | Inferior, middle frontal gyri |
| S57 | 74 | M | L | R | 65 | 0.60 | 0.59 | 17.65 | 15.16 | Putamen |
| S58 | 55 | F | L | R | 19 | 0.61 | 0.57 | 13.68 | 10.05 | Cingulate gyrus |
| S62 | 59 | M | R | L | 38 | 0.65 | 0.67 | 15.48 | 6.21 | Insula, inferior parietal lobe |
| S65 | 55 | M | R | L | 101 | 0.65 | 0.26 | 12.42 | 11.75 | Insula, putamen, precentral gyrus |
| S66 | 64 | M | L | R | 29 | 0.63 | 0.52 | 10.79 | 6.95 | Thalamus |
| S67 | 65 | F | L | R | 90 | 0.61 | 0.35 | 15.18 | 15.46 | Caudate, putamen, lentiform nucleus, ins |
| S68 | 68 | F | L | L | 136 | 0.60 | 0.47 | 27.09 | 20.56 | Superior temporal gyrus, insula |
| S69 | 58 | M | L | R | 17 | 0.63 | 0.67 | 16.29 | 8.90 | Pons |
| S70 | 63 | M | L | R | 28 | 0.63 | 0.54 | 14.69 | 11.67 | Cingulate gyrus |
|
| ||||||||||
| Mean ± SD: | 63.77 ± 6.42 | 65.77 ± 49.53 | 0.62 ± 0.02 | 0.52 ± 0.12 | 15.58 ± 4.97 | 11.68 ± 4.62 | ||||
|
| ||||||||||
| H05 | 64 | F | L | R | 0.58 | 0.6 | 9.07 | 6.43 | ||
| H06 | 72 | F | L | R | 0.64 | 0.63 | 14.60 | 9.56 | ||
| H07 | 67 | F | L | R | 0.68 | 0.66 | 12.98 | 10.13 | ||
| H08 | 63 | M | L | R | 0.61 | 0.6 | 7.24 | 6.10 | ||
| H09 | 60 | F | L | R | 0.67 | 0.67 | 7.98 | 7.49 | ||
| H10 | 51 | M | L | R | 0.64 | 0.64 | 17.94 | 7.54 | ||
| H11 | 68 | M | L | R | 0.65 | 0.63 | 11.45 | 10.05 | ||
| H13 | 80 | F | L | R | 0.65 | 0.64 | 8.38 | 6.26 | ||
| H14 | 69 | M | L | R | 0.62 | 0.64 | 9.97 | 7.78 | ||
|
| ||||||||||
| Mean ± SD: | 66 ± 8.06 | 0.64 ± 0.03 | 0.63 ± 0.02 | 11.07 ± 3.54 | 7.93 ± 1.61 | |||||
FA: fractional anisotropy, RMSE: root mean square error, Asym: asymmetry, Contra: contralesional, Ispi: ipsilesional, M: male, F: female, L: left, R: right, SD: standard deviation, y: years, mo: months, S: stroke, H: healthy
Inclusion/Exclusion Criteria
Individuals with a single middle cerebral artery ischaemic stroke occurring more than 6 months prior to participation were included in this study. Individuals were excluded from participation if they had any other known neurological impairments, scored below the 25th percentile on the Mini-Mental Status Exam,22 scored below 15 on the Fugl-Meyer Upper Extremity Motor Scale,23 or had any contraindications to magnetic resonance imaging (MRI). Additionally, participants required sufficient strength and motor control in order to grasp and manipulate a joystick to complete the skilled movements associated with our training task. Controls were excluded if they had known neurological impairments or contraindications to MRI.
Research Design
Each participant completed seven experimental sessions. The first session indexed baseline motor performance. Next, five practice sessions were completed on separate days, followed by a delayed retention test, which was performed on day 7. The data reported here were a part of a larger study that included multiple functional (f)MRI sessions. DTI data for this research were collected as a part of a single structural scanning battery that took place on day 7.
Visuomotor Pursuit Task
Participants sat in front of a computer monitor that displayed a white circle indicating the target and a red dot representing movement by the participant. Participants practiced tracking a moving target continuously using a joystick controlled by the hemiparetic arm (stroke group); healthy controls were matched for arm use (ie, right vs left) (Table 1). The participants’ forearms were fixed into foam-lined trays throughout the task, which necessitated movements into radial and ulnar deviation to control the joystick. On each training day, five tracking task blocks, composed of 10 tracking trials (20s each), were completed. Although the target and movement were visible, visual feed forward and feedback information regarding the upcoming movement pattern or previous tracking performance was not available (i.e., no pattern or trace was displayed on the computer screen). Each day participants were instructed to track the target as accurately as possible by controlling the position of the cursor with the joystick. At the completion of practice sessions, each participant completed 83.3 minutes of tracking training in total. The retention test consisted of a single block of the task (ten 20s tracking trials) completed in the functional (f)MRI scanner. This test was performed in order to assess improvement that had occurred as a result of previous training (as opposed to improvement within a single session). The time elapsed between each training session and between the final training session and retention test varied between 2–5 days.
Skill performance was quantified by root mean square error (RMSE) between target and response over time of each trial individually. RMSE was averaged across the first block of 10 (20s) trials for day 1 and day 7 assessments. A change score was calculated (Day 7 – Day 1) separately to assess improvement in tracking error associated with practice. Larger negative change scores represented greater reductions in visuomotor pursuit RMSE indicative of training-associated improvements in tracking skill.
