Abstract
Background
The initial trigger of inflammatory bowel disease (IBD) can be partly attributed towards the interaction and invasion of intestinal epithelial cells (IECs) and submucosal compartments. Identifying safe and economical methods to block these interactions may help prevent the onset of early colitis. Chitinase 3-like 1 is an inducible host protein that facilitates bacterial attachment and invasion on/into IECs. Therefore, we test the hypothesis of inhibiting CHI3L1 using the pan-chitinase inhibitor caffeine to reduce the likelihood of early colitis onset.
Methods
IEC lines were treated with caffeine (2.5 mM or 5 mM) and analyzed for CHI3L1 expression and the impact on bacterial invasion. In vivo, mice were treated with 2.5 mM caffeine and induced with 3.5% dextran sulfate sodium (DSS)-mediated colitis and subsequently analyzed colitis development.
Results
In vitro, caffeine treatment in IEC lines down-regulated CHI3L1 mRNA expression, which resulted in the reduction of bacterial invasion in a caffeine dose-dependent manner. In vivo, mice treated with caffeine displayed delayed response towards DSS-induced colitis, characterized by lower body weight loss, clinical and histological scores. Bacterial translocation into other organs and pro-inflammatory cytokines production were also reduced in the caffeine-treated mice with DSS-induced colitis. Caffeine treatment also resulted in the loss of CHI3L1-associated AKT signaling pathway activation both in vitro and in vivo.
Conclusion
Development of acute colitis is reduced upon caffeine treatment. The mechanism involves the down-regulation of CHI3L1 expression and its associated bacterial interaction effect. Therefore caffeine is proposed as a safe and economical candidate for successful IBD management.
Keywords: chitinase inhibitor, inflammatory bowel disease, AKT, host-microbial interactions
Introduction
Dysregulated host/microbial interactions and several biological pathways play a major role in the development of inflammatory bowel disease (IBD) including Crohn’s disease (CD) and ulcerative colitis (UC) [1, 2]. Recent genome-wide association studies showed that the risk of developing IBD is further enhanced in susceptible individuals [3–6]. Since the cause of IBD is currently unknown, there are no precise measures to prevent the onset of the disease.
Experimental murine models showed that during acute as well as chronic colitis, intestinal epithelial cells (IECs) is induced to express Chitinase 3-like 1 (CHI3L1) in response to pro-inflammatory cytokines (e.g. TNF-α and IL-6) [7]. This disease-associated CHI3L1 expression was also observed in colonic tissue samples obtained from CD and UC patients, but was undetected in normal control individuals [7]. Early expression of CHI3L1 on IECs promotes enteric bacterial adhesion on the mucosal epithelium and result in the enhancement of microbial invasion into the IECs and eventually into the submucosal layer, triggering the subsequent flare of intestinal inflammation [7]. CHI3L1 also functions to activate specific cellular signaling pathway in IECs, including PI3K/AKT [8]. Administration of anti-CHI3L1 antibody in vivo protects mice from DSS-induced acute colitis [7]. Therefore, intercepting CHI3L1 functions appears to be a plausible strategy to lower the onset and/or exacerbation of acute colitis.
Caffeine (1, 3, 7 trimethylxanthine) was identified as a pan-family 18 chitinase inhibitor, including acidic mammalian chitinase (AMCase) and CHI3L1. X-ray crystallography of the chitinase-caffeine complex showed the interaction between two linked caffeine moieties in the chitinase active site cleft [9]. Current known chitinase inhibitors including cyclic dipeptide CI-4, allosamidin and argifin, have high molecular weight, multiple stereocenters and high production cost, thus making them less suitable for chemotherapeutic purposes. In contrary, caffeine is commonly found in many natural sources including food and beverages and has also been reported to have mucosal protective effect [10]. The relatively low extraction/production cost and good tolerance levels makes caffeine an ideal candidate to target chitinase mediated IBD pathogenesis.
In this study, we investigate the beneficial effects of caffeine in the development of acute dextran sulfate sodium (DSS)-induced colitis in vitro and in vivo. We show that IECs down-regulate CHI3L1 mRNA levels in response to dose-dependent caffeine treatment. This down-regulation of CHIL31 results in a decrease bacterial invasion into both IECs and macrophages, accompanied with an apparent reduction in AKT activation. Mice treated with medium dose of caffeine (2.5 mM; equivalent to the concentration of caffeine in 2–3 cups of coffee) showed protection against acute DSS-induced colitis as compared to the control mice. These results suggest that caffeine is effective in preventing the onset of colitis through the suppression of CHI3L1 expression and biological function and thus reducing bacterial invasion and inflammatory initiation.
Materials and Methods
Cell culture and reagents
SW480 cell was obtained from the American Type Culture Collection (ATCC, Manassas, VA) and were cultured in DMEM (Cellgro, Lawrence, KS) supplemented with 10% (vol/vol) FBS (Atlanta Biologicals, Lawrenceville, GA) and antibiotics cocktail (Cellgro). Anhydrous caffeine (C6H10N4O2) was purchased from Sigma-Aldrich (Saint Louis, MO). 200 mg/ml caffeine stock solution was prepared by dissolving in boiling water. DSS was purchased from MP Biochemical, Santa Ana, CA.
Bacterial strain and culture condition
The adherent invasive Escherichia coli (AIEC) LF82 strain that was isolated originally from a chronic ileal lesion of a patient with CD was provided by Dr. Arlette Darfeuille-Michaud (Clermont-Ferrand, France). This strain was grown in Lauria-Bertani (LB) broth without shaking overnight at 37°C.
Animal experiments and husbandry
C57Bl/6 wild type (WT) mice (male, 6–8 weeks old) were purchased from Jackson Laboratories (Bar Harbor, ME) and maintained under specific pathogen-free facilities at Massachusetts General Hospital (MGH). All experimental protocols were reviewed and approved by the MGH Subcommittee on Research Animal Care, Assurance # A3596-01, and the experiments were performed under an approved protocol by the hospital SRAC (permit numbers #2011N17 and #2005N264).
In vitro caffeine treatment
SW480 or CMT93 cells (2×105) were seeded on a 24 well plate overnight and when cells are 90% confluent, 2.5 mM or 5 mM caffeine were added directly into the cell culture medium for 48 hours. In a separate experiment, 0.1 or 5 units of bacterial chitinase from Serratia marcescens (Sigma-Aldrich, St. Louis, MO) was pre-incubated with 2.5 mM or 5 mM caffeine for 48 hours under room temperature and directly added into the SW480 cell culture media and further incubated for additional 48 hours. Cells were washed in PBS and harvested for transcriptional analysis.
Clinical and histological evaluation of DSS-induced colitis
Subject mice were treated with 2.5 mM caffeine dissolved in the drinking water for 18 days (d0-d18), while control mice were given regular water. These mice were administered with 3.5% DSS for 5 days (d7-d12) and reverted back to water for another 6 days (d13-d18) in combination with or without 2.5 mM caffeine treatment. Mice were monitored daily for their body weight and clinical parameters including signs of diarrhea with soft stools (0 or 1), bloody stools (0 or 1), and hunching posture (0 or 1). Colonic tissue was removed from the middle part of the colon and used for histological evaluation in a blinded fashion as previously described [7].
