Abstract
The aim of the current study was to evaluate the cardioprotective ability of water (WE) and ethanolic (EE) papaya fruits extracts against cardiotoxicity induced by aflatoxin B1 (AFB1) in rats. Forty two female Sprague–Dawley rats were divided into six treatment groups and treated orally for 2 weeks as follow: control group, the group treated with WE (250 mg/kg b.w), the group treated with EE (250 mg/kg b.w), the group treated orally with AFB1 (17 μg/kg b.w) and the groups treated orally with AFB1 plus WE or EE. The results indicated that treatment with AFB1 resulted in oxidative stress in the heart manifested by the marked increase in cardiac malondialdehyde and calcium levels accompanied with a significant decrease in cardiac total antioxidant capacity. Serum nitric oxide and sodium levels, lactate dehydrogenase and creatine kinase isoenzyme activities were significantly increased, whereas, cardiac Na+/K+-ATPase activity and serum potassium were insignificantly affected. Supplementation with WE or EE effectively ameliorated most of the changes induced by AFB1. It could be concluded that both extracts attenuated the oxidative stress induced in heart tissue by AFB1 and WE was more pronounced due to the higher total phenolic contents than in the EE.
Keywords: Aflatoxin B1, Papaya, Heart, Oxidative stress, Antioxidant
Introduction
Aflatoxins (AFs) belong to a group of toxic and carcinogenic secondary metabolites produced by Aspergillus flavus and Aspergillus parasiticus (Rustom 1997; Bernabucci et al. 2011). AFs pose major health and economic problems worldwide, as evidenced by their frequent detection in food and agricultural commodities (Wild and Gong 2010). Aflatoxin B1 (AFB1) has been classified as a Group 1 carcinogen by the International Agency for Research on Cancer (IARC 2002). The biotransformation of AFB1 in the liver by microsomal enzymes results in the generation of toxic metabolites, including AFB1-8,9-epoxide. The toxic effects of AFs mostly arise from the binding of this particular epoxide derivative to DNA (Rawal et al. 2010). Reactive oxygen species such as the hydroxyl radical, superoxide anion and hydrogen peroxide, which are generated as a result of this metabolism, are also involved in the toxic mechanism of AFs (Choi et al. 2010; El-Agamy 2010). Ingestion of AFB1-contaminated food and feed is known to cause hepatotoxicity (Abdel-Wahhab et al. 2010), teratogenicity (Wangikar et al. 2005), immunotoxicity (IPCS-WHO 1998), kidney and heart damage (Abdulmajeed 2011) and even death in farm animals and humans (Guindon et al. 2007).
Heart diseases are the leading cause of death in developed countries. Experimental and clinical studies have shown that reactive oxygen species (ROS) are involved in the formation of lipid peroxides, damage of cell membrane and destruction of antioxidant capacity which thereby results in myocardial cell membrane destruction (Rajadurai and Prince 2007). Phytochemicals from fruits and vegetables have been shown to exert varied beneficial biological actions. For instance, flavonoids, the most common polyphenols found in plants, have been associated with the cardiovascular protective effects of various vegetables and fruits (Harborne and Williams 2000).
Papaya (Carica papaya L.) is a fruit that is well known for its nutritional and medicinal values. Papaya contains α-tocopherol (Ching and Mohamed 2001), lycopene (van Breemen and Pajkovic 2008), benzylisothiocyanate (Basu and Haldar 2008), proteolytic enzymes such as papain and chymopapain, cystatin, ascorbic acid, cyanogenic glucosides and glucosinolates (Seigler et al. 2002), alkaloids, such as carpain and carpasemine (Iyer et al. 2011), triterpenes, organic acids (Osuna-Torres et al. 2005) and flavonoids (Miean and Mohamed 2001). Moreover, the ethanolic extract (EE) is known to contain sulfurous compounds (benzyl isothiocyanate). Beside the total soluble phenols, Mahattanatawee et al. (2006) reported that papaya is rich in total ascorbic acid, total dietary fiber, and pectin. Moreover, Otsukia et al. (2010) reported that papaya is a good source of vitamins A, C, E and K as well as folate, in addition it is fat-free, cholesterol-free and low in sodium which suggests the potential beneficial effects. The current study was conducted to evaluate the protective role of water extract (WE) and ethanolic extract (EE) of Egyptian papaya fruit against AFB1-induced cardiotoxicity and in rats.
