Summary
Over the past few decades, one of the most salient lifestyle changes for us has been the use of computers. For many of us, manual interaction with a computer occupies a large portion of our working time. Through neural plasticity, this extensive movement training should change our representation of movements [e.g., 1, 2, 3], just like search engines affect memory [4]. However, how computer use affects motor learning is largely understudied. Additionally, as virtually all participants in studies of perception and actions are computer users, a legitimate question is whether insights from these studies bear the signature of computer-use experience. We compared non-computer users with age- and education-matched computer-users in standard motor learning experiments. We found people learned equally fast but non-computer users generalize significantly less across space, a difference negated by two weeks of intensive computer training. Our findings suggest that computer-use experience shaped our basic sensorimotor behaviors, and this influence should be considered whenever computer-users are recruited as study participants.
Results and Discussion
The average computer user produces 7,400 mouse clicks per week [5]. Computer use often involves globally linear transformations between the body movement and its screen representation, e.g., the mapping from hand movement to mouse cursor position. Hence, with long-term interaction with mice, computer users probably develop an expectation that visuomotor transformation between hand movement and its screen representation should remain consistent across work space. Hence, in line with recent findings that prior experience affects motor control [6-9] and motor generalization in particular [10, 11], our working hypothesis is that people without computer experience will generalize more locally in visuomotor learning and this difference should be negated with computer-use training.
To assess how such movement behaviors are affected by computer use, we recruited 18 Chinese migrant workers, 9 of them were regular computer users (control group, age 41.9±8.9 years) and 9 of them had never used a computer before (non-computer user group, age 38.2±10.1 years). We also assembled a control group made up of 9 college students (student group, age 21.9±2.4 years). All these naïve subjects were tested with a standard visuomotor gain adaptation experiment [12, 13] where subjects learned to move a cursor while their hand was hidden from view (Fig 1A). The gain between the hand displacement and the cursor displacement was modified in the training direction (Fig 1B). Subjects adapted to this visuomotor gain change and were subsequently tested in other directions to assess their directional generalization.
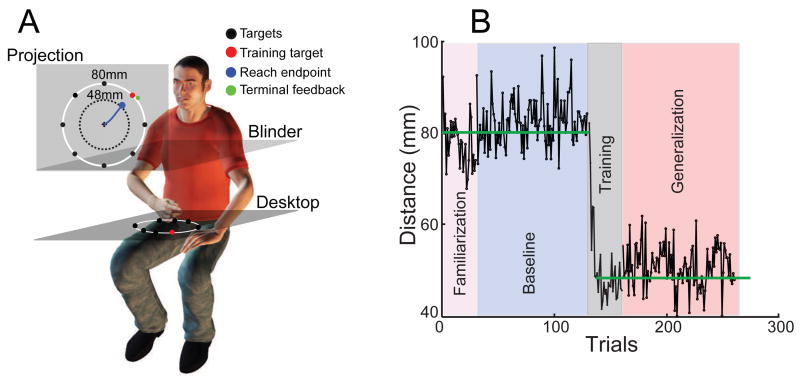
Figure 1.
Experimental setup and data from a typical subject. A) Illustration of experimental setup and movement targets arranged on the screen. With perturbed visuomotor gain, the terminal feedback is shown 1/0.6 further from the actual reach endpoint, i.e., people only need to move the unseen hand 48 mm to reach 80-mm targets. This terminal feedback is only shown for the training direction. Thus subjects only learned this sensorimotor gain in one direction and were then asked to generalize to other directions. B). Movement distance of all the reaches to the training target from a typical subject. The gain is 1 during the familiarization and the baseline phases and is 0.6 during the training (with feedback) and the generalization phase (without feedback). The distances of reaches to other targets (not shown) reflect how subjects generalize.
