Abstract
The purpose of this study was to investigate the pharmacological effect of fraction of Atractylodes lancea (Thunb.) DC. (A. lancea) extract. In this study, we isolated different polarity fractions, including petroleum ether (PE), ethyl acetate, n-butanol, and the remaining H2O fractions from the water extract of A. lancea. The antigastric cancer properties of the different fractions in BGC-823 and SGC-7901 cells were evaluated. Apoptotic cells were treated with PE fraction and stained with Hoechst 33342 and 5,5,6,6-tetrachloro-1,1,3,3-tetraethylbenzimidazolylcarbocyanine iodide. The cell cycle was analyzed via flow cytometry. The main compounds of PE fraction were determined by HPLC–ESI–MS. Results of this study showed that the PE fraction of A. lancea inhibited the growth of BGC-823 and SGC-7901 cells in a dose- and time-dependent manner. The morphological and mitochondrial transmembrane potential changes suggested that the cells showed preliminary apoptosis characteristics after treatment with the three different polarities. The main compounds of PE fraction include two sesquiterpene compounds: eudesm-4(15),7-diene-9α,11-diol and eudesm-4(15)-ene-7a,11-diol; three sesquiterpene lactone compounds: atractylenolid I, atractylenolid III and 3-β-acetyl-atractylenolid III and one polyacetylenic compound: 4,6,12-tetradecatriene-8,10-diyne-1,3,14-triol.
Keywords: Atractylodes lancea (Thunb.) DC., Water extracts, PE fraction, Gastric cells, Growth inhibitory
Introduction
Worldwide, gastric cancer is the second most common cause of mortality in cancer death (Parkin et al. 2005; Crew and Neugut 2006). East Asian countries, such as China, Japan, and Korea report the highest incidence of gastric cancer (Ferlay et al. 2010). The development of gastric cancer exhibits a multi-step process, ranging from chronic gastritis to atrophy, intestinal metaplasia, dysplasia, and finally, invasive cancer (Correa and Shiao 1994). Current comprehensive therapy of gastric cancer in China involves surgery and chemotherapy. Due to the adverse reactions and the resistance of tumor cells to the drugs (Gatti and Perego 2009), chemotherapy is not a preferred treatment. Several studies demonstrated that Chinese medicinal herbs combined with surgery and chemotherapy effectively improved the performance of anticancer treatments and reduced their toxic side effects (Dobos et al. 2005; Lin et al. 2012). In recent years, Chinese medicinal herbs have become an important source of potential anticancer agents (Naoyoshi et al. 2012; Zhang and Zhang 2011). Studies have revealed that Chinese medicinal herbs demonstrate anticancer effects by inducing apoptosis of cancer cells, enhancing the immune system, inducing cell differentiation, and inhibiting the telomerase activity and growth of tumors (Ruan et al. 2006; Adaramoye et al. 2011).
For a long time, rhizomes derived from Atractylodes lancea (Thunb.) DC. of the Compositae family have been used for the treatment of digestive disorders, rheumatic diseases, night blindness, and influenza (Tang and Eisenbrand 1992). A. lancea rhizomes are widely used as a traditional Chinese medicine to revitalize the spleen. In China and Japan, they are also used as stomachic. In Korean and Japanese pharmacopoeias, they are prescribed as gastric drugs (Kitajima et al. 2003). The mechanisms by which A. lancea exhibits antiulcer effects by improving the stomach damage is believed to be (Kubo et al. 1983) through inhibiting gastric secretion (Nogami et al. 1986) and gastric emptying (Nakai et al. 2003). In Chinese traditional medicine, A. lancea has always been used as a gastric cancer treatment with other Chinese medicines as a herbal medicine formula (Yuan et al. 2011). Earlier phytochemical investigations showed the presence of polyacetylenes, sesquiterpene, sesquiterpenoids, and sesquiterpene glycosides (Kano et al. 1989; Chen et al. 1997; Ding et al. 2000; Yahara et al. 1989) in A. lancea. The objective of the present study was to identify the anticancer activity and to assess the therapeutic potential of A. lancea water extract in gastric cancer using human gastric cancer cell lines BGC-823 and SGC-7901.