MRI Data Acquisition
MR acquisition was conducted at the UBC MRI Research Centre on a Philips Achieva 3.0T whole body MRI scanner (Phillips Healthcare, Andover, MD) using an eight-channel sensitivity encoding head coil (SENSE factor=2.4) and parallel imaging. A high-resolution anatomical scan (TR = 12.4 ms, TE = 5.4 ms, flip angle θ = 8°, FOV = 256 mm, 170 slices, 1 mm thickness) was collected. A diffusion weighted scan was conducted with a single shot echo-planar imaging (EPI) sequence (TR = 7465 ms, TE = 75 ms, FOV = 212 × 212mm, 60 slices, 2.2 mm slice thickness, voxel dimension =2.23mm). Diffusion weighting was applied across 15 independent non-collinear orientations (b = 1000 s/mm2) along with a non-weighted diffusion weighted image acquired (b=0). A gradient table was used for subsequent data analysis, computed using parameters of the diffusion-weighted images.24
MR Data Processing
Diffusion-weighted images (Fig. 1A) were visually inspected for excessive motion artifact or instrumental noise; if an image was deemed corrupt, it was removed prior to tensor calculation.25 Less than 1% of images were removed across all subjects. After tensor calculation, FA maps were produced based on the magnitude of diffusivity in three defined orientations and the mean diffusivity of each within a given tensor.5 Color- coded orientation maps were used to visualize the principal fiber orientation within each pixel (red: right-left, blue: superior-inferior, green: anterior-posterior).
Figure 1.
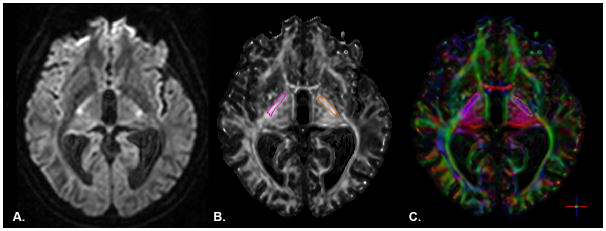
A) Axial slice of a raw diffusion weighted image. B) fractional anisotropy and C) color-coded orientation maps from a representative subject in the stroke group. Right (pink) and left (orange) posterior limb of the internal capsule regions of interest are overlaid in each image.
The ROIEditor software program (www.MRIStudio.org) was used to perform manual quantification of PLIC for each participant in subject-space. The PLIC was delineated bilaterally, beginning at the level of the anterior commissure and terminating at the inferior border of the corona radiata26 (Fig. 1B, C). The regions of interest (ROIs) were delineated using the FA and color maps and a white matter atlas.27 Lesions penetrating the PLIC were not excluded from the drawing procedure. Complete disruption of the affected PLIC was not observed for any participant. Mean FA values for the delineated region were computed by removing pixels with zero or negative FA values; the mean FA from the remaining voxels within the manually defined ROI mask was used for statistical analyses. We have previously shown that this approach has good reproducibility in individuals with stroke and healthy participants.7,16
Statistical Analyses
A two-way (Group × Day) repeated-measures analysis of variance (RM-ANOVA) was conducted to evaluate the differences in motor skill performance (i.e., RMSE) between the participants with stroke and healthy participants. The groups were then evaluated separately to measure the relationship between skill performance change and post-training FA values within the PLIC bilaterally. A one-way (Group) multiple analysis of variance (MANOVA) was conducted to assess differences in ipsilesional and contralesional post-training PLIC FA values in the stroke group compared to the non-dominant and dominant PLIC in the control group.
Hierarchical multiple linear regression analyses were performed to assess the amount of variance in RMSE change scores explained by PLIC FA values and demographic information. In the stroke group, post-stroke duration and age were entered together first into the model to account for stroke chronicity28–30 and age-related changes in learning capacity31 and white matter status.32 Post-training ipsilesional PLIC FA was the next predictor entered into the model, based on findings suggesting ipsilesional tract integrity is related to upper limb function,26 followed by contralesional post-training PLIC FA. In the healthy group, the same order of entry was used with age entered first into the model followed by non-dominant (trained) post-training PLIC FA and then dominant (untrained) post-training PLIC FA. We conducted the same analyses using hemispheric post-training PLIC FA asymmetry [(FAcontra − FAipsi)/(FAcontra + FAipsi)] as a single predictor where larger positive asymmetry values represented greater within-subject differences in post-training PLIC microstructural integrity. The analyses were also conducted to evaluate the relationship between naïve skill performance (day 1) and post-training FA values.
Zero-order, part and partial correlations were produced for each model. Colinearity between multiple predictors was evaluated using Variance Inflation Factor (VIF) and Tolerance levels. Values under 10 for VIF and above 0.1 for Tolerance were critical levels for acceptable colinearity.33 Residual statistics and plots were produced to ensure normality and homoscedasticity of the data.33,34 Durbin-Watson diagnostics were provided to ensure independence of the residuals. Influential cases and extreme outliers were determined using Cook’s distance (>1), studentized residuals (>3) and Mahalanobis distance (>10).33 No extreme outliers were detected. For each statistical test, significance level was set at p<0.05. All statistical procedures were conducted using SPSS software (SPSS 19.0).
Results
Comparisons between healthy and chronic stroke individuals
Across sessions, healthy participants demonstrated superior skill performance (indicated by lower RMSE values) compared to participants with stroke (Main effect of Group; F(1, 20)=6.09, p=0.023). Examining the change in skill across training, a group by time interaction was not observed (F(1, 20)=0.38, p=0.545). Both groups benefitted significantly from practice as shown by reduced RMSE values from day 1 to the retention test on day 7 (Main effect of Time; F(1, 20)=33.60, p<0.0005) (Fig. 2). Results from the MANOVA demonstrated differences in post-training FA between groups (Pillai’s Trace: F(2, 19)=4.34, p=0.028). This was attributed to difference in post-training FA in the ipsilesional PLIC. Post-training mean FA values in the contralesional PLIC did not differ between groups (F(1, 20)=2.24, p=0.151); however, post-training mean FA values for the ipsilesional PLIC were lower in the stroke group than in healthy controls (F(1, 20)=7.85, p=0.011) (Fig. 3).
Figure 2.