Materials and methods
Chemicals and kits
Aflatoxin B1 standards were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Kits for Creatine kinase isoenzyme (CK-MB) was purchased from Spinreact S.A. (Vall D’En Bas, Spain). Lactate dehydrogenase (LDH) kit was purchased from Stanbio Laboratory (Boerne, TX, USA). Total antioxidant capacity (TAC) and malondialdehyde (MDA) kits were purchased from Biodiagnostic Co. (Cairo, Egypt). Nitric oxide (NO) kit was purchased from R & D System GmbH (Wiesbaden, Germany). Calcium kit was purchased from Randox Laboratories LTD Co. (Antrim, UK). Sodium (Na+) potassium (K+) kits were purchased from Quimica Clinica Aplicada S.A. (Amposta, Spain). Other chemicals were of the highest purity commercially available.
Preparation of extracts
Carica papaya fruits were collected from a farm located in Qalubia region (Egypt). The fruits were identified in the Fruits Department, National Research Centre (NRC) and the voucher kept in the herbarium of NRC. The amount of plant used was 500 g. The fruits of papaya were peeled manually, cut into small pieces, dried and powdered. A crude WE was prepared by a maceration process (Rajkapoor et al. 2002). The filtrate was subjected to a lypholyzation process through freeze drier under pressure, 0.1–0.5 mbar and temperature −35 to −41 °C. The crude EE was prepared by soaking and stirring the powder in absolute ethanol (200 g/500 mL ethanol) for 3 days. The extract was filtered and the residue re-extracted twice using ethanol. The pooled extract was vacuum-dried at 40 °C using a rotary evaporator. Both the dry water and ethanolic extracts were stored at −20 °C until analysis. These procedures resulted in an approximate yield of 13 % (w/w) of EE and 27 % (w/w) of WE based on the dry weight.
Experimental animals
Adult female Sprague–Dawley rats (120–150 g) were purchased from the Animal House Colony of the National Research Centre (Cairo, Egypt). The environmental conditions were standardized with respect to temperature, humidity and light. All animals received human care in compliance with the standard institution’s criteria for the care and use of experimental animals. The safety measures recommended by WHO (1998) were taken when handling the AFB1.
Experimental design
The animals were distributed into six groups (7 rats/group) and orally treated daily for 2 weeks as follows: group 1, untreated control; group 2, rats treated orally with WE of C. papaya (250 mg/kg b.w) in corn oil; group 3, rats treated orally with EE of C. papaya (250 mg/kg b.w) in corn oil; group 4, rats were treated orally with AFB1 (17 μg/kg b.w) in corn oil; groups 5 and 6, rats treated orally with AFB1 and WE or EE, respectively. The animals were observed daily for signs of toxicity. At the end of the experimental period, rats were fasted overnight, fasting blood samples were collected from the retro-orbital venous plexus under diethyl ether anesthesia. The blood samples were left to clot and then centrifuged at 3,000 rpm for 15 min at 4 °C. The sera were separated and stored at −20 °C until the determination of creatine kinase isoenzyme (CK-MB) activity, lactate dehydrogenase (LDH) activity, nitric oxide (NO) level, sodium (Na+) and potassium (K+) concentration were determined according to the instructions of the kits.
After the collection of blood samples, all animals were rapidly sacrificed and the hearts were removed. The heart tissues were then immediately homogenized to give 10 % (w/v) homogenate in ice-cold buffer containing 50 mM Tris–HCl and 300 mM sucrose (pH 7.4) as described by Tsakiris et al. (2004). The homogenate was centrifuged at 3,000 rpm for 10 min in a cooling centrifuge at 0 °C. The supernatant (10 %) was used for the determination of Na+/K+-ATPase activity (Tsakiris et al. 2000), malondialdehyde (Ruiz-Larnea et al. 1994), total antioxidant capacity (Koracevic et al. 2001) and calcium levels (Barnett et al. 1973).
