Abstract
The effects of serial cell passaging on cell spreading, migration, and cell-surface ultrastructures have been less investigated directly. This study evaluated the effects of long-term serial cell passaging (totally 35 passages) on cultured human umbilical vein endothelial cells which were pre-stored at −80 °C as usual. Percentage- and spread area-based spreading assays, measurements of fluorescently labeled actin filaments, migration assay, and measurements of cell-surface roughness were performed and quantitatively analyzed by confocal microscopy or atomic force microscopy. We found that the abilities of cell spreading and migration first increased at early passages and then decreased after passage 15, in agreement with the changes in average length of actin filaments. Recovery from cold storage and effects of cell passaging were potentially responsible for the increases and decreases of the values, respectively. In contrast, the average roughness of cell surfaces (particularly the nucleus-surrounding region) first dropped at early passages and then rose after passage 15, which might be caused by cold storage- and cell passaging-induced endothelial microparticles. Our data will provide important information for understanding serial cell passaging and implies that for pre-stored adherent cells at −80 °C cell passages 5–10 are optimal for in vitro studies.
Keywords: Cell passaging, Cell spreading, Cell migration, Cell-surface roughness, Actin filaments, Atomic force microscopy (AFM), Human umbilical vein endothelial cells (HUVECs)
Introduction
As a critical component of the vasculature, vascular endothelial cells play vital roles in vascular integrity, functions, and the progression of vascular diseases (e.g. atherosclerosis). Cultured vascular endothelial cells are generally recruited as in vitro models for investigating effects of drugs or microbes, vascular permeability of molecules, adhesion or extravasation of peripheral blood cells or cancer cells, and others. Obviously, the physiological condition of cultured cells will more or less influence the quality of experimental data. To produce large number of cells for multiple assays in a study, repetitive cell passaging is inevitable. In some cases, long-term successive cell passaging will be performed for specific aims, such as in vitro cell aging research and cardiovascular tissue engineering applications. Therefore, it is essential and important to clarify the effects of serial cell passaging on the structure and physiological activities of cultured cells.
Over the past decades, the effects of cell passaging on biochemical properties of various types of endothelial cells derived from different species have been widely studied. For example, reduced proliferative capacity, increased oxidative stress, activation of the NFκ-B and p53 signaling pathways (Lee et al. 2010), reduced nitric oxide production and surfactant protein D (SP-D) expression (Lee et al. 2009) were detected in porcine coronary arterial endothelial cells after multiple passaging in vitro. Enhanced apoptosis of porcine pulmonary artery endothelial cells (Zhang et al. 2002) and declined arachidonic acid content and eicosanoid release in porcine aortic endothelial cells in response to agonist stimulation (Brown and Deykin 1991) were determined as well. The effects of cell passaging on endothelin-1 and prostaglandin F2α production of bovine luteal endothelial cells (Acosta et al. 2007) and on the responsiveness of bovine aortic endothelial cells to orbital shear stress (Kudo et al. 2005) were also evaluated. It has been reported that in vitro passaging causes the changes in enzyme activities and total protein in primate brain microvessel endothelial cells (Shi and Audus 1994) and in LDL uptake and expression of eNOS, MCP-1, von Willebrand factor (vWF), VCAM-1, ICAM-1, and E-selectin in primate femoral artery endothelial cells with or without cytokine stimulation (Shi et al. 2004). Expression of thymosin β-10 in human dermal endothelial cells (Vasile et al. 2001) or specific angiotensin II receptors (AT1 and AT2) in human glomerular endothelial cells (Cresci et al. 2003) was significantly influenced by in vitro passaging.
More research focused on human umbilical vein endothelial cells (HUVECs). For instance, Galustian et al. investigated the alterations in expression of actin cytoskeletal isoforms in HUVECs and human placental microvascular endothelial cells with cell passage (Galustian et al. 1995). Up-regulated expressions of endothelin and some extracellular matrix (ECM) proteins (e.g. fibronectin, plasminogen activator inhibitor, and others) were measured in HUVECs after multiple passaging in vitro (Grillari et al. 2000; Kumazaki et al. 1997). The synthesis and constitutive secretion of matrix metalloproteinase-9 (MMP-9) by early-passage HUVECs were analyzed by Arkell and Jackson (2003). Grillari’s group further compared the protein expression profiles of early-passage, senescent and immortalized HUVECs (Chang et al. 2005). Moreover, the significantly reduced immunogenicity of the sub-cultured HUVECs has been determined by Desai et al. (1995).
Until now, however, the effects of in vitro passaging on cell-surface ultrastructures and physiological behaviors of cultured endothelial cells, including cell spreading, migration, and others have been less investigated directly. This study focused on the effects of long-term serial cell passaging (~35 passages in total) on cell spreading, migration and cell-surface ultrastructures of cultured HUVECs.