Materials and methods
Materials
Dulbecco’s modified Eagle’s medium (DMEM) was provided by Gibco Industries Inc. (Grand Island, NY, USA). Penicillin, streptomycin, 3-(4,5-dimethyl-thiazol-2-yl)-2,5-diphenyl tetrazolium) (MTT), 5,5,6,6-tetrachloro-1,1,3,3-tetraethylbenzimidazolylcarbocyanine iodide (JC-1), the fluorescent probe propidium iodide (PI), Hoechst 33342, Trypsin/EDTA, and Triton X-100 were purchased from Sigma Co. (St. Louis, MO, USA). Fetal bovine serum (FBS) was provided by Hangzhou Sijiqing Biological Engineering Co. Ltd. (Hangzhou, PR China). BGC-823 and SGC-7901 cells were provided by the Institute of Life Science (Jiangsu University, China). All other chemicals and reagents used were of analytical grade.
Preparation for A. lancea extracts
Atractylodes lancea roots were collected from an area in Jurong, Jiangsu Province, China and identified by Professor Zhen Ouyang, School of Pharmacy, Jiangsu University, China. Oven-dried roots were ground to powder and passed through 40 mesh screen. Up to 20 g of the powder was mixed with 400 mL distilled water and subjected to reflux extraction for 2 h. The solution was centrifuged at 900×g for 15 min. The supernatant was collected, and the residue was re-extracted. Finally, the supernatant was filtered using filter paper and concentrated to dryness at 60 °C under reduced pressure. The concentrated extract was fractionated four times with an equal volume of petroleum ether (PE). The solution was partitioned using ethyl acetate (EtOAc) and n-butanol (n-BuOH). Finally, the remaining H2O was fractionated, and the isolated fractions were dried. All isolated fractions were subjected to cytotoxic and apoptosis assays.
Cell cultures
The BGC-823 and SGC-7901 cells were maintained at 37 °C in a humid atmosphere containing 5 % CO2 and cultured in DMEM with 5 % (v/v) FBS, 100 U/mL penicillin, and 100 μg/mL streptomycin. The culture medium was changed every 2 days.
Cell viability assay
Cell viability was determined using the MTT assay. The cells were seeded and allowed to grow for 24 h. Subsequently, the cells were treated with defatted fraction PE, EtOAc, n-BuOH, and the remaining H2O fractions (0, 0.0625, 0.125, 0.25, 0.5, and 1 mg/mL of each fraction). The control samples were not treated but received an equal volume of medium. After removing the medium, the cells were labeled with MTT solution for 4 h at 37 °C, and the resulting formazan was solubilized with DMSO (100 μL). Finally, the optical density (OD) of each well was immediately measured on an ELISA micro-plate reader (Bio-Rad) at 490 nm to detect cellular viability.
Morphological assessment of apoptotic cells
To assess the morphology of apoptotic cells, BGC-823 and SGC-7901 cells were stained with the DNA-binding dye Hoechst 33342. These cells (4 × 104) were cultured overnight in 24-well plates and treated with PE fraction (0.5 mg/mL) for 24 h. After removing the medium, the cells were washed twice with phosphate buffer saline (PBS). The cells were then incubated for 10 min with 20 mg/mL Hoechst 33342 to stain the nuclei. Morphological changes in the nuclear chromatin were observed under a fluorescence microscope (Ti-2000, Nikon, Japan).
Determination of mitochondrial transmembrane potential
The cells, BGC-823 and SGC-7901 (4 × 104) were cultured in 24-well plates and allowed to grow for 24 h. The cells were incubated with PE fraction (0.5 mg/mL) for 24 h. After removing the medium, the cells were washed twice with PBS. 100 μL JC-1 solution (3 μg/mL) was added to each well. The plates were incubated for 30 min and then washed twice with PBS after removing the solution. Finally, the OD of each well was immediately measured using a fluorescence enzyme standard instrument and observed under a fluorescence microscope (Ti-2000, Nikon, Tokyo, Japan).