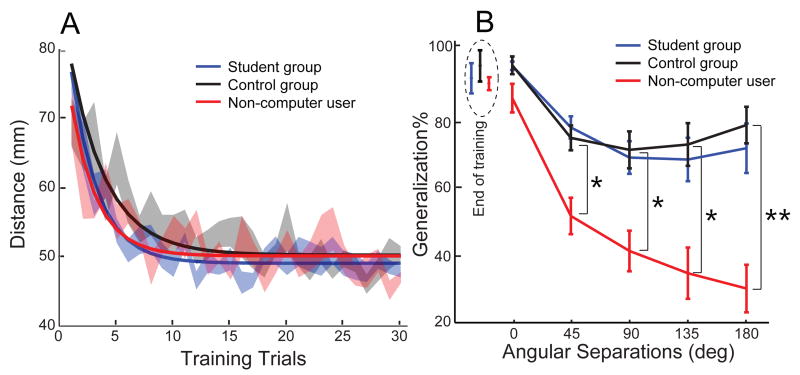
So how does computer use affect movement behavior? It affected neither the speed of learning (F(8,18) = .91, p = .53, one-way ANOVA) nor the degree of learning (F(8,18) = .77, p = .64; Fig. 2A). However, the computer users, compared to the non-computer user group, generalize much more into other directions (interaction effect F(8, 96) = 6.9, p < .0001; main effect on groups F(2, 24) = 12.5, p < .0001, 2-way ANOVA; Fig 2B). Interestingly, non-computer users still have a broad generalization as their generalization is significantly larger than zero even at the largest angular separation of 180° (30.0±6.9%; p < .001, one-sample t test). There is no difference between the computer group and the student group, suggesting that subjects of different ages behave similarly as long as they are computer users.
Figure 2.
Same learning speeds but different generalization. A). Average learning data during the training phase. The error bars denote SEMs across subjects. Solid lines are fitted exponential learning curves. B). The generalization as a function of difference in direction is assessed. The difference between computer-user groups and non-computer user group was significant at distant angles (p < .005, marked as *, p <.001, marked as **, simple main effect with Bonferroni correction).
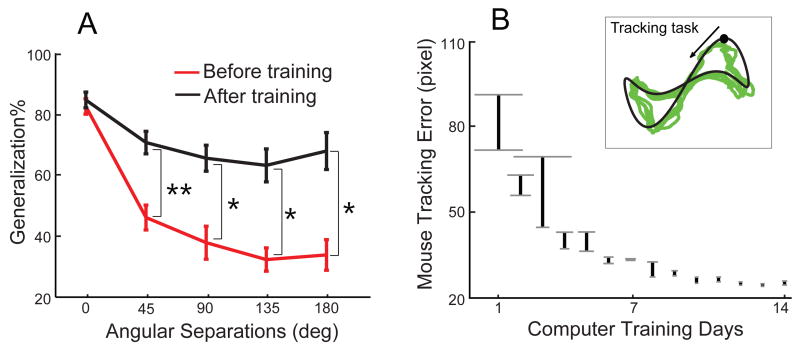
To establish the causal relationship, we recruited another group of 10 non-computer users and examined their generalization before and after intensive computer-use training. We realized that our movement interaction with computer is mostly via a computer mouse; this interaction involves a mapping between a movement space and a visual space, rather similar to experimental setting in our and others' studies. We thus chose to give subjects intensive training on using computer mouse. Before training, their generalization was again quite narrow and their generalization was not significant from that of the non-computer user group in Experiment 1 (Figure 3A; two-way mixed-design ANOVA, main effect on groups F(1,17) = .24, p = .63 and interaction effect F(4,68) = .25, p = .92). In the following 2 weeks, participants were instructed to play computer games (e.g., Pong) that require intensive mouse use, 2 hours each day. We also tested their mouse-use ability by asking them to track a moving cursor with mouse cursor (Figure 3B inset). This tracking task was performed before and after training on each day. The overall tracking error was reduced over days (Figure 3B). More importantly, participants exhibited significantly larger generalization when they were tested again after 14-day training (Figure 3A). Two-way repeated-measures ANOVA revealed significant main effect on timing (before vs after training, F(1,9) = 13.08, p < .01) and significant interaction effect (F(4,36) = 9.48, p < .0001). The generalization was significantly higher at all but the 0 angular separations (p < .01 or p < .005 for simple main effect tests). Two weeks of computer training converted the generalization patterns into those of computer users.
Figure 3.
Two weeks of computer use produces broad generalization curves. A) The generalization as a function of difference in direction before and after computer-use training. The difference induced by computer-use training was significant at distant angles (p < .01, marked as *, p < .005, marked as **, simple main effect). B). The mouse tracking error was reduced over 14 training days. The upper end of each vertical line denotes the error before training on each day and the lower end the error after the training. The width of grey horizontal lines denotes inter-subject variance (SEM). The trajectory of the moving target (black) and the mouse cursor trajectory of an exemplary trial (green) were shown in the inset.