Bacterial Translocation assay
Collected organ tissues were homogenized in Hank’s balanced salt buffer. After dilutions of the tissues homogenates were plated on LB agar plate and incubated at 37 °C overnight. Bacterial colony counting was examined as previously described [7].
Cytokine production determined by ELISA
TNFα, IFNγ, IL-10, IL-4 and IL-17F ELISA were performed according to manufacturer’s protocol (R&D systems, Minneapolis, MN). OD at 450 nm was read by Auto-Reader (Bio-Tec Instruments, Burlington, VT).
RT-PCR and Quantitative PCR
Total RNA was isolated with TRIzol (Invitrogen) according to manufactures instructions. Semi-quantitative reverse-transcription(RT)-PCR and real-time quantitative (Q)-PCR analyses were performed with the PTC-100 thermal cycler (Bio-Rad, Hercules, CA) and MX3000P quantitative PCR machine (Agilent Technologies, Santa Clara, CA), respectively, using primers listed in Table 1.
Table 1.
Primers for Reverse-Transcriptional- and Quantitative-PCR analyses
| Genes | Sense primers | Anti-sense primers | Size (bp) |
|---|---|---|---|
| hAMCase# | 5’-CGAATGGAACGATGTGACTC | 5’-GACTGGATATTGGAGATGCC | (bp) |
| hCHIT1 | 5’-TTCGGCACTCAGAAGTTCAC | 5’-AATCCAGGTTCTGGGCGATT | 371 |
| hCHI3L1 | 5’-GAAGACTCTCTTGTCTGTCGGA | 5’-AATGGCGGTACTGACTTGATG | 315 |
| hβ-actin | 5’-GTCTGCCTTGGTAGTGGATAATG | 5’-TCGAGGACGCCCTATCATGG | 108 |
| hGAPDH | 5’-TGTGGGCATCAATGGATTTGG | 5’-ACACCATGATTCCGGGTCAAT | 103 |
| mAMCase# | 5’-TGGACACACCTTCATCCTGA | 5’-AACAAGCCCTGCTTGACAAT | 116 |
| mCHIT1 | 5’CTACCTATGGACGCTCTTTCACCTT | 5’-ACAGCCGCCCTCCTCCACAGTTG | 586 |
| mCHI3L1 | 5’-TCCTGATGCTGCTCCAGAG | 5’-TATGCATGTTGTCGCTGCTG | 537 |
| mβ-actin | 5’-GTGGGCCGCTCTAGGCACCA | 5’-CGGTTGGCCTTAGGGTTCAGGGG | 160 |
| mGAPDH | 5’-AGGTCGGTGTGAACGGATTTG | 5’-GGGGTCGTTGATGGCAACA | 245 |
h represents human and m represents mouse.
Immunoblot analysis
Western blot analysis was performed as previously described [8]. Total or phosphorylation Akt (Thy308/Ser473), MAPK p42/p44 (Thr202/Tyr204), MAPK p38 (Thr180/Tyr182), JNK/JUNK (Thr183/Tyr185) and PI3K p85/p55 (Tyr458/Tyr199) were detected by antibodies obtained from Cell Signaling Technology (Danvers, MA).
Bacterial invasion assay, indirect immunofluorescence and confocal microscopic analyses
SW480 cells were seeded on 4 chamber polystyrene vessel tissue culture treated glass slides (BD Biosciences, Bedford, MA) and were transfected with pcDNA4 His/Max CHI3L1-TOPO vector using Lipofectamine 2000 (Life Technologies, Grand Island, NY) [7]. After 24 hours of transfection, cells were pre-treated for 1 hour with or without 2.5 or 5 mM caffeine followed by the infection with AIEC (MOI of 10) for 1 hour at 37°C. After the incubation, monolayers were washed with PBS and cultured 1 hour in the presence of 100 µg/ml gentamicin to kill any extracellular bacteria. Cells were washed with PBS twice and fixed with methanol and stained with mouse anti-Xpress® monoclonal antibody (Life Technologies) and rabbit anti-E. coli lipopolysaccharide (LPS) antibody (Dako, Carpinteria, CA) followed by FITC-horse anti-mouse IgG (Vector, Burlingame, CA) overnight. These antibodies were detected by FITC horse anti-mouse IgG (Vector, Burlingame, CA) and Alexa Fluor 647 goat anti-rabbit IgG (Life Technologies), which were incubated for an hour at RT. Macrophages from 10 week old C57Bl/6 mice were used for this study. At 5 days after intraperitoneal injection of 1 µg/g body weight thioglycolate (Difco, Houston, TX), peritoneal cavities of the mice were washed with ice-cold PBS, and free intraperitoneal cells were harvested. After centrifugation at 150 g for 5 min at 4 °C, a pellet of macrophages was suspended in RPMI 1640 with 4% FCS, which were seeded on 4 chamber polystyrene slides (BD Biosciences) for a few hours at 37 °C. Semi-adherent cells were pretreated with or without caffeine, infected with AIEC for 1 hour (MOI of 20) followed by gentamicin treatment as described above. Cells were washed with PBS, fixed with methanol and stained with anti-F4/80 monoclonal antibody (AbD Serotec, Raleigh, NC) and rabbit anti-E. coli LPS antibody (Dako) followed by FITC rabbit anti-rat IgG (Vector) and Alexa Fluor 647 goat anti-rabbit IgG (Life Technologies). The stained sections were examined with a confocal microscope (model Radiance 2000; Bio-Rad, Hercules, CA) using multi-tracking for two-color imaging. Image acquisition was performed with LaserSharp Scanning software (Bio-Rad).
Statistical analysis
Statistical significance was evaluated by Student’s t-test for parametric data, which are shown as the mean standard deviation (SD). Nonparametric data were analyzed using the Mann-Whitney U test. Differences were considered statistically significant at P< 0.05.