Chemical profiling of the PE fraction
Chemical profiling of the PE fraction was performed via high-performance liquid chromatography-electrospray ionization-mass spectrometry (HPLC–ESI–MS) (LXQ, Thermo Finnigan, Waltham, MA, USA). In a volumetric flask, approximately 4 mg of the PE fraction was added with 1 mL methanol. The mixture was subjected to ultrasound for 5 min to resolve the volume. The solvent was filtered using a microporous membrane (0.45 μm) (Hanbon Sci. & Tech., Jiangsu, China) and then injected into the HPLC–ESI–MS system. For the HPLC–ESI–MS, the HPLC column used was Kromasil–C18 (250 mm × 4.6 mm, 5 μm, Hanbon Sci. & Tech., China). The method of gradient elution was used. Methanol was used as the liquid phase (A) with 0.2 % glacial acetic acid of water solvent (B) under the following setting: 0–15 min, A: 45–60 %, B: 55–40 %; 15–30 min, A: 60–70 %, B: 40–30 %; 30–50 min, A: 70–80 %, B: 30–20 %; 50–60 min, A: 80–90 %, B: 20–10 %; 60–65 min, A: 90 %, B: 10 %. The elution rate was 0.7 mL/min, and the analytical column was maintained at 30 °C. Mass spectra were analyzed using a Finnigan LXQ instrument with an ESI source. Nitrogen was used as the sheath and auxiliary gas, whereas helium was used as the collision gas. Values of auxiliary gas flow rate were set at 10 arbitrary units, and capillary voltage was set at 30 V. Mass spectrometric data were collected from m/z 100–1,500 in positive ion mode.
Statistical analysis
One- and two-way analysis of variance and SPSS 16.0 were used for data analysis. All results were expressed as mean ± SEM or mean ± SD; p < 0.05 was considered to indicate statistical significance.
Results and discussion
Inhibitory effect on cell growth
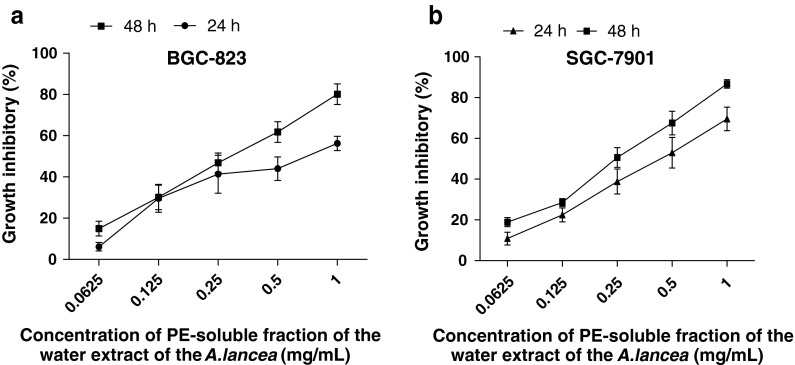
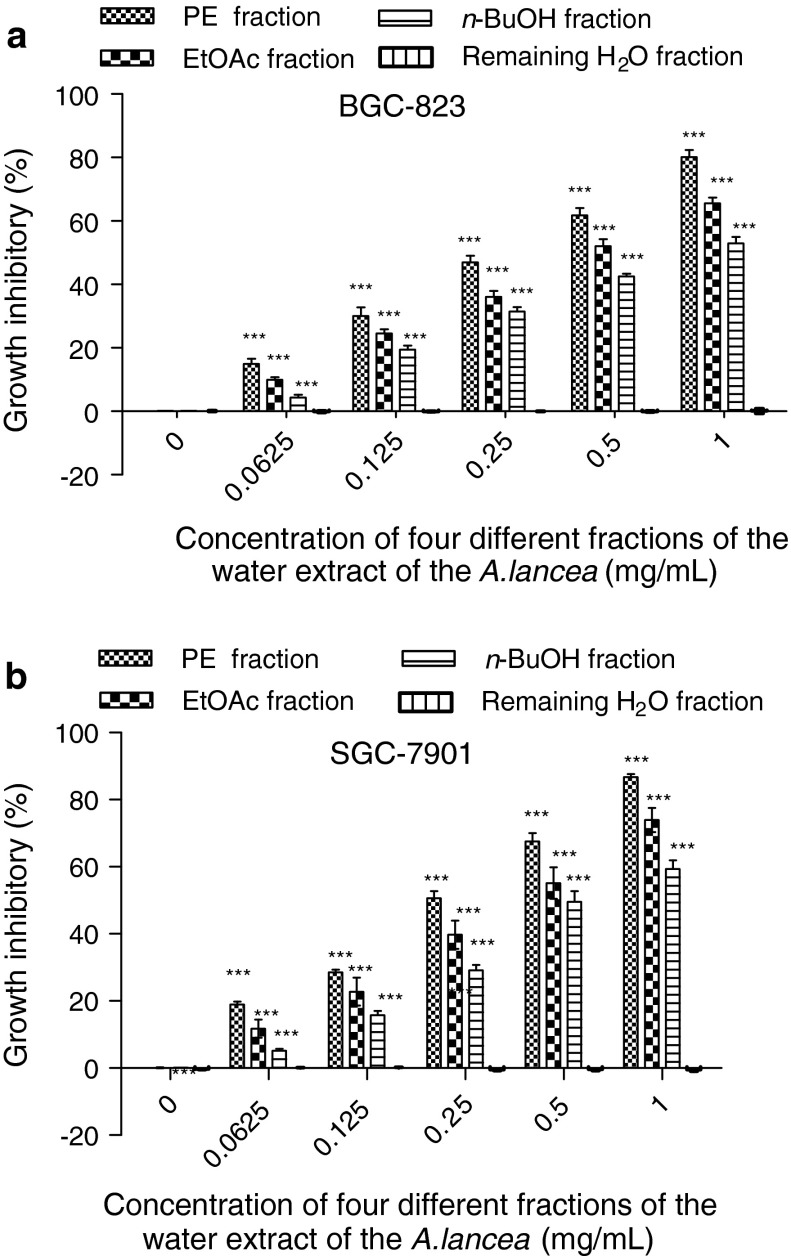