In sum, computer use leaves learning speed unaffected but leads to enhanced generalization into untrained directions. A possible reason for this change is that the gain mapping between mouse movements and cursor movements is uniform across different directions. Thus, long-term exposure to this sensorimotor mapping leads to our prior expectation of consistent transformation between manual actions and screen representations across directions. This prior expectation, in turn, leads to broad generalization in similar task settings. We postulate that this enhanced generalization is specific for visuomotor learning since computer use extensively involves visuomotor transformation. Furthermore, it has been shown that altered motor generalization in some neuropathological population is also task-specific [14].
It is interesting to note that normal participants (a.k.a, computer users) have a much broader generalization for visuomotor gain learning as compared to visuomotor rotation learning, another type of visumotor transformation [e.g., 15]. Can computer use explain this discrepancy? Our data shows that non-computer users exhibit broader generalization in gain learning than computer users in rotation learning, i.e., their gain generalization is significant even at 180 angles where rotation generalization is supposed to be absent. This suggests that computer use alone cannot explain the discrepancy between these two types of motor generalization. This behavioral distinction is consistent to the neurophysiological findings that separate neural substrates supports these two types of visuomotor learning [16].
Similar to existing studies on visuomotor generalization [3, 12, 17-22], we have analyzed the generalization of rather artificial movements on a plane. It is well possible that computer use is more important for such artificial movements than it would be for natural movements. However, our study informs the interpretation of the work that has been done so far. Future work can reveal how important naturalness is for the effects of generalization [10] and the importance of computer use in such a context.
The way subjects learn and generalize is often viewed as a reflection of the fundamental neural representation of movement [23-29]. Particularly, people usually reported limited generalization in various motor learning tasks [3, 17-19, 21, 30-32] and these patterns have been quantified to probe neural representations of movement learning [e.g., 3, 11, 17-19, 20, 26, 30-35] Hence, our finding suggests that computer use, through neural plasticity, changes movement representations. Our results also suggest that in typical movement experiments, at least those involving visuomotor perturbations, computer use affects the results. It is thus important to be cautious in generalizing behavioral findings on computer users to the overall population, just as psychologists recently acknowledged that data from selected Western subjects is not broadly representative across populations [36]. Computer use not only changes our lifestyle, it appears to also fundamentally affect the neural representation of our movements.
Experimental Procedures
All subjects were naïve to our research purpose and they provided written consent before experiments. All procedures were approved by the institutional review board of Peking University. Experiment 1 was a cross-sectional experiment with a non-computer user group (8 females and 1 male; age: 38.2±10.1; education: 4.9±2.1 years), an age- and education- matched control group (8 females and 1 male, age: 41.9±8.9; education: 6.6±2.5) and a student group (7 females and 2 males; age: 21.9±2.4; education: 15.3±1.9). Experiment 2 was a longitudinal experiment where 10 non-computer users were recruited (10 females; age 38.7±7.9 years; education: 7.9±3.1). Their motor generalization were accessed before and after a 14-day computer training. All subjects were screened for their computer use experience. Non-computer users were determined by one-on-one interview and they were required to use a computer mouse to open a file folder placed on the desktop of a Windows PC. They usually had trouble maneuvering the mouse to move the cursor, a hallmark of no experience of interacting with computers. All experiment sessions were schedule during the day. For quantifying generalization, subjects sat behind a desk and moved their right, dominant hand on the desktop. Their vision of the hand was blocked by a mirror placed horizontally at chest level. The movement of the index finger tip was measured at a frequency of 200Hz (Codamotion, Charnwood Dynamics). Visual feedback was projected on a vertically-placed back-projection screen about 100cm in front of the subject. On the screen, eight visual targets were arranged on an 80-mm-radius circle and separated 45° apart (Fig 1A). At the beginning of each trial, subjects rested their index finger on a 4-mm thin, smooth plastic disc glued on the desktop. This disc facilitated subjects returning to the center of the target circle without visual guidance. Once the finger was still for 100ms, one of the targets was highlighted to signal subjects to move their hand from the center to the target. A cursor, representing the finger position, was only visible within 1-cm around the circle center. On selected trials (see below), the cursor would re-appear when the reach stopped and this terminal feedback indicated the distance travelled by the finger/cursor. A beep, played at the trial end, signaled the subject to bring the finger back to the starting position for the next trial.