Statistical analysis
The data of the in vitro determinations were analyzed using the Student t test. The data of the in vivo study were subjected to one way analysis of variance (ANOVA). The analysis was performed using statistical analysis system (SAS) program software (copyright, 1998 by SAS Institute Inc., Cary, NC, USA). Duncan’s multiple range test was used to clarify the significance between the individual groups at probability level P ≤ 0.01 (Steel and Torrie 1960).
Results
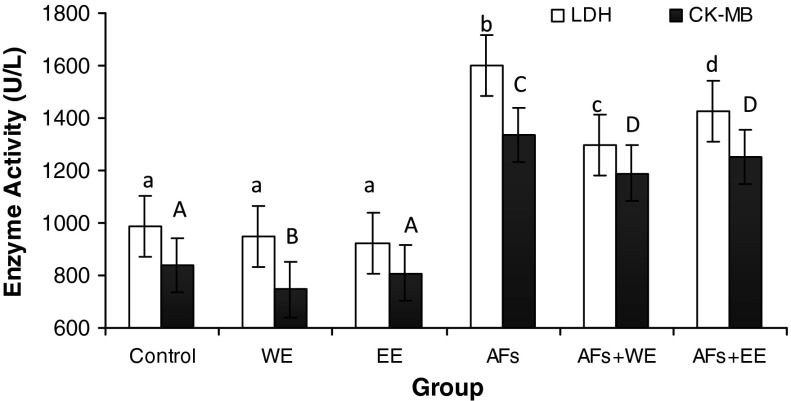
The results presented in Fig. 1 indicated that animals treated with AFB1 showed a significant increase in LDH and CK-MB activities compared to the control group or those treated with WE or EE alone. Animals treated with AFB1 and having received either WE or EE showed a significant improvement in the activity of LDA, however; CK-MB showed insignificant improvement. This improvement was more pronounced in the group treated with AFB1 and WE.
Fig. 1.
Effect of water (WE) and ethanolic (EE) extracts of papaya fruit on the activity of lactate dehydrogenase (LDH) and creatine kinase isoenzyme (CK-MB) in rats treated with AFB1 (Within each column, means superscript with different letters are significant differences at P ≤ 0.01.)
The current results also indicated that animals intoxicated with AFB1 showed significant increases in cardiac MDA and Ca++ levels accompanied with a significant decrease in TAC and ATPase (Table 1). Both extracts did not induce a significant effect on cardiac Ca++ levels and ATPase, however, they induced a significant decrease in MDA and a significant increase in TAC in cardiac tissue. Animals treated with AFB1 and treated with the extracts showed a significant improvement in all tested parameters in cardiac tissue. The data also showed that WE was more effective to reduce MDA, Ca++ levels and ATPase and normalized TAC in animals treated with AFB1. However, EE improved MDA and ATPase, increased TAC and normalized Ca++ levels in cardiac tissue of rats treated with AFB1 (Table 1).
Table 1.
Effect of water extract (WE) and ethanolic extract (EE) of papaya fruit on cardiac oxidant-antioxidant status and ATPase in rats treated with AFB1
| Groups parameters | Control | WE | EE | AFB1 | AFB1 + WE | AFB1 + EE |
|---|---|---|---|---|---|---|
| MDA (nmol/g) | 141.5 ± 8.25b | 88.5 ± 6.53c | 118.2 ± 13.63bc | 229.8 ± 6.0a | 104.7 ± 7.4bc | 115.5 ± 9.61bc |
| TAC (μmol/g) | 28.17 ± 1.82b | 44.50 ± 2.91a | 34.33 ± 3.50ab | 22.80 ± 2.0c | 27.50 ± 0.22bc | 35.0 ± 1.22ab |
| Ca++ (mg/g) | 31.2 ± 0.15ab | 32.7 ± 0.17ab | 32.6 ± 0.20ab | 36.8 ± 0.2a | 26.0 ± 0.07b | 31.5 ± 0.22ab |
| ATPase (μmol pi/h/g) | 304.0 ± 2.49a | 296.0 ± 3.54a | 310.8 ± 13.0a | 274.7 ± 12.30a | 286.2 ± 3.25a | 283.0 ± 2.88a |
Within each raw, means superscripts with different letters are significantly different compared to the control (P ≤ 0.01)
The data presented in Table 2 indicated that treatment with AFB1 resulted in a significant increase in serum NO level compared to the control or to the groups treated with the extracts alone. Treatment with WE alone induced a significant decrease in serum NO and K+ level, however; it did not induce a significant effect on serum Na+ level. On the other hand, treatment with EE alone resulted in a significant decrease in serum K+ level and Na+ level and did not induce a significant effect on serum NO compared to the control group. Animals treated with AFB1 and with the extracts showed a significant improvement in serum NO and K+ level, however; these extracts failed to normalize the serum Na+ level which was still lower compared to the control group.