This study determined inhibition of cancer cell proliferation and estimated the IC50 values of the different polarities of A. lancea extract on BGC-823 and SGC-7901 cells. The MTT assay showed that after the cells were treated with the PE, EtOAc, and n-BuOH fractions at 0, 0.0625, 0.125, 0.25, 0.5, and 1 mg/mL for 24 and 48 h these fractions exhibited growth inhibitory effects on BGC-823 cells in a dose-dependent manner and SGC-7901 cells in a dose- and time-dependent manner, respectively (Fig. 1a, b). The PE fraction of the extract was found to be the most effective in inhibitory capacity. The EtOAc fraction was much weaker than the PE fraction, whereas the n-BuOH fraction was weaker than the EtOAc fraction (Fig. 2a, b). BGC-823 and SGC-7901 cells were sensitive to the PE fraction.
Fig. 1.
The growth inhibition effect of PE fraction of A. lancea extracts at 24 and 48 h on the BGC-823 (a) and SGC-7901(b) cells (both treated with PE fraction of A. lancea extracts). Values are expressed as mean ± SD (n = 5)
Fig. 2.
The growth inhibition effects of different fractions of A. lancea extracts (0.0625, 0.125, 0.25, 0.5, 1 mg/mL) on BGC-823 (a) and SGC-7901(b) cells after 48 h. Values are expressed as mean ± SD (n = 5). (***p < 0.001, **p < 0.01, versus control group)
Morphology of apoptotic cells
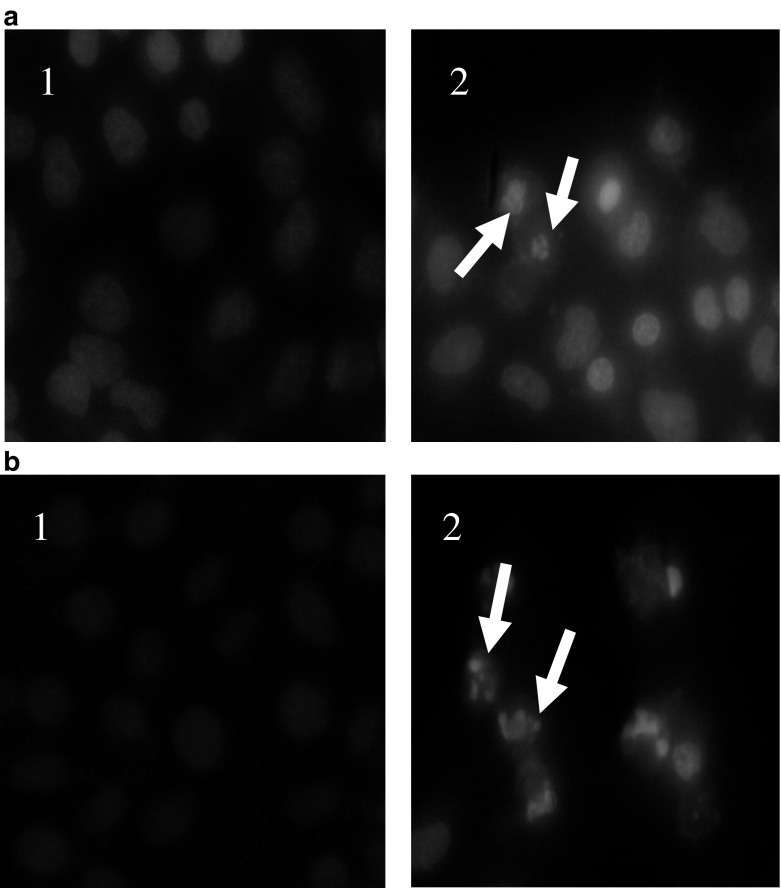
Morphological observation revealed that the chromatin of BGC-823 and SGC-7901 cells in the control group was normal and well distributed. By contrast, after treatment with the respective drug groups, the number of cells decreased, and the condensation and fragmentation of apoptotic cell nuclei were evident in BGC-823 and SGC-7901 cells. The results indicated that the PE fraction of A. lancea induced apoptotic death of BGC-823 and SGC-7901 cells (Fig. 3).