Materials and methods
Reagents, cell line, and cell culture
Human umbilical vein endothelial cells (HUVECs), purchased from ATCC (Manassas, VA, USA), were routinely cultured in DMEM medium (GIBCO, Life Technologies, Carlsbad, CA, USA) supplemented with 10 % (w/v) fetal calf serum (Hyclone, South Logan, UT, USA), 100 U/ml penicillin and 80 μg/ml streptomycin. The cells were sub-cultured every 2–3 days. To study the effects of cell passaging on physiological behaviors of cells, we artificially defined the cells stored in the −80 °C freezer as passage 0 and the cells after thawing and sub-culturing once as passage 1 (P1). So, the cells after sub-culturing for 5, 10, 15, 20, 25, 30, and 35 times were defined as passages 5 (P5), 10 (P10), 15 (P15), 20 (P20), 25 (P25), 30 (P30), and 35 (P35), respectively.
Cell spreading assays
Two methods were performed to investigate cell spreading, percentage-based spreading assay and spread area-based spreading assay. Suspended HUVECs in medium were plated on sterilized coverslips in the wells of a 6-well plate. After 0.5 h (for percentage-based spreading assay) or 24 h (for spread area-based spreading assay), the cells on the coverslips were rinsed gently with phosphate-buffered saline (PBS) to remove unattached cells, fixed by 4 % formaldehyde, rinsed again by PBS, and imaged by a confocal microscope. For the percentage-based spreading assay, the numbers of spread cells and total cells in each image were counted; and the percentage of the spread cells was calculated. For the spread area-based spreading assay, the spread area of each HUVEC cell was extracted by planimetry using the Zeiss LSM 710 Zen software equipped with the confocal microscope.
Cell migration assay
In vitro scratch assay was used to test cell migration as previously reported (Liang et al. 2007). Briefly, HUVECs were plated onto a petri dish and cultured in a CO2 incubator at 37 °C for around 24 h to create a confluent cell monolayer. Then, the cell monolayer was scraped in a straight line to create a “scratch” with a p100 pipet tip. After removal of the debris and replacement of the medium, a phase-contrast image of a section of the scratch was taken as a reference image by confocal microscope. Next, the cells were cultured for 6 h in the incubator followed by imaging sections of the scratch under confocal microscope. The number of the migrating cells in each side of the scratch in each image was counted.
Visualization and quantification of actin filaments
Cells were plated in a petri dish and cultured in the incubator (37 °C, 5 % CO2) for 24 h. After washing twice with PBS, the cells were fixed by 4 % paraformaldehyde for 20 min. The cells were washed three times with PBS. Then, 5 μg/ml Phalloidin-TRITC was used to label F-actin in the cells at room temperature for 60 min, following with PBS washes twice. The cells were imaged under confocal microscope and the length of each fluorescently labeled actin filament was measured.
Confocal microscopy
For cell spreading assay and cell migration assay, cells at different passages were fixed by 4 % formaldehyde and rinsed with PBS. The coverslips or petri dishes were mounted on the stage of a Zeiss inverted microscope and observed under an LSM710 confocal microscope (Carl Zeiss, Oberkochen, Germany). All images were obtained with a 20 × 0.50 Zeiss Plan-Neofluar objective for the spreading assay or a 10 × 0.30 Zeiss Plan-Neofluar objective for the migration assay; only DIC (differential interference contrast) images were obtained.
For fluorescence imaging of intracellular actin filaments, a 20 × 0.50 or 40 × 0.75 Zeiss Plan-Neofluar objective was recruited. The excitation and emission wavelengths for TRITC-phalloidin were 561 and 570–670 nm, respectively.
Atomic force microscopy (AFM)
Cells were plated on sterilized coverslips in the wells of a 6-well plate and cultured in a CO2 incubator at 37 °C for 24 h. Then, the cells on the coverslips were fixed by 2.5 % glutaraldehyde for 30 min, rinsed by PBS and then pure water, and air-dried in air. An Agilent AFM series 5500 (Agilent Technologies, Santa Clara, CA, USA) was recruited to image individual whole cells and cell-surface ultrastructures in tapping mode at room temperature with a lateral scan rate of ~0.5 Hz. The force constant of silicon nitride cantilevers (Length: ~225 μm; thickness: ~7 μm; mean width: ~38 μm. Agilent Technologies) was ~48 N/m. All the AFM images were analyzed using the Pico Image Basic 6.2 software equipped with the instrument.
Statistical analysis
All graphs were made by GraphPad Prism version 5.0 (GraphPad Software, La Jolla, CA, USA). Statistical analyses were performed using Student t test to determine the statistical significance for the values between different groups. A difference was regarded as significant when P < 0.05.