Results
Caffeine down-regulates CHI3L1 and AMCase mRNA levels
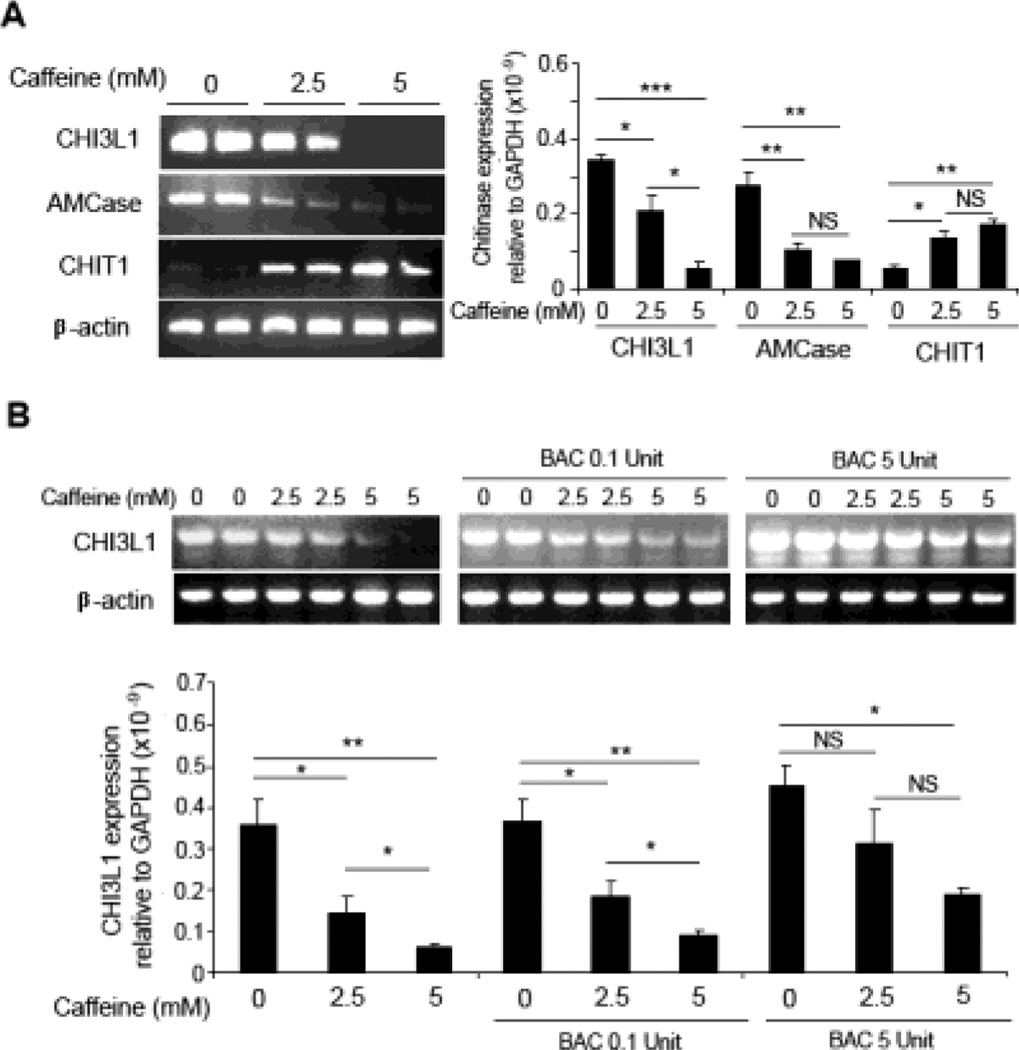
To examine the effects of caffeine on the family 18 chitinase transcription expression profiles, human SW480 IECs were treated with medium (2.5 mM) or high (5 mM) dose of caffeine for 48 hours. Subsequent semi-quantitative RT-PCR and real-time Q-PCR showed a down-regulation of the CHI3L1 and AMCase in a dose-dependent manner [Figure 1A]. Conversely CHIT1, which is endogenously lowly expressed in SW480 cells, were up-regulated after caffeine treatment [Figure 1A]. To confirm that this differential expression is a specific effect of caffeine, caffeine was pre-incubated with bacteria-derived chitinase for 48 hours before addition into the culture supernatant of SW480 cells for additional 48 hours. Subsequently, both RT-PCR and Q-PCR results showed that sequestering caffeine with 5 units of bacterial-derived chitinase reduced caffeine mediated down-regulation of CHI3L1 [Figure 1B]. Of note, caffeine concentrations lower than 1 mM does not have any significant influence on CHI3L1 expression in SW480 cells [Supplementary Figure 1A]. Together, these results suggest that caffeine not only inhibits the biological functions of the family 18 chitinases, but also affects their transcriptional expression levels.
Figure 1. Caffeine suppresses chitinase mRNA expression in IECs.
(A) SW480 cells were treated with 0, 2.5 or 5 mM caffeine for 48 hours incubation and detected for CHI3L1, AMCase and CHIT1 mRNA expression levels using RT-PCR (left) and Q-PCR (right). (B) Caffeine was first pre-incubated with bacteria-derived chitinase (BAC: 0.1 and 5 units) for 48 hours before adding into the culture medium of SW480 cells, followed by detection of CHI3L1 mRNA expression using RT-PCR (above) and Q-PCR (below). *P<0.05, **P<0.01, ***P<0.001, NS: no significant difference.
Caffeine prevents CHI3L1-mediated bacterial invasion into IECs and macrophages
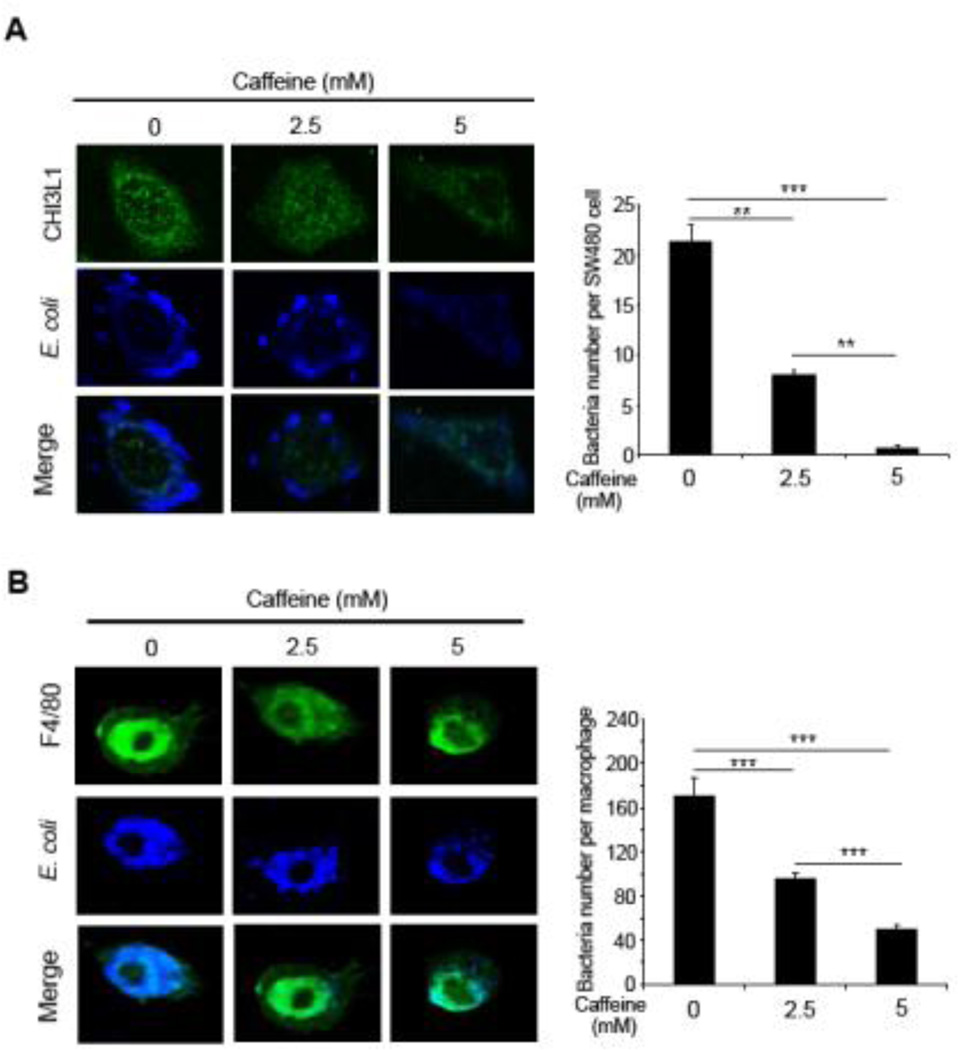
Our group previously demonstrated that CHI3L1 specifically enhance potentially pathogenic bacterial (e.g. AIEC) adhesion and invasion in IECs [7]. To investigate whether caffeine treatment can reduce this CHI3L1-mediated AIEC cellular invasion, SW480 IECs and mouse-derived peritoneal macrophages were treated with medium (2.5 mM) or high (5 mM) dose of caffeine and subsequently infected with AIEC for 1 hour, followed by gentamycin treatment to eliminate extracellular bacteria. Subsequent immunofluorescence staining using anti-E. coli LPS antibody and confocal analysis showed reduced number of intracellular bacterial after 2.5 mM caffeine treatment in both IECs [Figure 2A] and macrophages [Figure 2B], as compared to non-caffeine treated cells. Intracellular AIEC number was further reduced in cells that were treated with 5 mM caffeine [Figure 2A, B]. These results suggest that caffeine can prevent AIEC invasion into IECs and macrophages by down-regulating the expression levels of CHI3L1 mRNA and protein.