Fig. 3.
The appearance of apoptotic nuclei of BGC-823 (a) and SGC-7901 (b) cells treated with PE-fraction of A. lancea extracts (0.5 mg/mL). Cells were stained with Hoechst 33342 and observed under a fluorescence microscope. (1) Control group; (2) PE fraction
Changes in mitochondrial transmembrane potential
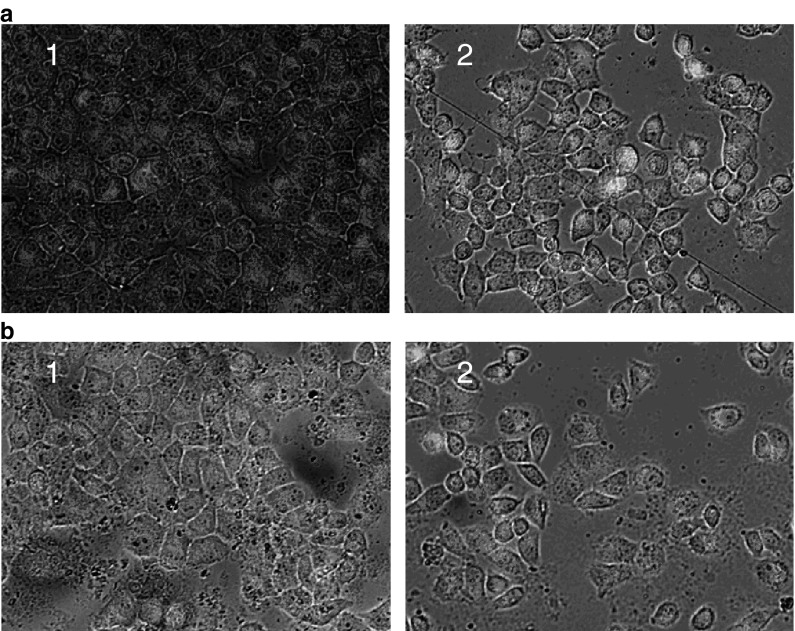
The mitochondrial transmembrane potential of the normal cells in the control group was higher than for the treated cells; JC-1 staining displayed jacinth fluorescence (Fig. 4). The jacinth fluorescence decreased and the green fluorescence increased in BGC-823 and SGC-7901 cells treated with the fraction extracts. This result indicated that the three polarity fractions could decrease the mitochondrial transmembrane potential of BGC-823 and SGC-7901 cells.
Fig. 4.
The mitochondrial transmembrane potential of BGC-823 (a) and SGC-7901 (b) cells treated with P-fraction of A. lancea extracts. Cells were stained with JC-1 and the color changed under a fluorescence microscope as function of a change in the transmembrane potential. (1) Control group; (2) PE-fraction
Chemical profiles of the active fraction
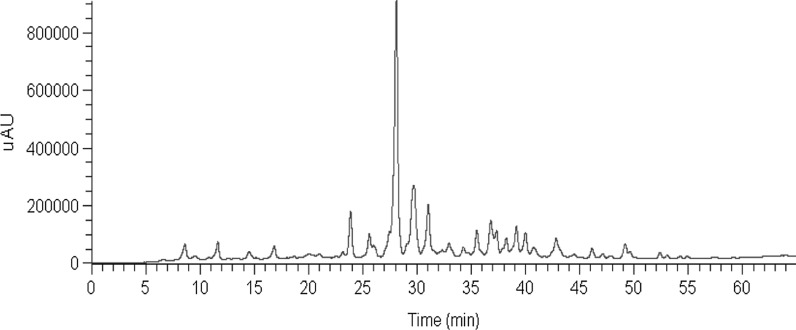
HPLC profiles of the PE fraction are shown in Fig. 5. All compounds exhibited quasi-molecular [M + H]+ and adduct [M + Na]+ ions in positive ion mode. The MS data and the main fragments in the MS spectra are presented in Table 1. The MS spectra of the dominant constituents of the PE fraction revealed six compounds. These compounds include two sesquiterpene compounds: eudesm-4(15),7-diene-9α,11-diol (Wang et al. 2008a, b) and eudesm-4(15)-ene-7a,11-diol (Wang et al. 2008a, b); three sesquiterpene lactone compounds: atractylenolid I (Meng et al. 2010; Endo et al. 1979), atractylenolid III (Wang et al. 2007; Li et al. 2002; Wu et al. 2004), and 3-β-acetyl-atractylenolid III (Wang et al. 2007); and one polyacetylenic compound: 4,6,12-tetradecatriene-8,10-diyne-1,3,14-triol (Nakai et al. 2003).