The assessment of generalization was conducted with 4 phases of trials (Fig 1B). In the familiarization phase, subjects moved to each target 6 times in a random sequence with terminal feedback. In the baseline phase, trials were organized in 50 blocks of 9 trials: every target was shown once with the exception of the training target (the upper-left target) which was shown twice. The terminal feedback was presented only for the reaches to the training target. For both familiarization and baseline phases, the gain between the hand movement and the cursor movement was 1, i.e., the terminal feedback was veridical. In the training phase, subjects reached to the training target with terminal feedback for 30 consecutive trials. Importantly the gain was modified from 1 to 0.6, creating a visuomotor perturbation. With this perturbation, subjects only needed to move 48 mm to reach the target. The last generalization phase was identical to the baseline phase except that the gain was kept at 0.6. As subjects never received visual feedback for reaches to targets other than the training target, we could assess their transfer of learning from the training direction to other directions. The amount of generalization is quantified as
where Dgeneralization and Dbaseline are average movement distances in the generalization phase and in the baseline phase, respectively. This generalization percentage was calculated for each direction separately and expressed as a function of angular separation from the training direction (Fig 2B). Subjects exhibited typical exponential learning during the training phase (Fig 2A). We fitted the learning data with an exponential function , where τ denotes the learning rate and b denotes the achieved learning level. When fitting parameters for each subject, the initial value of b was set at the learning achieved at the end of the training session (average error of the last 3 training trials). a was not a free parameter but the actual learning achieved during training; it was calculated for each participant as the average error before training (average of the last 3 baseline trials) minus the average error after training (average of the last 3 training trials).
The computer training in Experiment 2 involved subjects playing simple flash-based computer games that require frequent and precise mouse cursor movements, 2 hours each day for 14 consecutive days. Subjects were allowed to switch between 8 types of games and to take a break at their convenience. To quantify their improvement in using computer mouse, on each training day subjects also performed a modified pursuit rotor task, which required them to use mouse cursor to track a moving target on the computer monitor. The movement of the target followed a predefined trajectory whose horizontal position was a sine function (0.3Hz; 261 pixel in amplitude) and vertical position a sum of three sine functions (0.3, 0.6 and 0.9Hz; 150 pixel in amplitude). On the screen this tracking target spanned 522 and 750 pixels horizontally and vertically, respectively. Importantly, it moved with unpredictable and varying speeds (mean ± SD: 545±293 pixel/s) and thus discouraged subjects to improve the performance by simply remembering the trajectory. This task was organized as 20-s trials, 5 trials before and after the training on each day. The average, absolute distance between the target and the mouse cursor was computed as tracking error. The training was conducted in a lab setting under supervision of the experimenters.
For Experiment 1, between-group comparisons of generalization was performed with a two-way mixed-design ANOVA (3 groups × 5 angular separations). Comparisons of learning rate and learning extent were performed with one-way ANOVA. For Experiment 2, within-group comparisons between before- and after-training generalization was performed with a similar but repeated-measures ANOVA (2 timing × 5 angular separations). Across-experiments comparisons between two non-computer user groups were conducted via a two-way mixed-design ANOVA (2 groups × 5 angular separations). For both experiments, comparisons of generalizations between groups (Experiment 1) and between times (pre- and post-training) at specific angular separations were performed by using simple main effects with Bonferroni correction. Data entering two-way ANOVA tests were checked for normality. The significance level was set at α = .05.
Highlights.
We compared motor generalization for computer and non-computer users.
Non-computer users showed narrower generalization but with normal learning speed.
The narrower generalization was broadened by two-week computer training.
Computer use fundamentally affects the neural representation of our movements.
Acknowledgments
The study was supported by the National Institute of Neurological Disorders and Stroke Grants (Nos. R01NS-063399 and R01NS-057814), the National Natural Science Foundation of China (Nos. 61005082, 31000456, 31371020, J1103602, and 61020106005), the Fundamental Research Funds for the Central Universities, National High Technology Research and Development Program of China (863 Program, 2012AA011602).
Footnotes
Author contributions: K.W. and K.P.K designed experiments, X.Y., F.Z., G.K., C.Y and Q.W performed experiments, K.W., X.Y., and Q.W analyzed data, K.P.K and K.W wrote the paper.