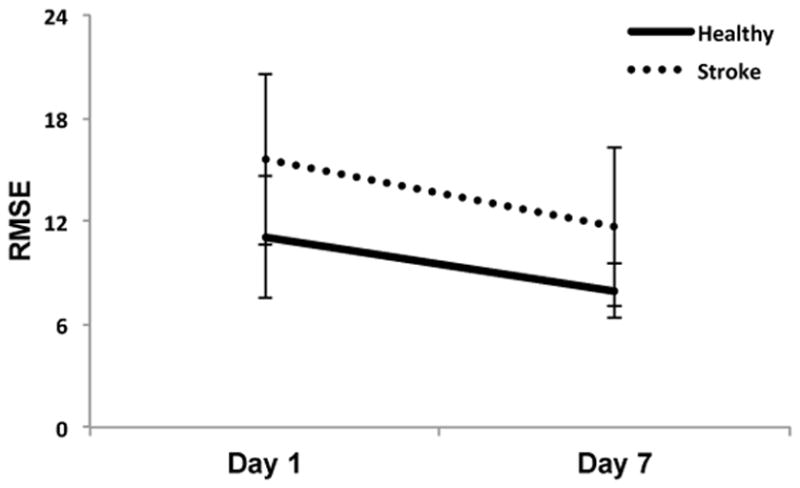
Difference in root mean square error (RMSE) for the visuomotor pursuit task from day 1 to the delayed retention test, which took place on day 7. Lower scores reflect more accurate visuomotor tracking and less error. Both groups demonstrated improved performance after completing the five training sessions. Error bars are standard deviation of the mean (SD).
Figure 3.
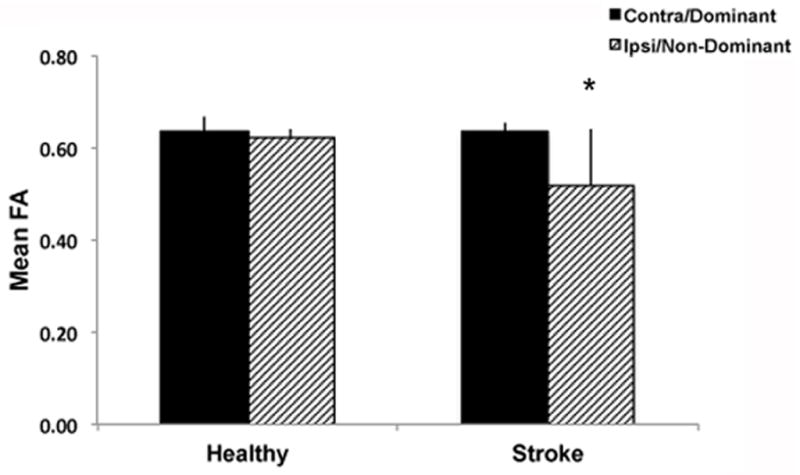
Mean post-training FA values from for dominant and non-dominant PLICs for healthy controls and ipsi- and contralateral PLICs for individuals with stroke. Reduced post-training mean FA was observed in the ipsilesional PLIC in the stroke group compared to the non-dominant PLIC in the healthy control group (*p<0.05). Error bars are standard deviation of the mean (SD).
Associations between visuomotor skill performance and diffusion measures
Results of the regression analyses conducted for both groups is summarized in Table 2. In the stroke group, the initial model (post-stroke duration, age) did not alone explain a significant amount of variance in change in RMSE associated with skill training or baseline skill performance. Adding post-training ipsilesional PLIC FA values produced a model that explained a unique amount of variance in response to skill training (R2=0.649, F(3,9) =5.55, p=0.020, βipsiFA: −1.073) but was not associated with baseline performance. Individuals that demonstrated greater improvements associated with practice (indicated by larger negative RMSE change scores) had higher post-training ipsilesional PLIC FA values. The bivariate relationship between change in RMSE and post-training ipsilesional PLIC FA is illustrated in Figure 4A. Note that when the amount of variance in change in RMSE explained by age and post-stroke duration are accounted for, post-training ipsilesional PLIC mean FA explains a larger proportion of variance in performance improvement (reduced RMSE) following training (Fig. 4B). Adding post-training contralesional PLIC FA to the model did not explain an additional amount of unique variance in change in performance, however the overall model remained significant (R2=0.746, F(4,8) =5.89, p=0.016).
Table 2a.
Summary of regression modeling of post-stroke duration and post-training PLIC integrity for baseline performance and motor learning
| Stroke Group | Predictors | R2 | F statistic | Significance | βPSD | βage | βipsi | βcontra |
|---|---|---|---|---|---|---|---|---|
| Baseline Performance | ||||||||
| Model 1 | PSD, age | 0.379 | 3.050 | 0.092 | 0.459 | 0.231 | ||
| Model 2 | PSD, age, FAIpsi | 0.446 | 2.415 | 0.134 | 0.730 | 0.122 | 0.347 | |
| Model 3 | PSD, age, FAIpsi, FAContra | 0.458 | 1.692 | 0.244 | 0.787 | 0.199 | 0.381 | 0.157 |
| Day 7 - Day 1 Change | ||||||||
| Model 1 | PSD, age | 0.008 | 0.041 | 0.960 | 0.049 | 0.053 | ||
| Model 2 | PSD, age, FAIpsi | 0.649 | 5.545 | 0.020* | −0.788* | 0.393 | −1.073* | |
| Model 3 | PSD, age, FAIpsi, FAContra | 0.746 | 5.885 | 0.016* | −0.948* | 0.175 | −1.167* | −0.441 |
|
| ||||||||
| Healthy Group | Predictors | R2 | F statistic | Significance | βage | βipsi | βcontra | |
| Baseline Performance | ||||||||
| Model 1 | Age | 0.142 | 1.163 | 0.317 | −0.377 | |||
| Model 2 | Age, FAIpsi | 0.190 | 0.705 | 0.531 | −0.366 | 0.219 | ||
| Model 3 | Age, FAIpsi, FAContra | 0.226 | 0.487 | 0.706 | −0.443 | −0.121 | 0.394 | |
| Day 7 - Day 1 Change | ||||||||
| Model 1 | Age | 0.241 | 2.226 | 0.179 | 0.491 | |||
| sdModel 2 | Age, FAIpsi | 0.241 | 0.954 | 0.437 | 0.491 | −0.003 | ||
| Model 3 | Age, FAIpsi, FAContra | 0.244 | 0.537 | 0.677 | 0.511 | 0.082 | −0.099 | |
Figure 4.