Results
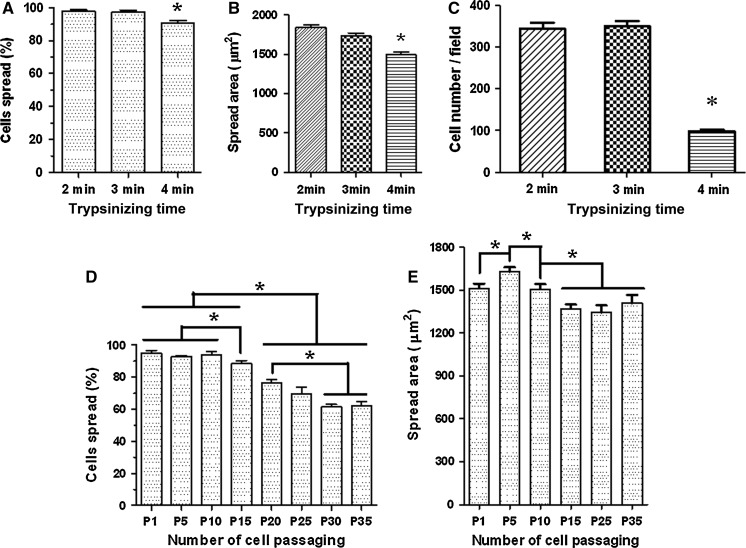
Prior to investigating the effects of cell passaging, we first determined the effects of trypsinization. Since 0.25 % trypsin is the commonly used concentration for digestion of adherent cells, here only the effects of trypsinizing time on cell spreading and migration of HUVECs were evaluated. After trypsinization with 0.25 % trypsin–EDTA for different periods (2, 3, and 4 min, respectively), the cells were re-plated on substrates and cultured for 30 min (for percentage-based spreading assay) or for 24 h (for spread area-based spreading assay and scratch-based migration assay). We found that compared with the 2 min and 3 min groups the average percentage of spread cells at 30 min (Fig. 1a) or the average spread area of cells at 24 h (Fig. 1b) was significantly lower in the 4 min group. Moreover, the scratch-based migration assay (Fig. 1c) showed that the number of the migrating cells in the scratch area dramatically decreased when the cells were trypsinized for 4 min or longer. The data indicated that trypsinization for more than 4 min significantly impaired cell spreading and migration abilities of HUVECs in our study. Therefore, trypsinization for 2–3 min was performed in the following cell passaging-related experiments.
Fig. 1.
a–c Effects of trypsinization on cell spreading and migration of HUVECs. After trypsinization with 0.25 % trypsin–EDTA for 2, 3, and 4 min, respectively, the cells were subject to percentage-based (a) or spread area-based (b) cell spreading assay and migration assay (c). d, e Effects of cell passaging on cell spreading of HUVECs by measuring percentage of spread cells (d) or spread areas of individual cells (e). Values are expressed as mean ± SEM (A, n ≥ 18; B, n ≥ 470; C, n ≥ 6; D, n ≥ 27; E, n ≥ 400; all from three independent experiments; asterisk, P < 0.05)
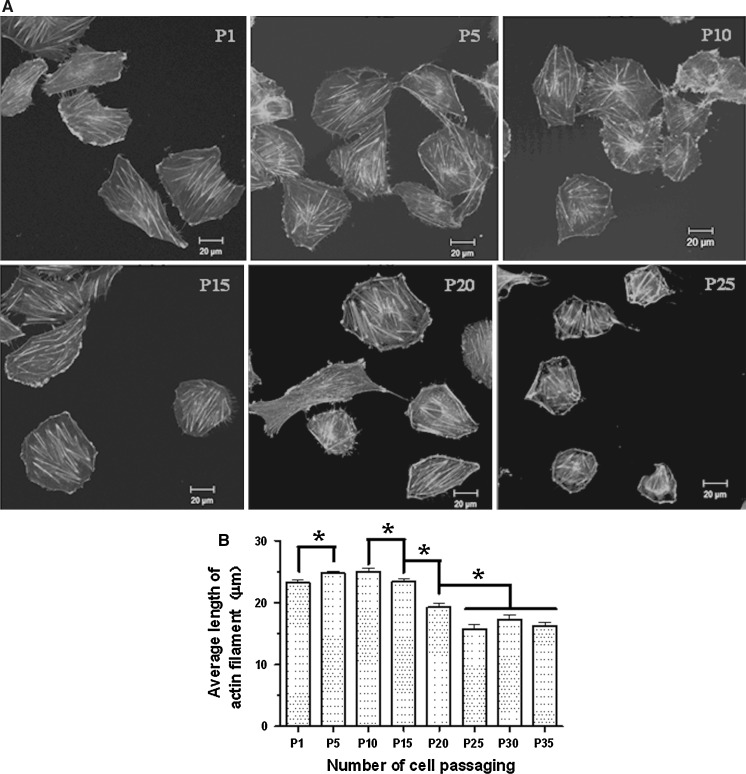
Two methods were recruited to evaluate the effects of cell passaging on cell spreading of HUVECs, percentage-based spreading assay and spread area-based spreading assay. Percentage-based spreading assay showed that the average percentages of spread cells before 10 passages were similar whereas the values gradually decreased after the 15th passage (Fig. 1d). Spread area-based spreading assay also showed a significant drop in the average spread area of HUVECs after the 15th passage although there was a temporary increase at passage 5 (Fig. 1e). Since actin cytoskeleton is the major driving force for cell spreading, the average length of actin filaments may indirectly reflect the status of cell spreading. So, filamentous actin cytoskeleton measurements were performed to confirm the data from the spreading assays. We used TRITC-conjugated phalloidin to label actin filaments (Fig. 2a) and quantify the average length of the filaments (Fig. 2b) since phalloidin selectively binds to F-actin. The actin cytoskeleton measurements also revealed a temporary increase in average length of actin filaments of HUVECs at passage 5 or 10 compared with the cells at passage 1 and gradual decreases after passage 15 (Fig. 2).