Figure 2. Caffeine blocked AIEC invasion into IECs and macrophages.
(A, B) SW480 cells (A) or mouse-derived peritoneal macrophages (B) were pre-treated with 0, 2.5 or 5 mM caffeine for 1 hour and inoculated with AIEC at MOI of 10 (for SW480) or 20 (for macrophages) for 1 hour at 37°C, followed by gentamicin (100 µg/ml) treatment for additional 1 hour to exclude extracellular bacteria. SW480 cells and macrophages were costained with anti-E coli LPS antibody and anti-CHI3L1 (Xpress®) or anti-F4/80 antibody respectively, followed by confocal microscopy analysis. Intracellular bacteria were quantified and shown. **P<0.01, and ***P<0.001
Caffeine inhibits AKT and PI3K pathway in a dose dependent manner but not barrier function in IECs
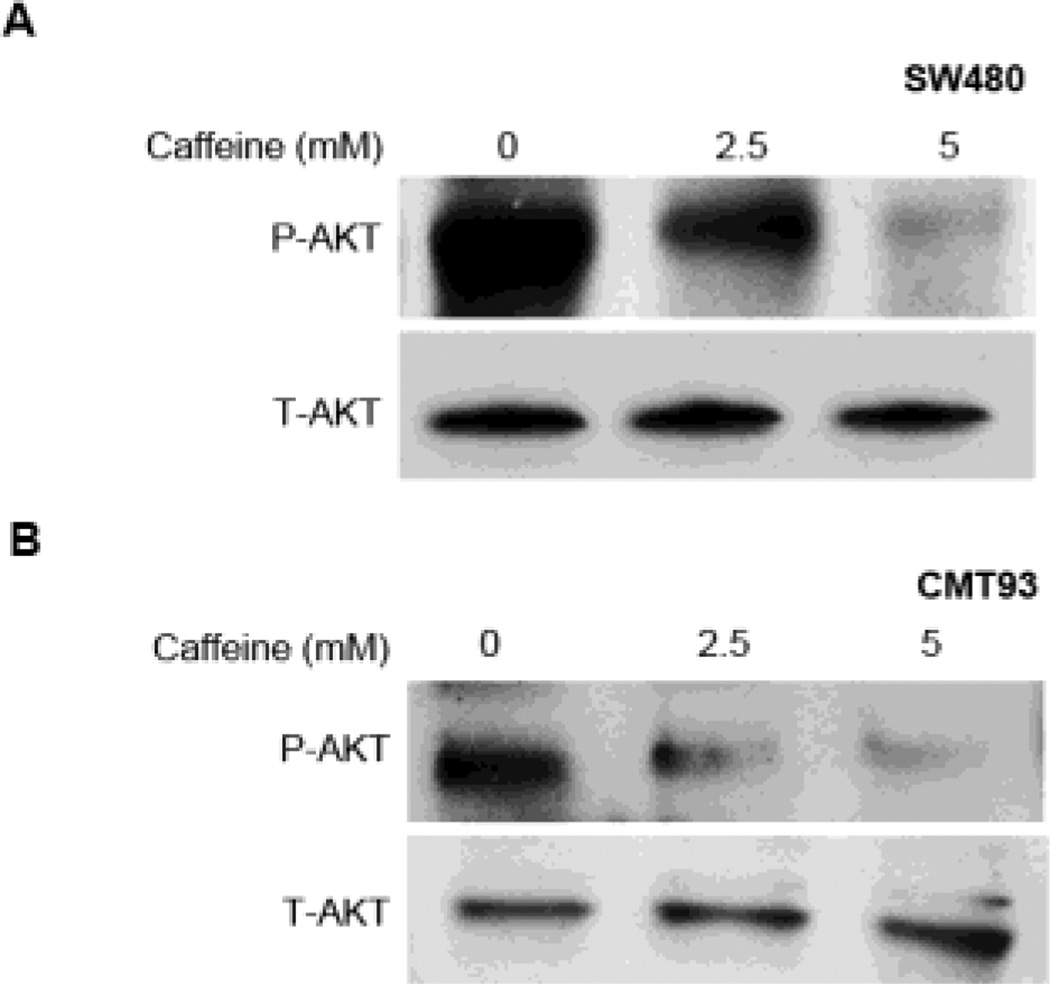
The effects of caffeine in CHI3L1-associated AKT signaling pathway activation was next investigated. Immunoblotting using anti-phosphorylated AKT antibody showed that human (SW480) and mouse (CMT93) IECs that were treated with medium (2.5 mM) doses of caffeine displayed reduced levels of AKT activation as compared to untreated cells [Figure 3A, B]. In addition, high-dose caffeine (5 mM) treatment in both human and mouse IECs results in an almost complete loss of AKT phosphorylation [Figure 3A, B], suggesting that AKT activation, at least in part mediated by CHI3L1, is suppressed via caffeine treatment. Similar dose-dependent inhibition on PI3K phosphorylation was also observed in CMT93 cells [Supplementary Figure 1B]. In contrast, caffeine does not have significant influence on the activation status of MAPK p42/p44, MAPK p38 or JNK/SAPK [Supplementary Figure 1B].
Figure 3. Caffeine suppresses AKT signaling pathway activation in IECs.
(A, B) SW480 (human) and CMT93 (mouse) IEC lines were treated with 0, 2.5 or 5 mM caffeine. Protein lysate were resolved using SDS-PAGE and detected for phosphorylated- or total-AKT using the respective specific antibodies.