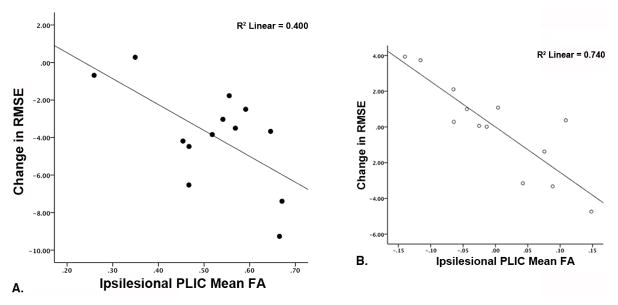
A) Scatterplot depicting the bivariate relationship between change in tracking performance (root mean square error, RMSE) and post-training ipsilesional PLIC FA values. Tracking performance improvements (more negative RMSE change scores) were correlated with higher post-training ipsilesional PLIC mean FA values. B) Partial residual plot illustrating the relationship between change RMSE and post-training ipsilesional PLIC FA after regressing each separately on age and post-stroke duration. The amount of variance in change in RMSE explained by post-training ipsilesional PLIC FA was increased after accounting for the variance in RMSE change explained by age and post-stroke duration.
In a separate model, adding post-training FA asymmetry as a predictor of change in motor skill learning produced a significant model (R2=0.573, F(3,9) =4.03, p=0.045, βFA asymmetry: 0.984) (Fig. 5A and B) where less asymmetry in post-training PLIC FA was associated with greater change in motor skill following training. No association was observed with baseline tracking performance for post-training FA asymmetry (p>0.05).
Figure 5.
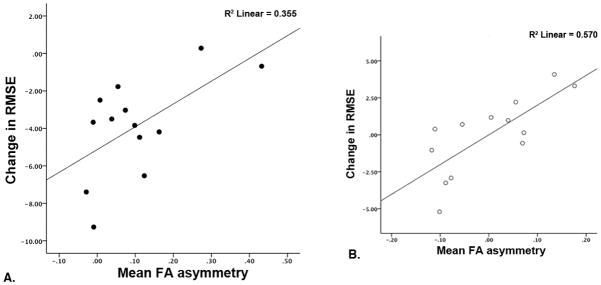
A) Scatterplot depicting the bivariate relationship between change in RMSE and post-training hemispheric PLIC FA asymmetry values. Performance improvements were associated with reduced post-training hemispheric PLIC FA asymmetry. B) Partial residual plot illustrating the relationship between change in RMSE and post-training hemispheric PLIC FA asymmetry after regressing each separately on age and post-stroke duration.
In the healthy group, regression analyses did not demonstrate a significant association between post-training FA values with change in skill or baseline performance (p>0.05) (Table 2) (Figure 6).
Figure 6.
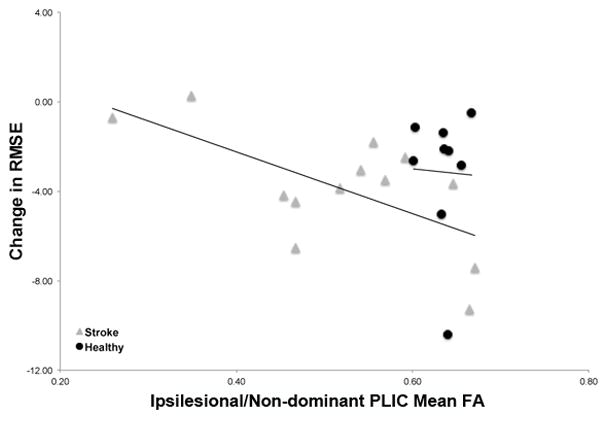
Scatterplot demonstrating the relationship between post-training FA values in the ipsilesional (stroke group) and non-dominant (healthy group) PLIC with change in RMSE following training. These data reiterate the significant relationship observed between post-training FA values in the ipsilesional PLIC and change in performance with increasing post-training FA associated with greater skill improvement (reduced RMSE values) in the stroke group. No significant relationship was observed in the healthy group of individuals.
Discussion
Consistent with past work, our results demonstrate that both individuals with stroke and age-matched healthy controls benefit from skilled practice and can demonstrate motor learning of a novel motor task.2,35 However, our data extend past work showing that magnitude of motor learning related change in the performance of a complex visuomotor task is associated with post-training ipsilesional PLIC status, indexed by FA, in individuals with chronic stroke. Interestingly, baseline motor performance was not related to post-training ipsilesional PLIC FA values, suggesting that white matter status within this region is linked to change in skilled motor behavior rather than naïve motor skill performance.
Numerous studies have shown that given adequate doses of practice individuals with stroke can learn new motor skills using either their hemiparetic1,2,36 or non-hemiparetic arm.3,37–40 Yet, the magnitude of change associated with motor learning after stroke is highly variable, and motor performance after stroke is rarely as accurate or as fast as that of age-matched healthy controls. Our findings suggest that post-training FA within the ipsilesional PLIC may be an important marker of motor learning related change in chronic stroke. It has begun to be considered in motor learning studies in healthy controls. When healthy individuals practice a new motor task, a strong relationship exists between skill development and baseline, pre-training FA within the corticospinal tract.41 Thus, future work may need to account for ipsilesional PLIC FA to individualize and optimize motor learning after stroke.