Author information: Reprints and permissions information is available at www.nature.com/reprints.
Authors have no completing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Karni A, Meyer G, Rey-Hipolito C, Jezzard P, Adams MM, Turner R, Ungerleider LG. The acquisition of skilled motor performance: fast and slow experience-driven changes in primary motor cortex. Proceedings of the National Academy of Sciences. 1998;95:861. doi: 10.1073/pnas.95.3.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Draganski B, May A. Training-induced structural changes in the adult human brain. Behavioural brain research. 2008;192:137–142. doi: 10.1016/j.bbr.2008.02.015. [DOI] [PubMed] [Google Scholar]
- 3.Paz R, Boraud T, Natan C, Bergman H, Vaadia E. Preparatory activity in motor cortex reflects learning of local visuomotor skills. NATURE NEUROSCIENCE. 2003;6:882–890. doi: 10.1038/nn1097. [DOI] [PubMed] [Google Scholar]
- 4.Sparrow B, Liu J, Wegner DM. Google effects on memory: Cognitive consequences of having information at our fingertips. Science. 2011;333:776. doi: 10.1126/science.1207745. [DOI] [PubMed] [Google Scholar]
- 5.Taylor K. An Analysis of Computer Use Across 95 Organisations in Europe, North America and Australasia. Wellnomics Limited 2007 [Google Scholar]
- 6.Liu X, Mosier KM, Mussa-Ivaldi FA, Casadio M, Scheidt RA. Reorganization of finger coordination patterns during adaptation to rotation and scaling of a newly learned sensorimotor transformation. Journal of Neurophysiology. 2011;105:454–473. doi: 10.1152/jn.00247.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diedrichsen J, White O, Newman D, Lally N. Use-dependent and error-based learning of motor behaviors. The Journal of Neuroscience. 2010;30:5159–5166. doi: 10.1523/JNEUROSCI.5406-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang VS, Haith A, Mazzoni P, Krakauer JW. Rethinking motor learning and savings in adaptation paradigms: model-free memory for successful actions combines with internal models. Neuron. 2011;70:787. doi: 10.1016/j.neuron.2011.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Slijper H, Richter J, Over E, Smeets J, Frens M. Statistics predict kinematics of hand movements during everyday activity. Journal of Motor Behavior. 2009;41:3–9. doi: 10.1080/00222895.2009.10125922. [DOI] [PubMed] [Google Scholar]
- 10.Yan X, Wang Q, Lu Z, Stevenson IH, Körding K, Wei K. Generalization of unconstrained reaching with hand-weight changes. Journal of Neurophysiology. 2013;109:137–146. doi: 10.1152/jn.00498.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krakauer JW, Mazzoni P, Ghazizadeh A, Ravindran R, Shadmehr R. Generalization of motor learning depends on the history of prior action. PLoS biology. 2006;4:e316. doi: 10.1371/journal.pbio.0040316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krakauer JW, Pine ZM, Ghilardi MF, Ghez C. Learning of visuomotor transformations for vectorial planning of reaching trajectories. J Neurosci. 2000;20:8916–8924. doi: 10.1523/JNEUROSCI.20-23-08916.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vindras P, Viviani P. Altering the visuomotor gain. Experimental Brain Research. 2002;147:280–295. doi: 10.1007/s00221-002-1211-9. [DOI] [PubMed] [Google Scholar]
- 14.Haswell CC, Izawa J, Dowell LR, Mostofsky SH, Shadmehr R. Representation of internal models of action in the autistic brain. NATURE NEUROSCIENCE. 2009;12:970–972. doi: 10.1038/nn.2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pine ZM, Krakauer JW, Gordon J, Ghez C. Learning of scaling factors and reference axes for reaching movements. NeuroReport-International Journal for Rapid Communications of Research in Neuroscience. 1996;7:2357–2362. doi: 10.1097/00001756-199610020-00016. [DOI] [PubMed] [Google Scholar]
- 16.Krakauer JW, Ghilardi MF, Mentis M, Barnes A, Veytsman M, Eidelberg D, Ghez C. Differential cortical and subcortical activations in learning rotations and gains for reaching: a PET study. Journal of Neurophysiology. 2004;91:924–933. doi: 10.1152/jn.00675.2003. [DOI] [PubMed] [Google Scholar]