Table 2.
Effect of water extract (WE) and ethanolic extract (EE) of papaya fruit on serum sodium (Na+), potassium (K+) and nitric oxide (NO) in rats treated with AFB1
| Groups Parameters | Control | WE | EE | AFB1 | AFB1 + WE | AFB1 + EE |
|---|---|---|---|---|---|---|
| NO (μmol/L) | 82.5 ± 3.28de | 66.7 ± 4.42e | 86.8 ± 2.60 cd | 139.6 ± 6.0a | 102.5 ± 4.0c | 122.0 ± 2.0b |
| Na+ (mmol/L) | 51.2 ± 2.70a | 48.0 ± 5.1ab | 42.3 ± 2.05abc | 36.8 ± 1.59c | 37.8 ± 1.38bc | 38.3 ± 2.86bc |
| K+ (mmol/L) | 5.24 ± 0.51ab | 3.34 ± 0.76b | 4.89 ± 0.31ab | 7.19 ± 0.32a | 5.50 ± 0.48ab | 6.70 ± 70.54a |
Within each raw, means superscripts with different letters are significantly different compared to the control (P ≤ 0.01)
Discussion
In the current study, we determined the cardiotoxicity of AFB1 and evaluated the protective role of the water and ethanolic extract of papaya fruits. The selected dose of AFB1 and the extracts were literature based (Abdel-Wahhab et al. 2006; Rajkapoor et al. 2002). It is well documented that oxidative stress is one of the important manifestation of AFB1 exposure. Increasing data suggest that physiological levels of ROS may play an important role in normal cell signaling. In contrast, under pathophysiological conditions, such as myocardial ischemia–reperfusion injury and cardiomyopathy, ROS production increases and exceeds the antioxidant defense of the cell. Thus, a large transient increase or a moderate sustained increase in ROS is suggested to be detrimental and to contribute to heart diseases and myocyte death (Madamanchi et al. 2005).
In the present study, CK-MB and LDH levels increased significantly in AFB1-treated animals which indicates the presence of damaging impact and toxicity to cardiac tissue and agrees with the previous reports suggesting that exposure to AFB1 causes heart defects (Pasha et al. 2007; Abdulmajeed 2011). CK-MB is the most sensitive and specific indicator available for the diagnosis of damage to the heart. The mechanism for the release of this marker into circulation seems to be from ventricular remodeling, ongoing cardiomyocyte degeneration, the presence of coronary artery disease and reduced coronary reserve (Potluri et al. 2004). LDH is considered as indicator of myocardial infarction. It is found in the cytoplasm of almost all body tissues, where its main function is to catalyze the oxidation of l-lactate to pyruvate and it is assayed as a measure of anaerobic carbohydrate metabolism (Yee et al. 2003).
Aflatoxin B1 induces oxidative stress in heart as indicated by the elevation of cardiac MDA concomitant with the significant decrease in tissue TAC. Several reports suggested that AFB1 exerts its toxicity through the formation of AFB1 8,9-epoxide and the generation of intracellular ROS, like superoxide anion, hydroxyl radical and hydrogen peroxide (Berg et al. 2004) during the metabolic processing of AFB1 by cytochrome P450 in the liver (Galvano et al. 2001). These species may attack the highly unsaturated fatty acids of the cell membrane of the heart to induce lipid peroxidation and eventually damage membranes (Schinella et al. 2002) and the subsequent release of its contents into circulation. It is well known that cardiomyocytes are particularly susceptible to attack by free radicals because the activity of antioxidant defense mechanism is lower than in other tissues (Singal and Kirshenbaum 1990). This may explain the observed elevation of cardiac lipid peroxidation marker (MDA) and the decrement of cardiac TAC in the animals treated with AFB1 alone in the current study.