Taken together with other work26,42,43 our data show the necessity for a certain level of residual white matter tissue to demonstrate motor learning related behavioral change after stroke. In fact, our data suggest that after stroke behavioral capacity for learning related change scales with post-training FA. Although it is possible that training altered FA values, our results show that individuals with higher post-training FA in the ipsilesional PLIC demonstrated greater magnitude of motor learning related change (Fig. 4A). \
Importantly, age and time post stroke alone were not significantly associated with motor learning related change in behavior; only when these factors were combined with post-training ipsilesional PLIC FA did the model explain a significant amount of variance in change in skill. This finding indicates that age and post stroke duration should not be used in isolation, to determine potential for motor learning related change. This is important information for individuals who design the content and course of rehabilitation interventions. A recent systematic review supports this contention and determined there is inconclusive evidence that time since stroke influences upper limb motor recovery.44 It has also been suggested that motor outcomes after stroke may be best predicted with diffusion-based information.6 Further we propose that altered white matter microstructural integrity within the ipsilesional PLIC may be one source of variability that could account for some degree of between-participant differences in outcomes across motor learning studies. This factor may need to be accounted for in the design and interpretation of experiments intended to ascertain the optimal choice of rehabilitation intervention, dose of practice, and feedback schedule to stimulate motor learning after stroke.
Our data confirm and extend those of Stinear et al26 showing that motor learning related change is associated with FA asymmetry between ipsi- and contralesional PLIC where an asymmetry greater than 0.25 was associated with lower potential for functional recovery and reduced response to an experimental block moving task. Given that even after a unilateral stroke white matter in both PLIC’s and motor function in both arms may be disrupted, indexing FA asymmetry provides a measure of white matter status that more fully characterizes upper extremity motor function. Interestingly, examination of our results shows that individuals with asymmetry of 0.20 or higher failed to demonstrate motor learning related change (Fig. 5A). It may be that the greater complexity and motor control requirements of our visuomotor pursuit motor learning task necessitated more symmetry between ipsi- and contralesional post-training PLIC FA values as compared to the relatively simple block moving task employed in past work. Importantly, it is consistently clear in the literature that greater asymmetry in PLIC FA after stroke contributes to both increased level of physical impairment (reduced FM scores)15,26 and reduced motor learning capacity.
There are limitations to this study. The sample of participants with stroke was relatively small and homogenous. Although this homogeneity may improve sensitivity to observing effects by reducing inter-subject variability, it could also limit its generalizability to all stroke populations. Sample size may have also reduced statistical power required to detect associations between white matter integrity and baseline skill performance. The sample size studied may also limit generalizability of the results from the multiple regression analyses. Future work should evaluate our preliminary findings in a larger participant cohort. Further, we were not powered to detect relationships between our variables within the healthy control group. Diffusion imaging was limited to one scan with a limited number of orientations, yet this is comparable to related investigations.14,15,45 However, our scan parameters may have decreased the signal-to-noise ratio and resolution, thus decreasing sensitivity to detecting small changes in diffusion behavior, especially at tissue boundaries and brain regions with crossing fibers. We focused on evaluating one DTI-based metric of white matter in the PLIC in an effort to minimize these potential limitations. We also limited our investigation to the PLIC because it was a region previously shown to have reduced integrity following stroke.14,15 Other investigations have used alternative analysis techniques to evaluate this subsection of tract and also other white matter regions within the brain.45–47 Currently there is no gold standard for DTI analysis techniques and all approaches have potential limitations and pitfalls48 that need to be considered when determine optimal imaging and analysis parameters in stroke.
Another limitation of this study was that diffusion imaging was performed following training. Currently, the time period required to observe training-induced white matter plasticity in healthy individuals is unclear. Studies showing change in white matter diffusion behavior following training generally occur over 5–8 weeks.9,10,13 Further, in a study specifically related to motor-based training, healthy individuals were trained on a serial reaction time motor skill task for 10 days within only a 2-week period and showed no FA differences following training.49 However, a recent investigation showed only two hours of training on a spatial memory and learning motor task produced short-term changes in hippocampal and parahippocampal white matter.50 Only one research report has documented white matter change in individuals with stroke; this work considered the impact of a functional electrical stimulation intervention on mean FA values.51 This work included only 3 participants and has yet to be replicated. Finally, DTI has not shown sensitivity to detect small, positive changes in white matter microstructure (i.e., increases in FA) that are likely associated with laboratory-based motor learning studies in older individuals or in people with chronic stroke; this result may stem in part from the relatively low doses of practice delivered in most motor learning studies.2,3,35,52 A newer imaging modality called multi-component T2 relaxation imaging (MCRI) has recently been shown to index a specific element of white matter microstructure, myelin, in individuals with chronic stroke.53 Although MCRI may allow the quantification of motor learning related change in white matter microstructure after stroke, it has yet to be fully investigated in this context. Thus, given the issues outline above, for the present study, we chose not to attempt to index change in FA but instead focused on understanding if post-training white matter tissue status might be associated with motor learning related change in behavior. Further work including longitudinal studies of training and white matter plasticity, that employ imaging techniques that are both sensitive to change and specific to brain structure such as MCRI, will be required to determine whether it is possible that training-induced white matter plasticity supports the acquisition of new motor skills.
Lastly, this investigation focused on one type of skill learning and a single training paradigm. Repeated daily practice sessions performing continuous tracking with the affected extremity have previously demonstrated implicit motor skill learning in individuals with stroke1 and have important implications for rehabilitation.54,55 Future inquiry should evaluate relationships between adaptive brain structure and function with response to different motor learning task paradigms using multimodal neuroimaging approaches.
Supplementary Material
Table 2b.