Fig. 2.
Effects of cell passaging on actin filaments of HUVECs. a Representative confocal images show the fluorescently labeled actin filaments in the cells at passages 1, 5, 10, 15, 20, and 25 (P1, P5, P10, P15, P20, and P25), respectively. b The average length of actin filaments at each passage. Values are expressed as mean ± SEM (n ≥ 350 from three independent experiments; asterisk, P < 0.05)
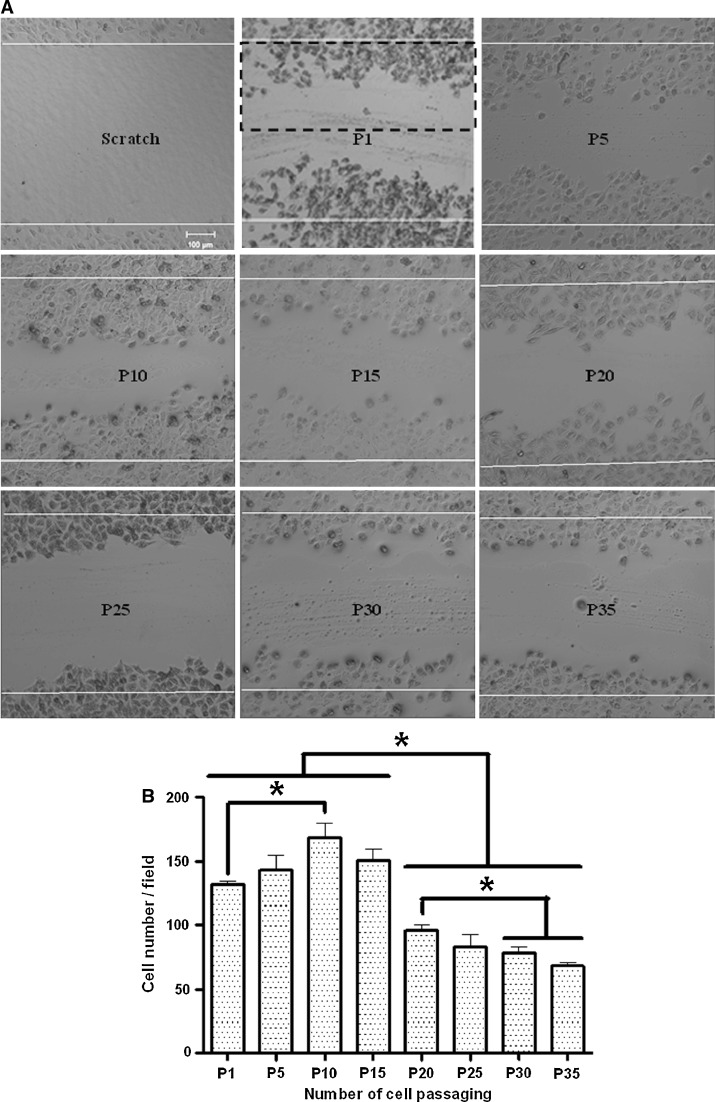
Then, we studied the effects of cell passaging on cell migration of HUVECs via the in vitro scratch assay (Fig. 3a). We found that the effects of cell passaging on cell migration were similar to the effects on cell spreading. The migration ability of HUVECs first increased before passage 10, and then started to decrease at passage 15 and dramatically dropped after passage 20 (Fig. 3b).
Fig. 3.