Furthermore, AFB1 increased serum NO and cardiac calcium level. It has been reported that AFB1 induces the production of NO through macrophage activation (Abdel-Wahhab et al. 2006). NO is a simple inorganic gaseous free radical. It is an important signaling molecule synthesized in endothelial cells and macrophages during the conversion of l-arginine to citrulline by nitric oxide synthase (Knowles and Moncada 1994). It plays an important role in the regulation of cardiac function (Danson et al. 2005). In addition to activating cyclic guanosine monophosphate (cGMP)-dependent signaling pathways, NO can directly modify sulfhydryl residues of proteins through S-nitrosylation, which has emerged as an important posttranslational protein modification based on prototypic redox mechanisms in signal transduction (Hess et al. 2005). Increased oxidative stress and the resultant dysregulation of NO are implicated in the pathogenesis of cardiovascular diseases. As nitrosative stress occurs with an increase in reactive nitrogen species (RNS) and ROS formed from oxidative stress where the peroxynitrite (OONO−), generated from NO and superoxide, is a very strong cytotoxic oxidant, which can irreversibly damage cells by oxidation of free thiols, nitration of tyrosine residues and lipid peroxidation (Huang et al. 2001).
In cardiac myocytes, ROS and RNS induce stress-signaling pathways involved in mitochondrial dysfunction and intracellular Ca2+ overload (Ungvari et al. 2005). Ca2+ is an important mediator of cell injury. It has been reported that an increased intracellular calcium level activates a number of enzymes with potential deleterious cellular effects. Moreover, increased intracellular Ca2+ levels resulted in increased mitochondrial permeability and induction of apoptosis (Ungvari et al. 2005; Park et al. 2008). ATPases are a class of enzymes that catalyzes the decomposition of adenosine triphosphate (ATP) into adenosine diphosphate (ADP) and a free phosphate ion. Na+/K+-ATPase (also known as the Na+/K+ pump) is an enzyme located in the plasma membrane (specifically an electrogenic transmembrane ATPase) in all animals (Tian et al. 2006). The active transport of sodium–potassium across the cell membrane is controlled by Na+/K+-ATPase enzyme, which is an integral plasma membrane protein responsible for a large part of the energy consumption constituting the cellular metabolic rate (Dixon et al. 1971). Na+/K+-ATPase pump which is fueled by oxidative energy continually transports K+ cation into the cell against the concentration gradient. This pump is a critical factor in controlling intracellular volume, maintaining and adjusting the ionic gradients on which nerve impulse transmission and contractility of cardiac and skeletal muscle depend (Scott et al. 1999). This suggestion is supported by the results of Abdulmajeed (2011) who demonstrated that aflatoxicosis interferes with the cellular energy supply of rat hearts through its inhibitory action on some markers of the energy metabolism indicated by a decrease in glucose and glycogen contents of the heart tissue and a reduction in the activities of some glycolytic enzymes such as phosphogluco-isomerase (PGI) and glyceraldehyde 3-phosphate dehydrogenase.
Another mechanism for AFB1-induced cardiotoxicity that might be taken into consideration is the toxic action of AFB1 on liver function which leads to a disturbance in the lipid metabolism resulting in increased levels of total cholesterol, triglycerides and low density lipoprotein in the blood stream (Abdel-Wahhab et al. 2006, 2010). This increment showed that aflatoxin may reduce the rate of cholesterol and triglycerides catabolism and increase the risk for atherosclerosis. This suggestion is asserted by the results of Tamer et al. (2002) who found a significant positive correlation between the concentration of both cholesterol and LDL in patients with myocardial infarction.