To elucidate whether caffeine treatment alters IECs barrier functions, trans-epithelial resistance (TER) and tight junction status were examined in caffeine treated T84 cells. No significant change in TER was observed in T84 cells after 2.5 mM caffeine treatment, but high dose (5 mM) caffeine treatment resulted in modest reduction in TER [Supplementary Figure 2A]. In contrast, immunofluorescence staining using anti-ZO1 antibody showed that both 2.5 and 5 mM caffeine treated T84 cells did not show significant change in tight junction integrity [Supplementary Figure 2B].
Caffeine treatment down-regulates colonic CHI3L1 and protect against acute colitis in a DSS-induced colitis model
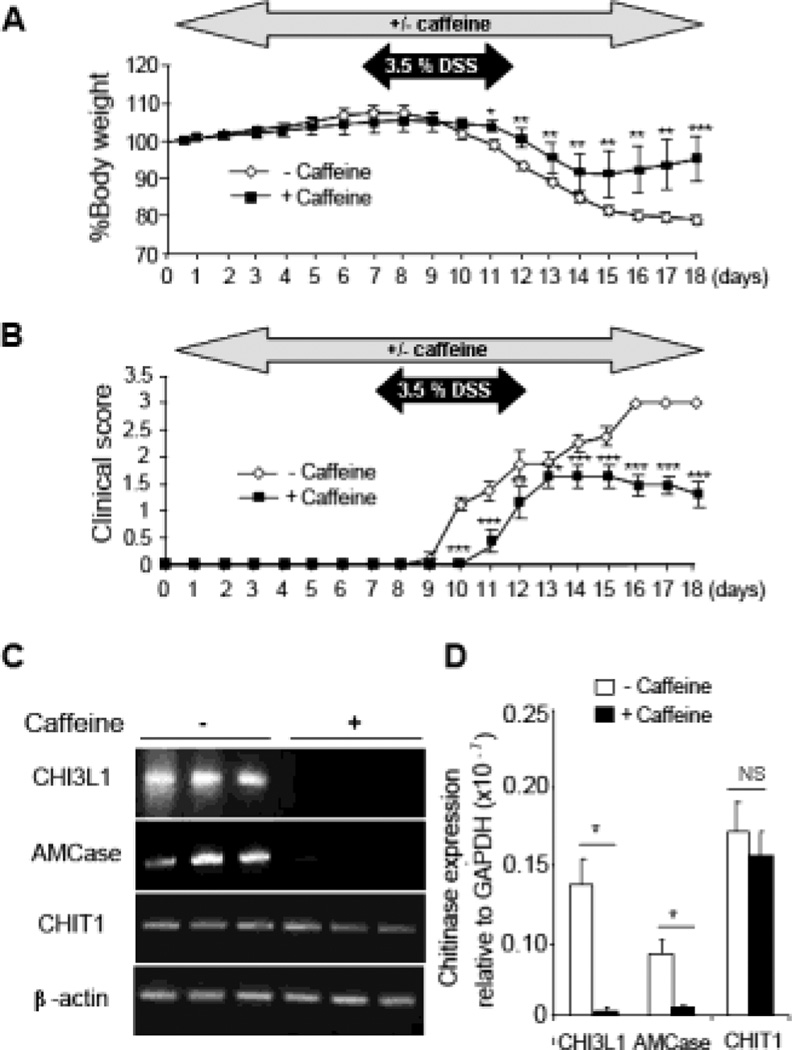
To examine the potential anti-inflammatory and anti-bacterial effects of caffeine in vivo, C57Bl/6 WT mice were first treated with a medium dose of caffeine (2.5 mM) dissolved in the drinking water for 7 days, whereas control mice were given regular water. Subsequently, 3.5% DSS was added into the water in parallel with caffeine for another 5 days and replaced with the regular water with or without caffeine for additional 7 days until the mice were sacrificed. Mice treated with caffeine showed delayed response to DSS and significantly enhanced recovery from acute colitis-induced by DSS as evaluated by the improvement of percent body-weight loss [Figure 4A] and clinical score [Figure 4B], suggesting a direct effect of caffeine in suppressing the onset manifestation of DSS-induced colitis. There were no significant differences in the body weight and clinical scores between non-DSS treated mice with or without caffeine supplementation [Supplementary Figure 3A].
Figure 4. Caffeine prevents onset of DSS-induced colitis in vivo.
(A, B) Mice were pre-treated with 2.5 mM caffeine for 7 days, followed by simultaneous treatment with 3.5% DSS for additional 5 days and finally with caffeine treatment only for the next 6 days before sacrificing. Percent body weight and clinical scores from the treated and untreated mice groups are shown. (C, D) Colonic mRNA expression of CHI3L1, AMCase and CHIT1 were determined using RT-PCR (C) and Q-PCR (D). Data are average of two independent experiments (n=8 in each group). *P<0.05, **P<0.01, ***P<0.001, NS: no significant difference.
Since up-regulation of CHI3L1 expression has been reported to be positively associated with the severity of colitis in both IBD patients and several murine models [7], colonic gene expression profile of the different mammalian chitinases in both caffeine treated- and non-treated mice groups were next determined. Consistent with the in vitro observations, RT-PCR and Q-PCR results showed a complete loss of both AMCase and CHI3L1 mRNA levels in caffeine-treated mice, as compared to non-treated controls [Figure 4C, D]. In contrast, CHIT1 gene expression is unaffected by caffeine treatment. Of note, CHI3L1 expression is almost undetectable in both caffeine-treated and non-treated mice in the absence of DSS treatment [Supplementary Figure 3B].