Effects of cell passaging on cell migration of HUVECs. a Representative confocal DIC images show the scratch (the first image) and the cells migrating into the scratch at passages 1, 5, 10, 15, 20, 25, 30, and 35 (P1, P5, P10, P15, P20, P25, P30, and P35, respectively). b The average number of migrating cells per field (the dashed rectangle indicates a field in the second image of the upper panel of a). Values are expressed as mean ± SEM (n ≥ 6 from three independent experiments; asterisk, P < 0.05)
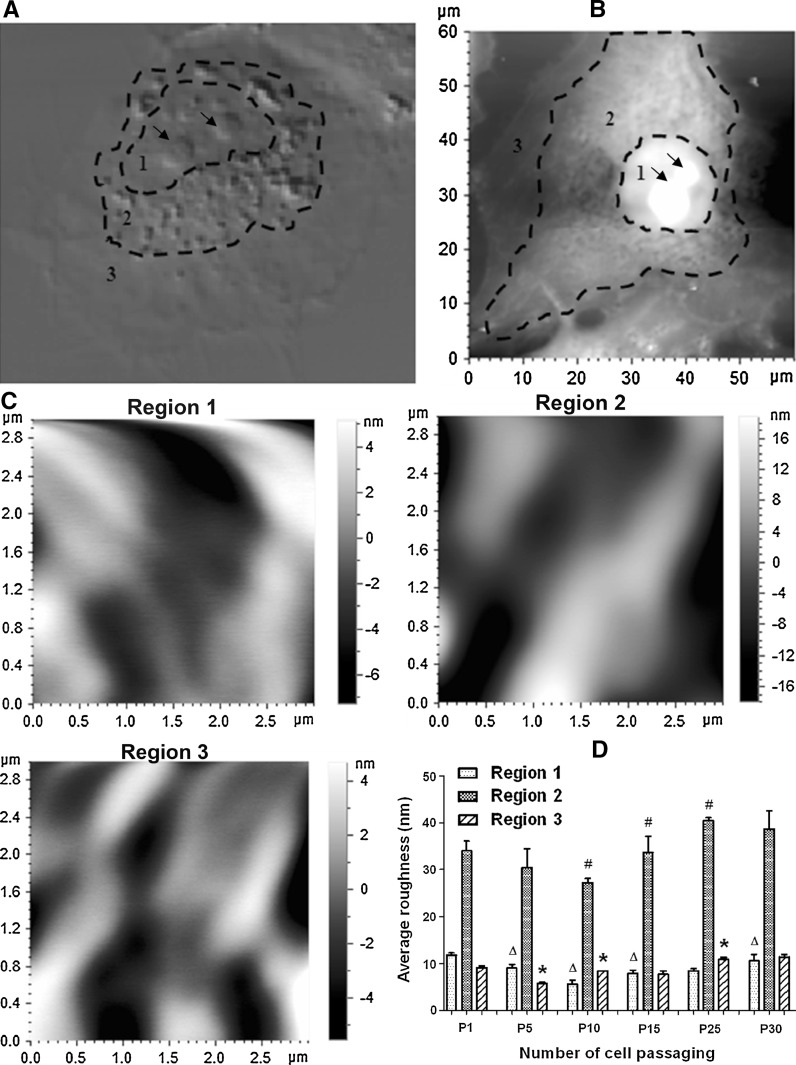
Next, we made use of our expertise of atomic force microscopy (AFM) (Chen 2012; Chen et al. 2011; Jin et al. 2011; Jin et al. 2012) to investigate the effects of cell passaging on cell-surface ultrastructures of HUVECs. Generally, there exist three regions with different degrees of roughness in the plasma membrane of a HUVEC cell (Shao et al. 2012): region 1 above the nucleus, region 2 surrounding the nucleus, and region 3 near cell boundary (Fig. 4a, b). Therefore, we utilized AFM to image the three regions (Fig. 4c) and measure the roughness of them separately (Fig. 4d). The statistical analysis indicated that region 2 was much rougher than the other two regions and that the average roughness value of region 2 first decreased and then went up dramatically with the increase in number of cell passaging (Fig. 4d). The changes in average roughness of region 1 or 3 also followed this pattern (Fig. 4d).
Fig. 4.
Effects of cell passaging on average cell-surface roughness of HUVECs. a A representative confocal DIC image shows the three regions with different degrees of roughness. b An AFM topographical image shows the three corresponding regions. c Representative AFM topographical images (3 μm × 3 μm) were enlarged from regions 1–3 of the cell in b, respectively. d The average roughness of each of the three regions on the cells at each passage (P1, P5, P10, P15, P25, or P30). During measurements of cell-surface roughness, the large protrusions in region 1 (as indicated by the arrows in a and b) were excluded since they were potentially caused by nucleoli. Values are expressed as mean ± SEM (n ≥ 6 from three independent experiments). triangle, ash, and asterisk correspond regions 1, 2, and 3, respectively, indicating the significant difference (P < 0.05) between a group and the previous group in average roughness of one of the three regions
Discussion
Serial cell passaging is an inevitable step during in vitro studies. Therefore, the effects of serial cell passaging on cellular structures or functions have been intensively evaluated, including expressions of intracellular or cell-surface or extracellular molecules, enzyme activities, oxidative stress, nitric oxide production, immunogenicity, proliferation, apoptosis, and related signaling pathways, as described in the Introduction section. Cell spreading and migration are important physiological behaviors of adherent cells, which are often evaluated during in vitro studies on cell biology, drug applications, cardiovascular tissue engineering applications, and others. However, the effects of serial cell passaging on cell spreading or migration have been less investigated directly.