Fig. 5.
HPLC profiles of the PE fractions of A. lancea extracts
Table 1.
The retention time, precursor ion and main fragment ions of the reference compounds in positive ion mode of MSn analysis
| Authentic compounds | RT (min) | Quasi-molecular ion (measured) | MSn data (measured) |
|---|---|---|---|
| Eudesm-4(15),7-diene-9α,11-diol | 28.09 | 235.16[M + 1]+ | 217.11 → 189.09 |
| 3β-acetyl-Atractylenolid III | 29.72 | 291.17[M + 1]+ | 231.09 → 213.04 |
| Atractylenolid III | 31.02 | 249.07[M + 1]+ | 231.04 → 203.15 |
| Eudesm-4(15)-ene-7a,11-diol | 32.98 | 237.09[M + 1]+ | 219.09 → 201.05 |
| Atractylenolid I | 36.37 | 231.11[M + 1]+ | 185.05 → 203.15 |
| 4,6,12-tetradecatriene-8,10-diyne-1,3,14-triol | 42.97 | 233.11[M + 1]+ | 215.16 → 187.01 |
Traditionally, Chinese medicine is water boiled to determine the possible effective compounds (Wang et al. 2010; Luan et al. 2010). In this study, the most suitable extraction and partitioning methods, as well as the most appropriate solvents were selected to extract specific classes of compounds to determine the composition of the extracts. Using the MTT assay, the antiproliferative effects of PE, EtOAc, n-BuOH, and H2O-souble fractions were determined. Data showed that the PE, EtOAc and n-BuOH fractions could inhibit the growth of BGC-823 and SGC-7901 cells. The growth inhibitory effect of the PE fraction was significant, whereas the H2O-souble fraction did not show any growth inhibitory effect on the cancer cells.
Current literature lack studies on the mechanism of action of A. lancea and its antigastric cancer activity. The present study demonstrated the inhibitory effect of PE fraction of A. lancea on the growth of BGC-823 and SGC-7901 cells. After treatment with the PE fraction, the nuclear condensation and DNA fragmentation of the cancer cells were prominent. These results suggested that BGC-823 and SGC-7901 cells treated with the PE fractions underwent typical apoptosis. Moreover, the results also showed that the mitochondrial transmembrane potential of the PE fraction treated BGC-823 and SGC-7901 cells decreased. The decreased mitochondrial transmembrane potential indicated cell apoptosis in the initial stage. The results suggested that the PE fraction induced cell apoptosis via the mitochondrial pathway.
Previous studies reported that sesquiterpenes exhibit activities against gastrointestinal diseases (Zhang et al. 1999). Cytotoxic activity of Sesquiterpenoids from Atractylodes ovata shows cytotoxic activity on Leukemia Cell Lines (Wang et al. 2002). Atractylenolid showed antitumor effects (Wang et al. 2006, 2008a, b) and contains components with gastrointestinal inhibitory effects (Zhang et al. 1999). Polyacetylenic compounds demonstrated improvement in the delay of gastric emptying (Nakai et al. 2003). In the present study, LC/MS results showed that the PE fraction of A. lancea mainly contains sesquiterpenes, esquiterpene lactone and polyacetylenes. The results suggested that sesquiterpenes, esquiterpene lactone and polyacetylenes are the active ingredients of A. lancea water extract.