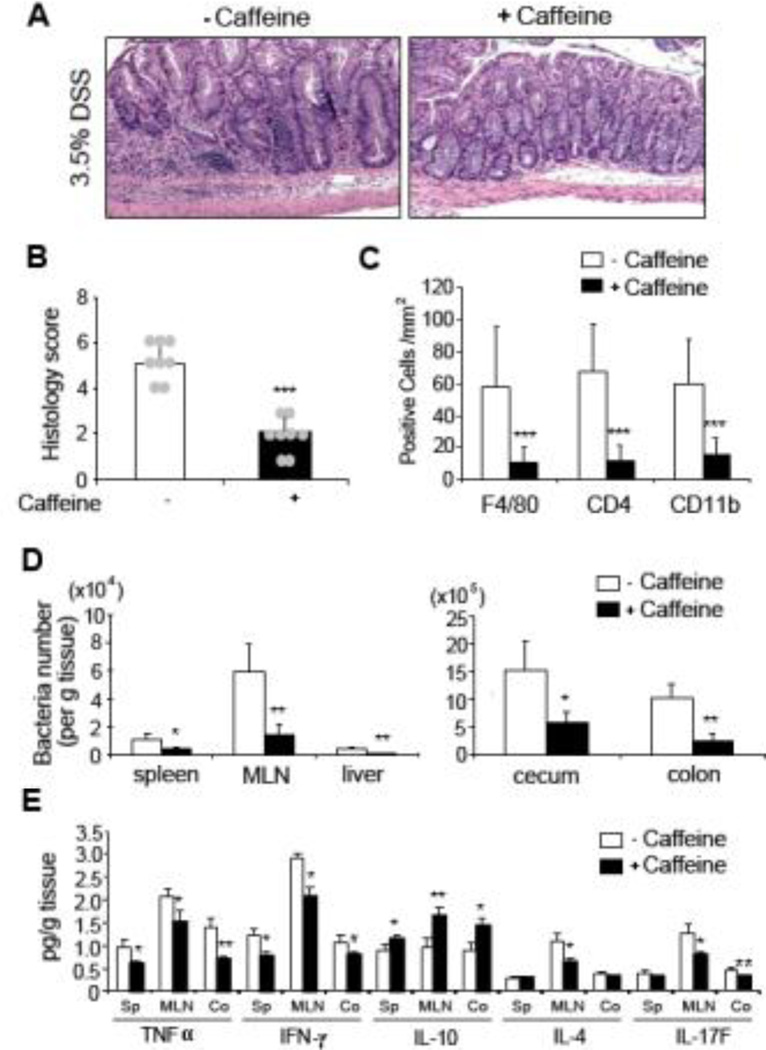
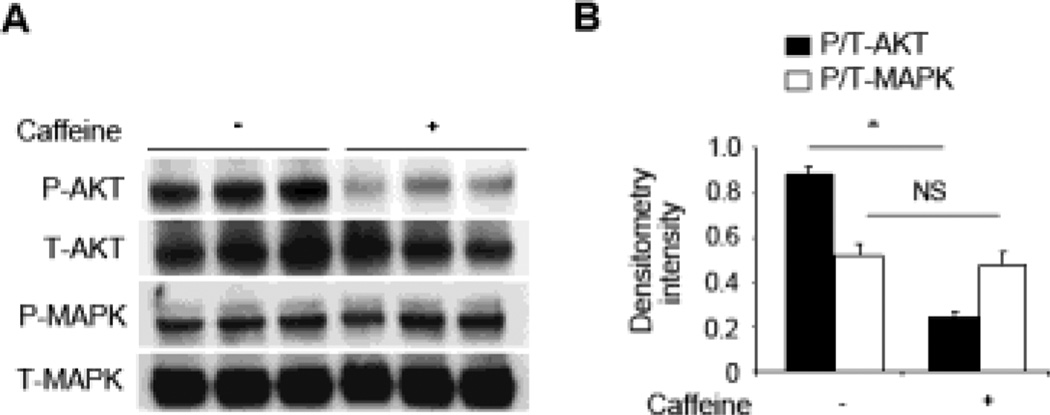
Colonic histological scores from caffeine-treated mice clearly displayed a milder form of DSS-induced colitis as compared to non-treated controls, indicated by a reduction in inflammatory cell infiltration [Figure 5A and 5B]. Of note, caffeine treated and non-treated mice without DSS do not show any signs of inflammation [Supplementary Figure 3C]. The extent of F4/80-, CD4- and CD11b-positive cell infiltration was also semi-quantified using immunohistochemistry, showing an approximately 80% reduction in caffeine-treated mice as compared to the control group [Figure 5C]. Significantly reduced bacterial translocation in the spleen, mesenteric lymph nodes (MLN), liver, cecum and colon were also observed in caffeine-treated mice as compared to the non-treated control mice, indicating a systemic protective effect in bacterial translocation upon caffeine treatment. Consistent with the disease severity, proinflammatory cytokines (TNFα and IL17F) productions, as well as IFNγ and IL-4 levels, were lower in the spleen, MLN and colon of caffeine-treated mice as compared to non-treated controls [Figure 5E]. In contrast, the anti-inflammatory cytokines IL-10 was much higher when mice were treated with caffeine in the examined organs. Consistent with the above in vitro observations, mice colonic AKT, but not MAPK, activation was markedly reduced in caffeine treated DSS-induced colitis mice when compared to the non-caffeine-treated controls [Figure 6].
Figure 5. Caffeine treatment reduces severity of DSS-induced early colitis.
(A, B) Colonic H&E staining (A) and histological scores (B) of caffeine treated and untreated DSS-treated mice are shown. 10×, objective. (C) Colonic sections were immuno-stained with anti-F4/80, -CD4 or -CD11b antibody and the number of positive cells per mm2 area were quantified. (D) Bacterial CFU from spleen, MLN, liver, cecum and colon homogenized extracts were quantified and shown. (E) Spleen, MLN, and colon cytokine production (TNFα IFNγ, IL-10, IL-4 and IL-17F) from the caffeine treated or untreated mice were determined using ELISA. Data are average of two independent experiments (n=8 in each group). *P<0.05, **P<0.01, ***P<0.001, NS: no significant difference.
Figure 6. Colonic Akt activation is suppressed by caffeine treatment.
(A) Colonic samples from mice with or without caffeine treatment were lysed and 30 µg/lane of protein lysate was subjected to immunoblot with anti-phospho (P)- or anti-total (T)- Akt or MAPK p42/p44 antibodies. The data are representative of three independent experiments. (B) Densitometry analysis of phosphorylation signals normalized to total-Akt (closed bars) or -MAPK p42/p44 (open bars) signals are shown. Data are average of two independent experiments (n=3 in each group). *P<0.05, NS: no significant difference.
To further dissect whether caffeine has prophylactic effect against DSS-induced colitis, as well as a role in DSS-induced colitis recovery, mice were subjected to caffeine treatment before, or after, DSS challenge, respectively. In both scenarios, caffeine treated mice showed milder DSS-induced colitis phenotype as compared to untreated mice, characterized by reduced body weight loss, lower clinical/histological scores and the ability to suppress CHI3L1 expression level [Supplementary Figures 4 and 5]. Inter-comparison of colitis severity in mice treated with caffeine, before, after, or continuously with respect to DSS challenge revealed that caffeine is most effective in controlling the degree of colitis when administered continuously, or after DSS during the recovery phase.
Taken together, these data suggest that caffeine has ameliorative effects in acute DSS-colitis that involves the down-regulation of CHI3L1.
Discussion
Current major treatment for IBD includes 5-aminosalicylates or glucocorticosteroids, as well as anti-TNFα agents (e.g. Infliximab) [11]. However, the therapeutic effectiveness of these treatments varies among individuals that may also be accompanied with adverse-effects. In this report, we demonstrate that caffeine can ameliorate acute colitis and the mode of action is through the down-regulation of host inducible CHI3L1 expression and functions, providing an alternative treatment method for the management of IBD.
Increased serum and tissue levels of CHI3L1 have been associated with poor prognosis of many diseases including inflammatory disorders such as rheumatoid arthritis and asthma [12, 13]. In the colon, CHI3L1 expression facilitates bacterial adhesion on the mucosal surface epithelium and invasion into IECs and macrophages, exacerbating the progression of colitis. Thus blocking CHI3L1 expression and protein functions poses an opportunity to control the extent of colitis development by disrupting the pathologic interactions between host cells and luminal microorganisms. As shown in this current report, continuous caffeine-treated mice have a more resistant response to DSS, characterized by less body weight loss and reduced anti-inflammatory cytokine productions. Since healthy intestinal epithelium is constantly regenerative, it is possible that regular consumption of appropriate dose of caffeinated food and beverage (e.g. coffee) may prevent intestinal inflammation, especially in IBD gene susceptible individuals.