In the current study, both ethanolic and water papaya extracts offered protection against the oxidative stress caused by AFB1 intoxication. This appeared from the restoration of the endogenous antioxidants, and decreased MDA and NO formation that were significantly altered by AFB1 intoxication, suggesting the antioxidant potentials of these extracts. The free radical trapping capacity of papaya extracts may reside mainly in their phenolic contents. These compounds possibly prevent ROS from acting on the macromolecules such as DNA, lipids and proteins suggesting a possible role of these extracts as a chain breaking antioxidant against lipid peroxidation. This in turn stabilizes the membrane permeability of cardiomyocytes and consequently prevents the excessive release of CK-MB and LDH enzymes into circulation, decreases the dramatic elevation of Ca+2 in cardiomyocytes and keeps normal Na+/K+-ATPase activity which controls the active transport of sodium and potassium across the cell membrane.
Noteworthy, the antioxidant activity of the water extract in the current study is more pronounced than that of the ethanolic extract. Perhaps this is due to the compositional difference of phenolics in the extracts (Harborne and Williams 2000). Calliste et al. (2001), suggested that phenolic compounds are distributed in different solvents according to the function of polarity. Based on this reason, water extract contains the most polar compounds such as triglycosyl flavonoids and high molecular weight compounds. These facts might explain the stronger scavenging and antioxidant activity of water extract compared with ethanolic extract of papaya.
It is well known that most phenolic compounds present in fruits and vegetables exhibit some level of antioxidant capacity (Harborne and Williams 2000). They appear to be able to regulate antioxidant enzyme gene transcription and intercellular signalling cascades involved in the regulation of cell growth, inflammation and many other processes (Stangeland et al. 2009). Additionally, several reports have demonstrated that the phenolic compounds exhibit antioxidant, anti-inflammatory, anti-mutagenic, anti-carcinogenic, anti-bacterial properties (Negi et al. 2008; Fresco et al. 2010) (Jeong et al. 2010; Jayakumar and Kanthimathi 2011) and possess hypotensive actions (Eno et al. 2000) which are in agreement with their vasorelaxant effects (Runnie et al. 2004).
Conclusion
The current study indicated that both WE and EE extracts have potential protective effects against AFB1-induced cardiotoxicity via diverse mechanisms at different cellular processes. The cardioprotective activities of the water extract were stronger than ethanolic extract. The potency of these extracts could provide a chemical basis for some of the health benefits claimed for papaya extracts in folk medicine against cardiovascular diseases.
Acknowledgments
This work was supported by National Research Center, Dokki, Cairo, Egypt project # S90402.
Conflict of interest
The authors declare that there is no conflict of interest.
References
- Abdel-Wahhab MA, Ahmed HH, Hagazi MM. Prevention of aflatoxin B1-initiated hepatotoxicity in rat by marine algae extracts. J Appl Toxicol. 2006;26:229–238. doi: 10.1002/jat.1127. [DOI] [PubMed] [Google Scholar]
- Abdel-Wahhab MA, Hassan NS, El-Kady AA, Khadrawy YA, El-Nekeety AA, Mohamed SR, Sharaf HA, Mannaa FA. Red ginseng extract protects against aflatoxin B1 and fumonisins-induced hepatic pre-cancerous lesions in rats. Food Chem Toxicol. 2010;48:733–742. doi: 10.1016/j.fct.2009.12.006. [DOI] [PubMed] [Google Scholar]