Conclusion
The results showed that the PE fraction of A. lancea demonstrated significant growth inhibitory effects on BGC-823 and SGC-7901 cells. The cytotoxic mechanism was related to the effects of the extract on apoptosis and cell cycle arrest. Apoptosis was induced through mitochondria-dependent and death receptor-dependent apoptotic pathways. The possible components of A. lancea responsible for its resistance to gastric cancer included sesquiterpenes, esquiterpene lactone and polyacetylenes. However, more experimentation is needed to isolate and identify the effective chemical composition using separation and anticancer evaluation methods. The results of this study suggest that A. lancea deserves further study as a potential anticancer drug.
Acknowledgments
This work is supported by Bureau of traditional Chinese medicine of Jiangsu Province (No. LZ09086; HZ07005).
Conflict of interest
The authors have declared that there is no conflict of interest.
References
- Adaramoye OA, Sarkar J, Singh N. Antiproliferative action of Xylopia aethiopica fruit extract on human cervical cancer cells. Phytother Res. 2011;25:1558–1563. doi: 10.1002/ptr.3551. [DOI] [PubMed] [Google Scholar]
- Chen ZL, Cao WY, Zhou G. A sesquiterpene lactam from Atractylodes macrocephala. Phytochemistry. 1997;45:765–767. doi: 10.1016/S0031-9422(97)00036-8. [DOI] [Google Scholar]
- Correa P, Shiao YH. Phenotypic and genotypic events in gastric carcinogenesis. Cancer Res. 1994;54:1941–1943. [PubMed] [Google Scholar]
- Crew KD, Neugut AI. Epidemiology of gastric cancer. World J Gastroenterol. 2006;12:354–362. doi: 10.3748/wjg.v12.i3.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding H, Wu YC, Lin HC. Phytochemical and pharmacological studies on Chinese Changzhu. J Chin Chem Soc. 2000;47:561–566. [Google Scholar]
- Dobos GJ, Tan L, Cohen MH. Are national quality standards for traditional Chinese herbalmedicine sufficient? Current governmental regulations for traditional Chinese herbal medicine in certain Western countries and China as the Eastern origin country. Complementary Ther Med. 2005;13:183–190. doi: 10.1016/j.ctim.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Endo K, Taguchi T, Taguchi F. Antiinflammatory principles of Atractylodes rhizomes. Chem Pharm Bull. 1979;27:2954–2958. doi: 10.1248/cpb.27.2954. [DOI] [PubMed] [Google Scholar]
- Ferlay J, Shin HR, Bray F. Estimates of wordwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- Gatti L, Perego P. Cellular resistance to oxaliplatin and drug accumulation defects. Cancer Drug Discov Dev. 2009;2:115–124. [Google Scholar]
- Kano Y, Komatsu KI, Saito KI. A new polyacetylene compound from Atractylodes rhizome. Chem Pharm Bull. 1989;37:193–194. doi: 10.1248/cpb.37.193. [DOI] [Google Scholar]
- Kitajima J, Kamoshita A, Ishikawa T. Glycosides of Atractylodes lancea. Chem Pharm Bull. 2003;51:673–678. doi: 10.1248/cpb.51.673. [DOI] [PubMed] [Google Scholar]
- Kubo M, Nogami M, Nishimura M. Studies of crude drugs about its origin, process and quality. I. The preventive effects of Chinese crude drug ‘Zhu’ on experimental stomach ulcer and its pharmacological evaluation (1) Yakugaku Zasshi. 1983;103:442–448. [PubMed] [Google Scholar]
- Li X, Wang JH, Li X. Studies on the chemical consitutent of Atractylodes chinensis (DC.) Koidz. J Shengyang Pharm Univ. 2002;9:178. [Google Scholar]
- Lin HP, Kuo LK, Chuu CP. Combined treatment of curcumin and small molecule inhibitors suppresses prolif-eration of A549 and H1299 human non-small-cell lung cancer cells. Phytother Res. 2012;26:122–126. doi: 10.1002/ptr.3523. [DOI] [PubMed] [Google Scholar]
- Luan JL, Duan HY, Liu Q. Inhibitory effects of norcantharidin against human lung cancer cell growth and migration. Cytotechnology. 2010;62:349–355. doi: 10.1007/s10616-009-9250-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng H, Li GY, Dai RH. Chemical constituents of Atractylodes chinensis (DC.) Koidz. Biochem Syst Ecol. 2010;38:1220–1223. doi: 10.1016/j.bse.2010.12.023. [DOI] [Google Scholar]