Current clinical benefits of caffeine have been demonstrated in the treatment of several diseases in patients such as Parkinson’s disease (PD) and sleep-apnea of prematurity. Caffeine was shown to negatively associate with the risk of developing PD and clinical trials reports that PD patients who received caffeine treatment showed motor- and nonmotor-associated improvements [14–16]. Other disease in which caffeine exerts positive effects includes stroke, traumatic brain injury, Alzheimer's disease, Huntington's disease and multiple sclerosis [17]. In the gastrointestinal tract, caffeine has been shown to have protective effect in gastric mucosal damage induced by non-steroidal anti-inflammatory drugs (NSAID) [10]. Another report studying 41,836 postmenopausal women for 15 years showed that high coffee consumption is inversely correlated to the severity of inflammatory diseases [18]. In this study, we provide direct evidence that caffeine ameliorates colitis and defined the mechanism involved, attributed towards down-regulation of CHI3L1 through the significant suppression of AKT- and PI3K-signaling pathway. This information provides preclinical insights that can be potentially utilized for bed-side translation into future human clinical trials for IBD treatment.
Of note, optimizing drug doses appears to be a critical factor to balance drug safety versus efficacy. In the case of PD, the maximum tolerability dose of caffeine was reported to be 100 to 200 mg bis in diem (i.e. twice a day) [15]. In this current study, medium dose (2.5 mM) of caffeine treatment in colitic mice is effective in suppressing CHI3L1 expression accompanied by an ameliorative effect on colitis phenotype. However in vitro, we observed that cells receiving high dose of caffeine (5 mM; approximately equivalent to 5 cups of coffee) begin to show compromise in IEC-permeability although the tight junction is still intact at the dose [Supplementary Figure 3A]. Current clinical advisory to IBD patients to avoid caffeine aims to reduce bowel movements stimulation and diuretic effect that might lead to dehydration. Therefore, we propose that a caffeine concentration between 1 mM to a maximum of 2.5 mM (equivalent to 2–3 cups of coffee) is an appropriate/ideal dose for colitis treatment, based on our study data.
In addition, caffeine has been known to have anti-tumor effect [19]. Since CHI3L1 has been reported to be associated with many forms of tumors and cancers, suppressing CHI3L1 expression and functions using caffeine may be one of the safe and appropriate strategies to counter these diseases [20]. During IBD, CHI3L1 is inducibly expressed on IECs, which then further up-regulates during the development of colitis-associated cancer [21]. CHI3L1-mediated cell proliferative can be suppressed with caffeine treatment in a dose-dependent manner, thereby controlling and retarding tumor formation (Lee IA and Mizoguchi E, unpublished observations). This suggests that caffeine can also be utilized in IBD patients to control colitis-associated cancer development and prevention.
When using caffeine to treat IBD, the route of administration is an important aspect to address which thereby affect its pharmacokinetics. The two major route of caffeine administration includes oral and rectal enema. Although caffeine rectal enema is an ancient medical procedure that is still currently being used today, it was documented that such enema have risk of proctocolitis, polymicrobial enteric septicemia, electrolyte imbalance and maybe even fatal [22–25]. Like oral administration, caffeine enemas also results in absorption into systemic circulation in the rectum and colon [26]. Therefore, whether caffeine exerts its effect directly on the intestine, or via bloodstream circulation, remains to be determined. It was shown in human subjects that caffeine enema has 3.5 folds lower plasma caffeine concentration as compared to orally administered, though half-life of the two groups was not statistically any different [26]. In this study, we chose to orally administer caffeine into DSS-induced colitic mice to test if this convenient mode of drug delivery can achieve the desired improvement in colitis condition. The observation that orally administered caffeine can indeed ameliorate colitis suggest that this route of drug delivery is preferred, as compared to rectal enema, so as to lower the above mentioned risk involved in rectal caffeine enema and also to eliminate patient’s discomfort level.
To reduce potential systemic side effects that might arise from oral administration of caffeine, controlled release of ingested caffeine is an attractive method to achieve topical effect of caffeine in the intestine. Several options are available that comes in the form of capsule coated caffeine. For instance, a combination of pH- and time-based coated caffeine capsule that contains a first layer of pH dependent polymer EUDRAGIT RL/RS which will produce slow release of caffeine, together with a second layer of EUDRAGIT FS 30D that delays onset of release until the capsule reaches the intestine, has been reported [27]. This may achieve effective site specificity of drug delivery during the treatment of colitis. Another reported method of site specific delivery of caffeine utilizes the physiological luminal pressure in the intestinal tract that cause the disintegration of ethylcellulose coated gelatin capsule containing caffeine [28]. By leveraging on these technologies, together with the current findings that caffeine can ameliorates colitis, one can better further optimize the treatment of IBD using caffeine for future clinical use.
In conclusion, we identified a preventive role of caffeine in IBD by targeting selected mammalian chitinase expression and functions. The data can serve as the basis for the potential utilization of caffeine in antibiotics-resistant bacterial infection and acute/chronic intestinal inflammation for clinical settings in the near future.
Supplementary Material
Acknowledgment
This work has been supported by National Institute of Health (DK 80070, DK74454, DK64289 and DK43351, DK-068181, DK-033506, AI093588), and grants from the Eli and Edythe L. Broad Medical Foundation and American Gastroenterological Association Foundation to EM. IAL was supported by the National Research Foundation of Korea (Seoul, South Korea) and DL has been awarded the international fellowship grant supported by A*STAR Graduate Academy (Singapore).