- Abdulmajeed NA. Therapeutic ability of some plant extracts on aflatoxin B1 induced renal and cardiac damage. Arab J Chem. 2011;4:1–10. doi: 10.1016/j.arabjc.2010.06.005. [DOI] [Google Scholar]
- Barnett RN, Skodon SB, Goldberg NH. Performance of “kits” used for clinical chemical analysis of calcium in serum. Am J Clin Pathol. 1973;59:836–845. doi: 10.1093/ajcp/59.6.836. [DOI] [PubMed] [Google Scholar]
- Basu A, Haldar S. Dietary isothiocyanate mediated apoptosis of human cancer cells is associated with Bcl-xL phosphorylation. Int J Oncol. 2008;33:657–663. [PubMed] [Google Scholar]
- Berg D, Youdim MB, Riederer P. Redox imbalance. Cell Tissue Res. 2004;318:201–213. doi: 10.1007/s00441-004-0976-5. [DOI] [PubMed] [Google Scholar]
- Bernabucci U, Colavecchia L, Danieli PP, Basirico L, Lacetera N, Nardone A, Ronchi B. Aflatoxin B1 and fumonisin B1 affect the oxidative status of bovine peripheral blood mononuclear cells. Toxicol In Vitro. 2011;25:684–691. doi: 10.1016/j.tiv.2011.01.009. [DOI] [PubMed] [Google Scholar]
- Calliste CA, Trouillas P, Allais DP, Simon A, Duroux JL. Free radical scavenging activities measured by electron spin resonance spectroscopy and B16 cell antiproliferative behaviors of seven plants. J Agric Food Chem. 2001;49:3321–3327. doi: 10.1021/jf010086v. [DOI] [PubMed] [Google Scholar]
- Ching LS, Mohamed S. Alpha-tocopherol content in 62 edible tropical plants. J Agric Food Chem. 2001;49:3101–3105. doi: 10.1021/jf000891u. [DOI] [PubMed] [Google Scholar]
- Choi KC, Chung WT, Kwon JK, Yu JY, Jang YS, Park SM, Lee SY, Lee JC. Inhibitory effects of quercetin on aflatoxin B1-induced hepatic damage in mice. Food Chem Toxicol. 2010;48:2747–2753. doi: 10.1016/j.fct.2010.07.001. [DOI] [PubMed] [Google Scholar]
- Danson EJ, Choate JK, Paterson DJ. Cardiac nitric oxide: emerging role of nNOS in regulating physiological function. Pharmacol Ther. 2005;106:57–74. doi: 10.1016/j.pharmthera.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Dixon MF, Nimmo J, Prescott LF. Experimental paracetamol-induced hepatic necrosis: a histopathological study. J Pathol. 1971;103:225–229. doi: 10.1002/path.1711030404. [DOI] [PubMed] [Google Scholar]
- El-Agamy DS. Comparative effects of curcumin and resveratrol on aflatoxin B1-induced liver injury in rats. Arch Toxicol. 2010;84:389–396. doi: 10.1007/s00204-010-0511-2. [DOI] [PubMed] [Google Scholar]
- Eno AE, Owo OI, Itam EH, Konya RS. Blood pressure depression by the fruit juice of Carica papaya (L.) in renal and DOCA-induced hypertension in the rat. Phytother Res. 2000;14:235–239. doi: 10.1002/1099-1573(200006)14:4<235::AID-PTR574>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Fresco P, Borges F, Marques MP, Diniz C. The anticancer properties of dietary polyphenols and its relation with apoptosis. Curr Pharm Des. 2010;16:114–134. doi: 10.2174/138161210789941856. [DOI] [PubMed] [Google Scholar]
- Galvano F, Piva A, Ritieni T, Galvano G. Dietary strategies to counteract the effects of mycotoxins: a review. J Food Protect. 2001;64:120–131. doi: 10.4315/0362-028x-64.1.120. [DOI] [PubMed] [Google Scholar]
- Guindon KA, Bedard LL, Massey TE. Elevation of 8-hydroxydeoxyguanosine in DNA from isolated Mouse lung cells following in vivo treatment with aflatoxin B1. Toxicol Sci. 2007;98:57–62. doi: 10.1093/toxsci/kfm073. [DOI] [PubMed] [Google Scholar]
- Harborne JB, Williams CA. Advances in flavonoid research since 1992. Phytochem. 2000;55:481–504. doi: 10.1016/S0031-9422(00)00235-1. [DOI] [PubMed] [Google Scholar]
- Hess DT, Matsumoto A, Kim SO, Marshall HE, Stamler JS. Protein S-nitrosylation: purview and parameters. Nat Rev Mol Cell Biol. 2005;6:150–166. doi: 10.1038/nrm1569. [DOI] [PubMed] [Google Scholar]