- Nakai Y, Kido T, Hashimoto K, Kase Y, Sakakibara I, Higuchi M, Sasaki H. Effect of the rhizomes of Atractylodes lancea and its constituents on the delay of gastric emptying. Ethnopharmacology. 2003;84:51–55. doi: 10.1016/S0378-8741(02)00260-X. [DOI] [PubMed] [Google Scholar]
- Naoyoshi N, Mari I, Mari K. In vitro cytotoxic effect of ethanol extract prepared from Sporophyll of Undaria pinnatifida on human colorectal cancer cells. Phytother Res. 2012;26:191–196. doi: 10.1002/ptr.3527. [DOI] [PubMed] [Google Scholar]
- Nogami M, Moriura T, Kubo M, Tani T. Studies on the origin, processing and quality of crude drugs. II. Pharmacological evaluation of the Chinese crude drug ‘zhu’ in experimental stomach ulcer (2). Inhibitory effect of extract of Atractylodes lancea on gastric secretion. Chem Pharm Bull (Tokyo) 1986;34:3854–3860. doi: 10.1248/cpb.34.3854. [DOI] [PubMed] [Google Scholar]
- Parkin DM, Bray F, Ferlay J, Pisani P (2005) Global Cancer Statistics, 2002. Cancer J Clin 55:74–108 [DOI] [PubMed]
- Ruan WJ, Lai MD, Zhou JG. Anticancer effects of Chinese herbal medicine, science or myth? J Zhejiang Univ SCIENCE B. 2006;12:1006–1014. doi: 10.1631/jzus.2006.B1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W, Eisenbrand G. Chinese drugs of plant origin: chemistry, pharmacology, and use in traditional and modern medicine. Berlin and New York: Springer; 1992. [Google Scholar]
- Wang CC, Chen LG, Yang LL. Cytotoxic activity of sesquiterpenoids from Atractylodes ovata on leukemia cell lines. Planta Med. 2002;68:204–208. doi: 10.1055/s-2002-23144. [DOI] [PubMed] [Google Scholar]
- Wang CC, Lin SY, Cheng HC. Pro-oxidant and cytotoxic activities of atractylenolide I in human promyeloleukemic HL-60 cells. Food Chem Toxicol. 2006;8:1308–1315. doi: 10.1016/j.fct.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Wang LY, Duan JA, Qian SH. Studies on the chemical consitutent of Atractylodes lancea (Thunb.) DC. Chin Tradit Herbal Drugs. 2007;38:499–500. [Google Scholar]
- Wang CH, Wang SC, Chen QH. A capillary gas chromatography-selected ion monitoring mass spectrometry method for the analysis of atractylenolide I in rat plasma and tissues, and application in a pharmacokineticstudy. Chromatogr B Analyt Technol Biomed Life. 2008;3:215–222. doi: 10.1016/j.jchromb.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Wang HX, Liu CM, Liu Q. Three types of sesquiterpenes from rhizomes of Atractylodes lancea. Phytochemistry. 2008;69:2088–2094. doi: 10.1016/j.phytochem.2008.04.008. [DOI] [PubMed] [Google Scholar]
- Wang LP, Duan HY, Wang YS. Inhibitory effects of Lang-du extract on the in vitro and in vivo growth of melanoma cells and its molecular mechanisms of action. Cytotechnology. 2010;62:357–366. doi: 10.1007/s10616-010-9283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Han RB, Luo HS. Studies on the chemical constituents of Actractycodes japonica Koidz ex Kitam. J Yanbian Univ (Nat Sci) 2004;30:29–34. [Google Scholar]
- Yahara S, Higashi T, Iwaki K. Studies on the constituents of Atractylodes lancea. Chem Pharm Bull. 1989;37:2995–3000. doi: 10.1248/cpb.37.2995. [DOI] [Google Scholar]
- Yuan ZH, Huang MH, Chen Yuan Y (2011) Kind of treatment of liver, stomach, lung, and cancer of the blood and preparation methods that combination of Chinese herbal medicine, CN102205059A (in Chinese)
- Zhang LL, Zhang SL. Modulating Bcl-2 family proteins and caspase-3 in induction of apoptosis by paeoniflo-rin in human cervical cancer cells. Phytother Res. 2011;25:1551–1557. doi: 10.1002/ptr.3534. [DOI] [PubMed] [Google Scholar]
- Zhang YQ, Xu SB, Lin C. Gastrointestinal inhibitory effects of sesquiterpene lactones from Atractylodes macrocephala. Zhong Yao Cai. 1999;12:636–640. [PubMed] [Google Scholar]