Our study directly evaluated the effects of long-term serial cell passaging (ranging from passage 1 through passage 35) on cell spreading and migration via percentage-based spreading assay or spread area-based spreading assay and in vitro scratch assay, respectively. Interestingly, we found that both the spreading and migration abilities of HUVECs first increased slightly and then decreased dramatically with the increase in number of cell passaging. Since the cells at passage 0 were thawed out from an −80 °C freezer, under which condition cellular physiological activities are generally at a relatively low level, it might take some time (i.e. a couple of cell passaging) for the cells to recover. Therefore, the recovery of cellular physiological activities was potentially responsible for the slight increase in spreading and migration abilities of the cells at the early passages (<passage 10); and the dramatic decreases in cell spreading and migration after passage 15 were the actual effects of cell passaging. The dynamic changes in cell spreading during serial cell passaging were also confirmed by the data on the average length of actin filaments whose dynamic changes followed the same pattern. Besides filamentous actin cytoskeleton, alternations in expression of related molecules (such as adhesion molecules, extracellular molecules, cell-surface receptors, and others) might also contribute to the decreases in cell spreading and migration during cell passaging as aforementioned.
The effects of serial cell passaging on cell-surface ultrastructures have also been less evaluated. We investigated the dynamic changes in the average roughness of three different regions on cell surfaces during cell passaging. Surprisingly but interestingly, the changes in cell-surface roughness did not follow the same pattern as cell spreading and migration; in contrast, the average roughness of cell surfaces, particularly the average roughness of region 2, first decreased at the early passages and then rose after passage 15. In region 2, there were many cell-surface particles with sizes ranging from several hundreds nanometers to 1 micron, which seemed to be the so-called endothelial microparticles (EMPs) (Gyorgy et al. 2011), the membrane vesicles on endothelial cells. It has been reported that cold storage can induce increased membrane vesiculation (Bode and Knupp 1994). Moreover, in vitro aging, apoptosis, injury, and others have been found to promote the formation or release of endothelial microparticles (Bode et al. 1991; Chironi et al. 2009; Dignat-George and Boulanger 2011; Leroyer et al. 2010). Therefore, potentially by enhancing membrane vesiculation, cold storage and cell passaging might be responsible for the relatively high average roughness values before passage 5 and the gradual increases after passage 15, respectively.
Some previously published data also support our data from other physiological aspects. For example, it has been reported that most in vitro cultured endothelial cells reached senescence after approximately 10 passages for adult baboon endothelial cells (Shi et al. 2004) and after approximately 15 passages for human endothelial cells (Campisi 2001; Rubin 1997). A significant increase in acidic β-galactosidase expression of HUVECs and a reduction of telomere length in HUVECs were found after 11 passages (Vasa et al. 2000). While studying the different effects of various matrixes (gelatin-, fibrin-, and composite-coated polystyrene) on physiological functions of HUVECs at different passages, Prasad Chennazhy and Krishnan found that the physiological functions of HUVECs, including the monolayer formation, percentage of apoptotic cells, vWF expression, and others, changed dramatically by the 10th or 15th passage (Prasad Chennazhy and Krishnan 2005). Cresci et al. studied the expression of Ang II receptors (AT1 and AT2 receptors) in human glomerular endothelial cells at different passages (2, 4, 9, and 15p) and found that AT1 increased dramatically at the 9th and 15th passages whereas AT2 significantly decreased from the 9th passage (Cresci et al. 2003).
Taken together, ignoring the effects of cold storage, all data indicated that cell passaging significantly and dramatically influenced the spreading, migration, elongation of intracellular actin filaments, and cell-surface ultrastructures (or membrane vesiculation) of HUVECs after passage 15. It implies that the HUVEC cells at a passage of less than 10 are optimum for studies. If cells are taken from a freezer, the number of cell passaging is recommended to be 5–10 to avoid the influences of cold storage and cell passaging on cellular properties as much as possible. Moreover, our data provide important information for further understanding cell passaging.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (30900340 and 31260205), the Open Fund of the State Key Laboratory of Optoelectronic Materials and Technologies (Sun Yat-sen Unversity; KF2010-MS-07), and the Natural Science Foundation of Jiangxi Province (2010GZN0138).
Footnotes
Huanhuan Liao and Hui He contributed equally to this work.