Summary of regression modeling of post-stroke duration and FA asymmetry for baseline performance and motor learning
| Stroke Group | Predictors | R2 | F statistic | Significance | βPSD | βage | βasym | |
|---|---|---|---|---|---|---|---|---|
| Baseline Performance | ||||||||
| Model 1 | PSD, age | 0.379 | 3.050 | 0.092 | 0.459 | 0.231 | ||
| Model 2 | PSD, age, FA asym | 0.440 | 2.362 | 0.139 | 0.707 | 0.077 | −0.325 | |
| Day 7 - Day 1 Change | ||||||||
| Model 1 | PSD, age | 0.008 | 0.041 | 0.960 | 0.049 | 0.053 | ||
| Model 2 | PSD, age, FA_asym | 0.573 | 4.025 | 0.045* | −0.701 | 0.519 | 0.984* | |
|
| ||||||||
| Healthy Group | Predictors | R2 | F statistic | Significance | βage | βasym | ||
| Baseline Performance | ||||||||
| Model 1 | Age | 0.142 | 1.163 | 0.317 | −0.377 | |||
| Model 2 | Age, FA_asym | 0.201 | 0.756 | 0.510 | −0.467 | 0.258 | ||
| Day 7 - Day 1 Change | ||||||||
| Model 1 | Age | 0.241 | 2.226 | 0.179 | 0.491 | |||
| Model 2 | Age, FA_asym | 0.243 | 0.965 | 0.433 | 0.508 | −0.05 | ||
PLIC: posterior limb internal capsule, PSD: post-stroke duration, ipsi: ipsilateral, contra: contralateral, asym: asymmetry
Clinical Implications.
Our findings indicate that post-training diffusion-based measures of white matter microstructural integrity of ipsilesional primary motor output tracts are closely associated with response to motor skill training in well-recovered individuals with stroke in the chronic phase of recovery. Importantly, demographic information in isolation did not predict motor learning related change. These results imply that regional white matter diffusion characteristics may be an important factor to consider when interpreting or predicting response to rehabilitation or when designing studies that consider motor learning related change.
Acknowledgments
This work was supported by the National Institutes of Health [NS051714 to L.A.B.] and the Canadian Institutes of Health Research [MOP-106651 to L.A.B.]. Support was also provided to LAB by the Canada Research Chairs and the Michael Smith Foundation for Health Research. The Natural Sciences and Engineering Research Council of Canada provided support to KEB and the Heart and Stroke Foundation of Canada supported MRB.
References
- 1.Meehan SK, Randhawa B, Wessel B, Boyd LA. Implicit sequence-specific motor learning after subcortical stroke is associated with increased prefrontal brain activations: an fMRI study. Human brain mapping. 2011 Feb;32(2):290–303. doi: 10.1002/hbm.21019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boyd LA, Vidoni ED, Wessel BD. Motor learning after stroke: is skill acquisition a prerequisite for contralesional neuroplastic change? Neuroscience letters. 2010 Sep 20;482(1):21–25. doi: 10.1016/j.neulet.2010.06.082. [DOI] [PubMed] [Google Scholar]
- 3.Boyd LA, Quaney BM, Pohl PS, Winstein CJ. Learning implicitly: effects of task and severity after stroke. Neurorehabil Neural Repair. 2007 Sep-Oct;21(5):444–454. doi: 10.1177/1545968307300438. [DOI] [PubMed] [Google Scholar]
- 4.Le Bihan D, Mangin J-F, Poupon C, et al. Diffusion Tensor Imaging: Concepts and Applications. Journal of Magnetic Resonance Imaging. 2001;13:534–546. doi: 10.1002/jmri.1076. [DOI] [PubMed] [Google Scholar]
- 5.Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson B. 1996 Jun;111(3):209–219. doi: 10.1006/jmrb.1996.0086. [DOI] [PubMed] [Google Scholar]
- 6.Jang SH. Prediction of motor outcome for hemiparetic stroke patients using diffusion tensor imaging: A review. NeuroRehabilitation. 2010;27(4):367–372. doi: 10.3233/NRE-2010-0621. [DOI] [PubMed] [Google Scholar]
- 7.Borich MR, Wadden KP, Boyd LA. Establishing the reproducibility of two approaches to quantify white matter tract integrity in stroke. Neuroimage. 2012 Feb 1;59(3):2393–2400. doi: 10.1016/j.neuroimage.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Draganski B, Gaser C, Busch V, Schuierer G, Bogdahn U, May A. Neuroplasticity: Changes in grey matter induced by training - Newly honed juggling skills show up as a transient feature on a brain-imaging scan. Nature. 2004 Jan 22;427(6972):311–312. doi: 10.1038/427311a. [DOI] [PubMed] [Google Scholar]
- 9.Gebauer D, Fink A, Kargl R, et al. Differences in brain function and changes with intervention in children with poor spelling and reading abilities. PLoS One. 2012;7(5):e38201. doi: 10.1371/journal.pone.0038201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Engvig A, Fjell AM, Westlye LT, et al. Memory training impacts short-term changes in aging white matter: a longitudinal diffusion tensor imaging study. Hum Brain Mapp. 2012 Oct;33(10):2390–2406. doi: 10.1002/hbm.21370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Floel A, de Vries MH, Scholz J, Breitenstein C, Johansen-Berg H. White matter integrity in the vicinity of Broca’s area predicts grammar learning success. Neuroimage. 2009 Oct 1;47(4):1974–1981. doi: 10.1016/j.neuroimage.2009.05.046. [DOI] [PubMed] [Google Scholar]
- 12.Schlaug G, Marchina S, Norton A. Evidence for plasticity in white-matter tracts of patients with chronic Broca’s aphasia undergoing intense intonation-based speech therapy. Ann N Y Acad Sci. 2009 Jul;1169:385–394. doi: 10.1111/j.1749-6632.2009.04587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scholz J, Klein MC, Behrens TE, Johansen-Berg H. Training induces changes in white-matter architecture. Nat Neurosci. 2009 Nov;12(11):1370–1371. doi: 10.1038/nn.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stinear CM, Barber PA, Smale PR, et al. Functional potential in chronic stroke patients depends on corticospinal tract integrity. Brain. 2007 Jan;130(Pt 1):170–180. doi: 10.1093/brain/awl333. [DOI] [PubMed] [Google Scholar]