- 17.Ghahramani Z, Wolpert DM, Jordan MI. Generalization to local remappings of the visuomotor coordinate transformation. The Journal of Neuroscience. 1996;16:7085–7096. doi: 10.1523/JNEUROSCI.16-21-07085.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Imamizu H, Uno Y, Kawato M. Internal representations of the motor apparatus: implications from generalization in visuomotor learning. Journal of Experimental Psychology: Human Perception and Performance. 1995;21:1174. doi: 10.1037//0096-1523.21.5.1174. [DOI] [PubMed] [Google Scholar]
- 19.Mattar AA, Ostry DJ. Modifiability of generalization in dynamics learning. Journal of Neurophysiology. 2007;98:3321–3329. doi: 10.1152/jn.00576.2007. [DOI] [PubMed] [Google Scholar]
- 20.Thoroughman KA, Taylor JA. Rapid reshaping of human motor generalization. The Journal of Neuroscience. 2005;25:8948–8953. doi: 10.1523/JNEUROSCI.1771-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taylor JA, Hieber LL, Ivry RB. Feedback-dependent generalization. Journal of Neurophysiology. 2013;109:202–215. doi: 10.1152/jn.00247.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Izawa J, Shadmehr R. Learning from sensory and reward prediction errors during motor adaptation. PLoS computational biology. 2011;7:e1002012. doi: 10.1371/journal.pcbi.1002012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singh K, Scott SH. A motor learning strategy reflects neural circuitry for limb control. NATURE NEUROSCIENCE. 2003;6:399–403. doi: 10.1038/nn1026. [DOI] [PubMed] [Google Scholar]
- 24.Mattar AAG, Gribble PL. Motor learning by observing. Neuron. 2005;46:153–160. doi: 10.1016/j.neuron.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 25.Berniker M, Körding K. Estimating the sources of motor errors for adaptation and generalization. Nature Neuroscience. 2008;11:1454–1461. doi: 10.1038/nn.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Poggio T, Bizzi E. Generalization in vision and motor control. Nature. 2004;431:768–774. doi: 10.1038/nature03014. [DOI] [PubMed] [Google Scholar]
- 27.Thoroughman KA, Shadmehr R. Learning of action through adaptive combination of motor primitives. Nature. 2000;407:742–747. doi: 10.1038/35037588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Houde JF, Jordan MI. Sensorimotor adaptation in speech production. Science. 1998;279:1213–1216. doi: 10.1126/science.279.5354.1213. [DOI] [PubMed] [Google Scholar]
- 29.Ghahramani Z, Wolpert DM. Modular decomposition in visuomotor learning. Nature. 1997;386:392–395. doi: 10.1038/386392a0. [DOI] [PubMed] [Google Scholar]
- 30.Hwang EJ, Donchin O, Smith MA, Shadmehr R. A gain-field encoding of limb position and velocity in the internal model of arm dynamics. PLoS biology. 2003;1:e25. doi: 10.1371/journal.pbio.0000025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shabbott BA, Sainburg RL. Learning a visuomotor rotation: simultaneous visual and proprioceptive information is crucial for visuomotor remapping. Experimental Brain Research. 2010;203:75–87. doi: 10.1007/s00221-010-2209-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu HG, Smith MA. The generalization of visuomotor learning to untrained movements and movement sequences based on movement vector and goal location remapping. The Journal of Neuroscience. 2013;33:10772–10789. doi: 10.1523/JNEUROSCI.3761-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Donchin O, Francis JT, Shadmehr R. Quantifying generalization from trial-by-trial behavior of adaptive systems that learn with basis functions: theory and experiments in human motor control. The Journal of Neuroscience. 2003;23:9032–9045. doi: 10.1523/JNEUROSCI.23-27-09032.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shadmehr R, Mussa-Ivaldi FA. Adaptive representation of dynamics during learning of a motor task. The Journal of Neuroscience. 1994;14:3208–3224. doi: 10.1523/JNEUROSCI.14-05-03208.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gandolfo F, Mussa-Ivaldi F, Bizzi E. Motor learning by field approximation. Proceedings of the National Academy of Sciences. 1996;93:3843–3846. doi: 10.1073/pnas.93.9.3843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Henrich J, Heine SJ, Norenzayan A. Most people are not WEIRD. Nature. 2010;466:29–29. doi: 10.1038/466029a. [DOI] [PubMed] [Google Scholar]