References
- Acosta TJ, Yoshioka S, Komiyama J, Lee SH, Grazul-Bilska AT, Skarzynski DJ, Okuda K. Effects of storage and passage of bovine luteal endothelial cells on endothelin-1 and prostaglandin F2alpha production. J Reprod Dev. 2007;53:473–480. doi: 10.1262/jrd.18142. [DOI] [PubMed] [Google Scholar]
- Arkell J, Jackson CJ. Constitutive secretion of MMP9 by early-passage cultured human endothelial cells. Cell Biochem Funct. 2003;21:381–386. doi: 10.1002/cbf.1037. [DOI] [PubMed] [Google Scholar]
- Bode AP, Knupp CL. Effect of cold storage on platelet glycoprotein Ib and vesiculation. Transfusion. 1994;34:690–696. doi: 10.1046/j.1537-2995.1994.34894353465.x. [DOI] [PubMed] [Google Scholar]
- Bode AP, Orton SM, Frye MJ, Udis BJ. Vesiculation of platelets during in vitro aging. Blood. 1991;77:887–895. [PubMed] [Google Scholar]
- Brown ML, Deykin D. Passage state affects arachidonic acid content and eicosanoid release in porcine aortic endothelial cells. Arterioscler Thromb. 1991;11:167–173. doi: 10.1161/01.ATV.11.1.167. [DOI] [PubMed] [Google Scholar]
- Campisi J. From cells to organisms: can we learn about aging from cells in culture? Exp Gerontol. 2001;36:607–618. doi: 10.1016/S0531-5565(00)00230-8. [DOI] [PubMed] [Google Scholar]
- Chang MW, Grillari J, Mayrhofer C, Fortschegger K, Allmaier G, Marzban G, Katinger H, Voglauer R. Comparison of early passage, senescent and hTERT immortalized endothelial cells. Exp Cell Res. 2005;309:121–136. doi: 10.1016/j.yexcr.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Chen Y. Elucidation and identification of double-tip effects in atomic force microscopy studies of biological structures. J Surf Eng Mater Adv Technol. 2012;2:238–247. [Google Scholar]
- Chen Y, Zeng G, Chen SS, Feng Q, Chen ZW. AFM force measurements of the gp120-sCD4 and gp120 or CD4 antigen-antibody interactions. Biochem Biophys Res Commun. 2011;407:301–306. doi: 10.1016/j.bbrc.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chironi GN, Boulanger CM, Simon A, Dignat-George F, Freyssinet JM, Tedgui A. Endothelial microparticles in diseases. Cell Tissue Res. 2009;335:143–151. doi: 10.1007/s00441-008-0710-9. [DOI] [PubMed] [Google Scholar]
- Cresci B, Giannini S, Pala L, Mavilia C, Manuelli C, Cappugi P, Maggi E, Rotella CM. AT1 and AT2 receptors in human glomerular endothelial cells at different passages. Microvasc Res. 2003;66:22–29. doi: 10.1016/S0026-2862(03)00009-8. [DOI] [PubMed] [Google Scholar]
- Desai JK, Thompson MM, Eady SL, James RF, Bell PR. Immunomodulation of cultured vascular endothelial cells by serial cell passage. Eur J Vasc Endovasc Surg. 1995;10:101–107. doi: 10.1016/S1078-5884(05)80205-9. [DOI] [PubMed] [Google Scholar]
- Dignat-George F, Boulanger CM. The many faces of endothelial microparticles. Arterioscler Thromb Vasc Biol. 2011;31:27–33. doi: 10.1161/ATVBAHA.110.218123. [DOI] [PubMed] [Google Scholar]
- Galustian C, Dye J, Leach L, Clark P, Firth JA. Actin cytoskeletal isoforms in human endothelial cells in vitro: alteration with cell passage. In Vitro Cell Dev Biol Anim. 1995;31:796–802. doi: 10.1007/BF02634122. [DOI] [PubMed] [Google Scholar]
- Grillari J, Hohenwarter O, Grabherr RM, Katinger H. Subtractive hybridization of mRNA from early passage and senescent endothelial cells. Exp Gerontol. 2000;35:187–197. doi: 10.1016/S0531-5565(00)00080-2. [DOI] [PubMed] [Google Scholar]
- Gyorgy B, Szabo TG, Pasztoi M, Pal Z, Misjak P, Aradi B, Laszlo V, Pallinger E, Pap E, Kittel A, Nagy G, Falus A, Buzas EI. Membrane vesicles, current state-of-the-art: emerging role of extracellular vesicles. Cell Mol Life Sci. 2011;68:2667–2688. doi: 10.1007/s00018-011-0689-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin H, Ma S, Song B, Ma L, Pi J, Chen X, Chen Y, Cai J. Liposome impaired the adhesion and spreading of HEK293 cells: an AFM study. Scanning. 2011;33:413–418. doi: 10.1002/sca.20265. [DOI] [PubMed] [Google Scholar]