- 15.Qiu M, Darling WG, Morecraft RJ, Ni CC, Rajendra J, Butler AJ. White matter integrity is a stronger predictor of motor function than BOLD response in patients with stroke. Neurorehabil Neural Repair. 2011 Mar-Apr;25(3):275–284. doi: 10.1177/1545968310389183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Borich MR, Mang C, Boyd LA. Both projection and commissural pathways are disrupted in individuals with chronic stroke: investigating microstructural white matter correlates of motor recovery. BMC Neurosci. 2012 Aug 29;13(1):107. doi: 10.1186/1471-2202-13-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schaechter JD, Fricker ZP, Perdue KL, et al. Microstructural status of ipsilesional and contralesional corticospinal tract correlates with motor skill in chronic stroke patients. Hum Brain Mapp. 2009 Nov;30(11):3461–3474. doi: 10.1002/hbm.20770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Caeyenberghs K, Leemans A, Geurts M, et al. Correlations Between White Matter Integrity and Motor Function in Traumatic Brain Injury Patients. Neurorehabil Neural Repair. 2011 Jul-Aug;25(6):492–502. doi: 10.1177/1545968310394870. [DOI] [PubMed] [Google Scholar]
- 19.Sullivan EV, Rohlfing T, Pfefferbaum A. Longitudinal study of callosal microstructure in the normal adult aging brain using quantitative DTI fiber tracking. Dev Neuropsychol. 2010;35(3):233–256. doi: 10.1080/87565641003689556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bosnell RA, Kincses T, Stagg CJ, et al. Motor Practice Promotes Increased Activity in Brain Regions Structurally Disconnected After Subcortical Stroke. Neurorehabil Neural Repair. 2011 Sep;25(7):607–616. doi: 10.1177/1545968311405675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cramer SC. Repairing the human brain after stroke: I. Mechanisms of spontaneous recovery. Ann Neurol. 2008 Mar;63(3):272–287. doi: 10.1002/ana.21393. [DOI] [PubMed] [Google Scholar]
- 22.Folstein M, Folstein S, McHugh P. “Mini-mental state:” A practical method for grading the cognitive state of patients for the clinician. J Psychiat Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 23.Fugl-Meyer AR, Jaasko L, Leyman I, Olsson S, Steglind S. The post-stroke hemiplegic patient: a method for evaluation of physical performance. Scand J Rehab Med. 1975;7:13–31. [PubMed] [Google Scholar]
- 24.Farrell JA, Landman BA, Jones CK, et al. Effects of signal-to-noise ratio on the accuracy and reproducibility of diffusion tensor imaging-derived fractional anisotropy, mean diffusivity, and principal eigenvector measurements at 1.5 T. J Magn Reson Imaging. 2007 Sep;26(3):756–767. doi: 10.1002/jmri.21053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiang H, van Zijl PC, Kim J, Pearlson GD, Mori S. DtiStudio: resource program for diffusion tensor computation and fiber bundle tracking. Comput Methods Programs Biomed. 2006 Feb;81(2):106–116. doi: 10.1016/j.cmpb.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 26.Stinear CM, Barber PA, Smale PR, Coxon JP, Fleming MK, Byblow WD. Functional potential in chronic stroke patients depends on corticospinal tract integrity. Brain. 2007 Jan;130(Pt 1):170–180. doi: 10.1093/brain/awl333. [DOI] [PubMed] [Google Scholar]
- 27.Oishi K, Faria A, van Ziji P, Mori S. MRI Atlas of Human White Matter. Academic Press; 2011. [Google Scholar]
- 28.Jablonka JA, Burnat K, Witte OW, Kossut M. Remapping of the somatosensory cortex after a photothrombotic stroke: dynamics of the compensatory reorganization. Neuroscience. 2010 Jan 13;165(1):90–100. doi: 10.1016/j.neuroscience.2009.09.074. [DOI] [PubMed] [Google Scholar]
- 29.Feydy A, Carlier R, Roby-Brami A, et al. Longitudinal study of motor recovery after stroke - Recruitment and focusing of brain activation. Stroke; a journal of cerebral circulation. 2002;33(6):1610–1617. doi: 10.1161/01.str.0000017100.68294.52. [DOI] [PubMed] [Google Scholar]
- 30.Duncan PW, Goldstein LB, Matchar D, Divine GW, Feussner J. Measurement of motor recovery after stroke. Outcome assessment and sample size requirements. Stroke. 1992 Aug;23(8):1084–1089. doi: 10.1161/01.str.23.8.1084. [DOI] [PubMed] [Google Scholar]
- 31.Boyd LA, Vidoni ED, Siengsukon CF. Multidimensional motor sequence learning is impaired in older but not younger or middle-aged adults. Physical Therapy. 2008 Mar;88(3):351–362. doi: 10.2522/ptj.20070131. [DOI] [PubMed] [Google Scholar]
- 32.Lebel C, Rasmussen C, Wyper K, Andrew G, Beaulieu C. Brain microstructure is related to math ability in children with fetal alcohol spectrum disorder. Alcohol Clin Exp Res. 2010 Feb;34(2):354–363. doi: 10.1111/j.1530-0277.2009.01097.x. [DOI] [PubMed] [Google Scholar]
- 33.Field AP. Discovering statistics using SPSS. 3. Los Angeles, CA, USA: Sage; 2009. [Google Scholar]
- 34.Howell DC. Statistical Methods for Psychology. Belmont, California: Thomson Wadsworth; 2007. [Google Scholar]