Abbreviations used in this paper
- AIEC
adherent-invasive Escherichia coli
- Akt
protein kinase B
- AMCase
acidic mammalian chitinase
- CFU
colony-forming unit
- CHI3L1
chitinase 3-like 1
- DSS
dextran sulfate sodium
- IBD
inflammatory bowel disease
- IEC
intestinal epithelial cells
- LB
Luria-Bertani
- MLN
mesenteric lymph nodes
- MAPK
mitogen-activated protein kinase
- MOI
multiplicity of infection
- PD
Parkinson’s disease
- PI3K
phosphoinositide 3-kinase
- RT-PCR
semi-quantitative reverse-transcription polymerase chain reaction
- Q-PCR
quantitative real time polymerase chain reaction
- WT
wild type
References
- 1.Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448(7152):427–434. doi: 10.1038/nature06005. [DOI] [PubMed] [Google Scholar]
- 2.Hamilton MJ, Snapper SB, Blumberg RS. Update on biologic pathways in inflammatory bowel disease and their therapeutic relevance. J Gastroenterol. 2012;47(1):1–8. doi: 10.1007/s00535-011-0521-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Franke A, McGovern DP, Barrett JC, Wang K, Radford-Smith GL, Ahmad T, et al. Genome-wide meta-analysis increases to 71 the number of confirmed Crohn's disease susceptibility loci. Nat Genet. 2010;42(12):1118–1125. doi: 10.1038/ng.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Franke A, Balschun T, Karlsen TH, Hedderich J, May S, Lu T, et al. Replication of signals from recent studies of Crohn's disease identifies previously unknown disease loci for ulcerative colitis. Nat Genet. 2008;40(6):713–715. doi: 10.1038/ng.148. [DOI] [PubMed] [Google Scholar]
- 5.Anderson CA, Boucher G, Lees CW, Franke A, D'Amato M, Taylor KD, et al. Metaanalysis identifies 29 additional ulcerative colitis risk loci, increasing the number of confirmed associations to 47. Nat Genet. 2011;43(3):246–252. doi: 10.1038/ng.764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jostins L, Ripke S, Weersma RK, Duerr RH, McGovern DP, Hui KY, et al. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature. 2012;491(7422):119–124. doi: 10.1038/nature11582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mizoguchi E. Chitinase 3-like-1 exacerbates intestinal inflammation by enhancing bacterial adhesion and invasion in colonic epithelial cells. Gastroenterology. 2006;130(2):398–411. doi: 10.1053/j.gastro.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 8.Chen CC, Llado V, Eurich K, Tran HT, Mizoguchi E. Carbohydrate-binding motif in chitinase 3-like 1 (CHI3L1/YKL-40) specifically activates Akt signaling pathway in colonic epithelial cells. Clin Immunol. 2011;140(3):268–275. doi: 10.1016/j.clim.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schuttelkopf AW, Andersen OA, Rao FV, Allwood M, Lloyd C, Eggleston IM, et al. Screening-based discovery and structural dissection of a novel family 18 chitinase inhibitor. J Biol Chem. 2006;281(37):27278–27285. doi: 10.1074/jbc.M604048200. [DOI] [PubMed] [Google Scholar]
- 10.Koyama R, Kataoka H, Tanaka Y, Nakatsugi S, Furukawa M. Effect of caffeine on ibuprofen-induced gastric mucosal damage in rats. J Pharm Pharmacol. 1999;51(7):817–824. doi: 10.1211/0022357991773014. [DOI] [PubMed] [Google Scholar]
- 11.Ford AC, Moayyedi P, Hanauer SB. Ulcerative colitis. Bmj. 2013;346:f432. doi: 10.1136/bmj.f432. [DOI] [PubMed] [Google Scholar]
- 12.Verheijden GF, Rijnders AW, Bos E, Coenen-de Roo CJ, van Staveren CJ, Miltenburg AM, et al. Human cartilage glycoprotein-39 as a candidate autoantigen in rheumatoid arthritis. Arthritis Rheum. 1997;40(6):1115–1125. doi: 10.1002/art.1780400616. [DOI] [PubMed] [Google Scholar]
- 13.Bara I, Ozier A, Girodet PO, Carvalho G, Cattiaux J, Begueret H, et al. Role of YKL-40 in bronchial smooth muscle remodeling in asthma. Am J Respir Crit Care Med. 2012;185(7):715–722. doi: 10.1164/rccm.201105-0915OC. [DOI] [PubMed] [Google Scholar]
- 14.Ross GW, Abbott RD, Petrovitch H, Morens DM, Grandinetti A, Tung KH, et al. Association of coffee and caffeine intake with the risk of Parkinson disease. Jama. 2000;283(20):2674–2679. doi: 10.1001/jama.283.20.2674. [DOI] [PubMed] [Google Scholar]
- 15.Altman RD, Lang AE, Postuma RB. Caffeine in Parkinson's disease: a pilot open-label, dose-escalation study. Mov Disord. 2011;26(13):2427–2431. doi: 10.1002/mds.23873. [DOI] [PubMed] [Google Scholar]
- 16.Postuma RB, Lang AE, Munhoz RP, Charland K, Pelletier A, Moscovich M, et al. Caffeine for treatment of Parkinson disease: a randomized controlled trial. Neurology. 2012;79(7):651–658. doi: 10.1212/WNL.0b013e318263570d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen JF, Chern Y. Impacts of methylxanthines and adenosine receptors on neurodegeneration: human and experimental studies. Handb Exp Pharmacol. 2011;(200):267–310. doi: 10.1007/978-3-642-13443-2_10. [DOI] [PubMed] [Google Scholar]
- 18.Andersen LF, Jacobs DR, Jr, Carlsen MH, Blomhoff R. Consumption of coffee is associated with reduced risk of death attributed to inflammatory and cardiovascular diseases in the Iowa Women's Health Study. Am J Clin Nutr. 2006;83(5):1039–1046. doi: 10.1093/ajcn/83.5.1039. [DOI] [PubMed] [Google Scholar]
- 19.Ku BM, Lee YK, Jeong JY, Ryu J, Choi J, Kim JS, et al. Caffeine inhibits cell proliferation and regulates PKA/GSK3beta pathways in U87MG human glioma cells. Mol Cells. 2011;31(3):275–279. doi: 10.1007/s10059-011-0027-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eurich K, Segawa M, Toei-Shimizu S, Mizoguchi E. Potential role of chitinase 3-like-1 in inflammation-associated carcinogenic changes of epithelial cells. World J Gastroenterol. 2009;15(42):5249–5259. doi: 10.3748/wjg.15.5249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen CC, Pekow J, Llado V, Kanneganti M, Lau CW, Mizoguchi A, et al. Chitinase 3-like-1 expression in colonic epithelial cells as a potentially novel marker for colitis-associated neoplasia. Am J Pathol. 2011;179(3):1494–1503. doi: 10.1016/j.ajpath.2011.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keum B, Jeen YT, Park SC, Seo YS, Kim YS, Chun HJ, et al. Proctocolitis caused by coffee enemas. Am J Gastroenterol. 2010;105(1):229–230. doi: 10.1038/ajg.2009.505. [DOI] [PubMed] [Google Scholar]
- 23.Lee CJ, Song SK, Jeon JH, Sung MK, Cheung DY, Kim JI, et al. Coffee enema induced acute colitis. Korean J Gastroenterol. 2008;52(4):251–254. [PubMed] [Google Scholar]
- 24.Margolin KA, Green MR. Polymicrobial enteric septicemia from coffee enemas. West J Med. 1984;140(3):460. [PMC free article] [PubMed] [Google Scholar]
- 25.Eisele JW, Reay DT. Deaths related to coffee enemas. Jama. 1980;244(14):1608–1609. [PubMed] [Google Scholar]
- 26.Teekachunhatean S, Tosri N, Rojanasthien N, Srichairatanakool S, Sangdee C. Pharmacokinetics of Caffeine following a Single Administration of Coffee Enema versus Oral Coffee Consumption in Healthy Male Subjects. ISRN Pharmacol. 2013;2013:147238. doi: 10.1155/2013/147238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bott C, Rudolph MW, Schneider AR, Schirrmacher S, Skalsky B, Petereit HU, et al. In vivo evaluation of a novel pH- and time-based multiunit colonic drug delivery system. Aliment Pharmacol Ther. 2004;20(3):347–353. doi: 10.1111/j.1365-2036.2004.02033.x. [DOI] [PubMed] [Google Scholar]
- 28.Muraoka M, Hu Z, Shimokawa T, Sekino S, Kurogoshi R, Kuboi Y, et al. Evaluation of intestinal pressure-controlled colon delivery capsule containing caffeine as a model drug in human volunteers. J Control Release. 1998;52(1–2):119–129. doi: 10.1016/s0168-3659(97)00201-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.