- Jin H, Pi J, Huang X, Huang F, Shao W, Li S, Chen Y, Cai J. BMP2 promotes migration and invasion of breast cancer cells via cytoskeletal reorganization and adhesion decrease: an AFM investigation. Appl Microbiol Biotechnol. 2012;93:1715–1723. doi: 10.1007/s00253-011-3865-3. [DOI] [PubMed] [Google Scholar]
- Kudo FA, Warycha B, Juran PJ, Asada H, Teso D, Aziz F, Frattini J, Sumpio BE, Nishibe T, Cha C, Dardik A. Differential responsiveness of early- and late-passage endothelial cells to shear stress. Am J Surg. 2005;190:763–769. doi: 10.1016/j.amjsurg.2005.07.017. [DOI] [PubMed] [Google Scholar]
- Kumazaki T, Wadhwa R, Kaul SC, Mitsui Y. Expression of endothelin, fibronectin, and mortalin as aging and mortality markers. Exp Gerontol. 1997;32:95–103. doi: 10.1016/S0531-5565(96)00080-0. [DOI] [PubMed] [Google Scholar]
- Lee MY, Sorensen GL, Holmskov U, Vanhoutte PM. The presence and activity of SP-D in porcine coronary endothelial cells depend on Akt/PI3 K, Erk and nitric oxide and decrease after multiple passaging. Mol Immunol. 2009;46:1050–1057. doi: 10.1016/j.molimm.2008.09.027. [DOI] [PubMed] [Google Scholar]
- Lee MY, Wang Y, Vanhoutte PM. Senescence of cultured porcine coronary arterial endothelial cells is associated with accelerated oxidative stress and activation of NFkB. J Vasc Res. 2010;47:287–298. doi: 10.1159/000265563. [DOI] [PubMed] [Google Scholar]
- Leroyer AS, Anfosso F, Lacroix R, Sabatier F, Simoncini S, Njock SM, Jourde N, Brunet P, Camoin-Jau L, Sampol J, Dignat-George F. Endothelial-derived microparticles: biological conveyors at the crossroad of inflammation, thrombosis and angiogenesis. Thromb Haemost. 2010;104:456–463. doi: 10.1160/TH10-02-0111. [DOI] [PubMed] [Google Scholar]
- Liang CC, Park AY, Guan JL. In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat Protoc. 2007;2:329–333. doi: 10.1038/nprot.2007.30. [DOI] [PubMed] [Google Scholar]
- Prasad Chennazhy K, Krishnan LK. Effect of passage number and matrix characteristics on differentiation of endothelial cells cultured for tissue engineering. Biomaterials. 2005;26:5658–5667. doi: 10.1016/j.biomaterials.2005.02.024. [DOI] [PubMed] [Google Scholar]
- Rubin H. Cell aging in vivo and in vitro. Mech Ageing Dev. 1997;98:1–35. doi: 10.1016/S0047-6374(97)00067-5. [DOI] [PubMed] [Google Scholar]
- Shao W, Jin H, Huang J, Qiu B, Xia R, Deng Z, Cai J, Chen Y (2012) AFM investigation on Ox-LDL-induced changes in cell spreading and cell-surface adhesion property of endothelial cells. Scanning. doi:10.1002/sca.21040 [DOI] [PubMed]
- Shi F, Audus KL. Biochemical characteristics of primary and passaged cultures of primate brain microvessel endothelial cells. Neurochem Res. 1994;19:427–433. doi: 10.1007/BF00967320. [DOI] [PubMed] [Google Scholar]
- Shi Q, Aida K, Vandeberg JL, Wang XL. Passage-dependent changes in baboon endothelial cells–relevance to in vitro aging. DNA Cell Biol. 2004;23:502–509. doi: 10.1089/1044549041562294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasa M, Breitschopf K, Zeiher AM, Dimmeler S. Nitric oxide activates telomerase and delays endothelial cell senescence. Circ Res. 2000;87:540–542. doi: 10.1161/01.RES.87.7.540. [DOI] [PubMed] [Google Scholar]
- Vasile E, Tomita Y, Brown LF, Kocher O, Dvorak HF. Differential expression of thymosin beta-10 by early passage and senescent vascular endothelium is modulated by VPF/VEGF: evidence for senescent endothelial cells in vivo at sites of atherosclerosis. FASEB J. 2001;15:458–466. doi: 10.1096/fj.00-0051com. [DOI] [PubMed] [Google Scholar]
- Zhang J, Patel JM, Block ER. Enhanced apoptosis in prolonged cultures of senescent porcine pulmonary artery endothelial cells. Mech Ageing Dev. 2002;123:613–625. doi: 10.1016/S0047-6374(01)00412-2. [DOI] [PubMed] [Google Scholar]