- 35.Boyd LA, Edwards JD, Siengsukon CS, Vidoni ED, Wessel BD, Linsdell MA. Motor sequence chunking is impaired by basal ganglia stroke. Neurobiology of learning and memory. 2009 Jul;92(1):35–44. doi: 10.1016/j.nlm.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 36.Winstein CJ, Merians AS, Sullivan KJ. Motor learning after unilateral brain damage. Neuropsychologia. 1999;37:975–987. doi: 10.1016/s0028-3932(98)00145-6. [DOI] [PubMed] [Google Scholar]
- 37.Pohl PS, Winstein CJ. Practice effects on the less-affected upper extremity after stroke. Archives of physical medicine and rehabilitation. 1999;80(6):668–675. doi: 10.1016/s0003-9993(99)90170-3. [DOI] [PubMed] [Google Scholar]
- 38.Vidoni ED, Boyd LA. Preserved motor learning after stroke is related to the degree of proprioceptive deficit. Behav Brain Funct. 2009 Aug 28;5(1):36. doi: 10.1186/1744-9081-5-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boyd L, Winstein C. Explicit information interferes with implicit motor learning of both continuous and discrete movement tasks after stroke. J Neurol Phys Ther. 2006 Jun;30(2):46–57. doi: 10.1097/01.npt.0000282566.48050.9b. discussion 58–49. [DOI] [PubMed] [Google Scholar]
- 40.Boyd LA, Winstein CJ. Providing explicit information disrupts implicit motor learning after basal ganglia stroke. Learn Mem. 2004 Jul-Aug;11(4):388–396. doi: 10.1101/lm.80104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Song S, Sharma N, Buch ER, Cohen LG. White Matter Microstructural Correlates of Superior Long-term Skill Gained Implicitly under Randomized Practice. Cerebral cortex. 2012 Jul;22(7):1671–1677. doi: 10.1093/cercor/bhr247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lotze M, Beutling W, Loibl M, et al. Contralesional motor cortex activation depends on ipsilesional corticospinal tract integrity in well-recovered subcortical stroke patients. Neurorehabilitation and neural repair. 2012 Jul;26(6):594–603. doi: 10.1177/1545968311427706. [DOI] [PubMed] [Google Scholar]
- 43.Schaechter JD, Fricker ZP, Perdue KL, et al. Microstructural status of ipsilesional and contralesional corticospinal tract correlates with motor skill in chronic stroke patients. Human brain mapping. 2009 Nov;30(11):3461–3474. doi: 10.1002/hbm.20770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Coupar F, Pollock A, Rowe P, Weir C, Langhorne P. Predictors of upper limb recovery after stroke: a systematic review and meta-analysis. Clin Rehabil. 2012 Apr;26(4):291–313. doi: 10.1177/0269215511420305. [DOI] [PubMed] [Google Scholar]
- 45.Sterr A, Shen S, Szameitat AJ, Herron KA. The role of corticospinal tract damage in chronic motor recovery and neurorehabilitation: a pilot study. Neurorehabilitation and neural repair. 2010 Jun;24(5):413–419. doi: 10.1177/1545968309348310. [DOI] [PubMed] [Google Scholar]
- 46.Lindenberg R, Renga V, Zhu LL, Betzler F, Alsop D, Schlaug G. Structural integrity of corticospinal motor fibers predicts motor impairment in chronic stroke. Neurology. 2010 Jan 26;74(4):280–287. doi: 10.1212/WNL.0b013e3181ccc6d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bosnell RA, Kincses T, Stagg CJ, et al. Motor practice promotes increased activity in brain regions structurally disconnected after subcortical stroke. Neurorehabilitation and neural repair. 2011 Sep;25(7):607–616. doi: 10.1177/1545968311405675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jones DK. Precision and accuracy in diffusion tensor magnetic resonance imaging. Topics in magnetic resonance imaging: TMRI. 2010 Apr;21(2):87–99. doi: 10.1097/RMR.0b013e31821e56ac. [DOI] [PubMed] [Google Scholar]
- 49.Kwon YH, Nam KS, Park JW. Identification of cortical activation and white matter architecture according to short-term motor learning in the human brain: functional MRI and diffusion tensor tractography study. Neurosci Lett. 2012 Jun 27;520(1):11–15. doi: 10.1016/j.neulet.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 50.Sagi Y, Tavor I, Hofstetter S, Tzur-Moryosef S, Blumenfeld-Katzir T, Assaf Y. Learning in the fast lane: new insights into neuroplasticity. Neuron. 2012 Mar 22;73(6):1195–1203. doi: 10.1016/j.neuron.2012.01.025. [DOI] [PubMed] [Google Scholar]
- 51.Boespflug EL, Storrs JM, Allendorfer JB, Lamy M, Eliassen JC, Page S. Mean diffusivity as a potential diffusion tensor biomarker of motor rehabilitation after electrical stimulation incorporating task specific exercise in stroke: a pilot study. Brain Imaging Behav. 2011 Dec 28; doi: 10.1007/s11682-011-9144-1. [DOI] [PubMed] [Google Scholar]
- 52.Boyd LA, Vidoni ED, Siengsukon CF. Multidimensional motor sequence learning is impaired in older but not younger or middle-aged adults. Phys Ther. 2008 Mar;88(3):351–362. doi: 10.2522/ptj.20070131. [DOI] [PubMed] [Google Scholar]
- 53.Borich MR, MacKay AL, Vavasour IM, Rauscher A, Boyd LA. White matter myelin water fraction is reduced in chronic stroke. Neuroimage: Clinical. 2013 doi: 10.1016/j.nicl.2013.04.006. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Boyd LA, Winstein CJ. Impact of explicit information on implicit motor-sequence learning following middle cerebral artery stroke. Phys Ther. 2003 Nov;83(11):976–989. [PubMed] [Google Scholar]
- 55.Boyd LA, Winstein CJ. Cerebellar stroke impairs temporal but not spatial accuracy during implicit motor learning. Neurorehabilitation and neural repair. 2004;18:134–143. doi: 10.1177/0888439004269072. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