- Huang KT, Han TH, Hyduke DR, Vaughn MW, Van Herle H, Hein TW, Zhang C, Kuo L, Liao JC. Modulation of nitric oxide bioavailability by erythrocytes. Proc Natl Acad Sci USA. 2001;98:11771–11776. doi: 10.1073/pnas.201276698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IARC Some traditional herbal medicines, some mycotoxins, naphthalene and styrene, IARC monographs on the evaluation of carcinogenic Risks to Humans. IARC. 2002;82:171–300. [PMC free article] [PubMed] [Google Scholar]
- IPCS-WHO (1998) Aflatoxins. In: WHO (ed) International programme on chemical safety, food additives, series 40. World Health Organization, Geneva, pp 1–73
- Iyer D, Sharma BK, Patil UK. Effect of ether- and water-soluble fractions of Carica papaya ethanol extract in experimentally induced hyperlipidemia in rats. Pharm Biol. 2011;49:1306–1310. doi: 10.3109/13880209.2011.596210. [DOI] [PubMed] [Google Scholar]
- Jayakumar R, Kanthimathi MS. Inhibitory effects of fruit extracts on nitric oxide-induced proliferation in MCF-7 cells. Food Chem. 2011;126:956–960. doi: 10.1016/j.foodchem.2010.11.093. [DOI] [Google Scholar]
- Jeong JH, Jung H, Lee S, Lee H, Hwang KT, Kim T. Anti-oxidant, anti-proliferative and anti-inflammatory activities of the extracts from black raspberry fruits and wine. Food Chem. 2010;123:338–344. doi: 10.1016/j.foodchem.2010.04.040. [DOI] [Google Scholar]
- Knowles RG, Moncada S. Nitric oxide synthases in mammals. Biochem J. 1994;298:249–258. doi: 10.1042/bj2980249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koracevic D, Koracevic G, Djordjevic V, Andrejevic S, Cosic V. Method for the measurement of antioxidant activity in human fluids. J Clin Pathol. 2001;54:356–361. doi: 10.1136/jcp.54.5.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madamanchi NR, Vendrov A, Runge MS. Oxidative stress and vascular disease. Arterioscler Thromb Vasc Biol. 2005;25:29–38. doi: 10.1161/01.ATV.0000161050.77646.68. [DOI] [PubMed] [Google Scholar]
- Mahattanatawee K, Manthey JA, Luzio G, Talcott S, Goodner KL, Baldwin EA. Total antioxidant activity and fiber content of select Florida-grown tropical fruits. J Agric Food Chem. 2006;54:7355–7363. doi: 10.1021/jf060566s. [DOI] [PubMed] [Google Scholar]
- Masuda Y, Yano I, Sumida S, Murano T. Studies on the function of cell membrane. 10th report: effects of CCl4 on the marker enzyme activities and fine structures of rat liver plasma membranes and microsomes in vitro. J Pharmacol. 1975;25:151–160. [Google Scholar]
- Miean KH, Mohamed S. Flavonoid (myricetin, quercetin, kaempferol, luteolin, and apigenin) content of edible tropical plants. J Agric Food Chem. 2001;49:3106–3112. doi: 10.1021/jf000892m. [DOI] [PubMed] [Google Scholar]
- Negi PS, Jayaprakasha GK, Jena BS. Antibacterial activity of the extracts from the fruit rinds of Garcinia cowa and Garcinia pedunculata against food borne pathogens and spoilage bacteria. LWT-Food Sci Technol. 2008;41:1857–1861. doi: 10.1016/j.lwt.2008.02.009. [DOI] [Google Scholar]
- Osuna-Torres L, Tapia-Pérez ME, Aguilar-Contreras A. Plantas medicinales de la medicina tradicional mexicana para tratar afecciones gastrointestinales: Estudio etnobotánico fitoquımico y farmacológico. Barcelona: Universidat de Barcelona; 2005. [Google Scholar]
- Otsukia N, Dangb NH, Kumagaia E, Kondoc A, Iwataa S, Morimotoa C. Aqueous extract of Carica papaya leaves exhibits anti-tumor activity and immunomodulatory effects. J Ethnopharmacol. 2010;127:760–767. doi: 10.1016/j.jep.2009.11.024. [DOI] [PubMed] [Google Scholar]
- Park KH, Kim SY, Gul R, Kim BJ, Jang KY, Chung HT, Sohn DH. Fatty acids ameliorate doxorubicin-induced intracellular Ca2+ increase and apoptosis in rat cardiomyocytes. Biol Pharma Bull. 2008;31:809–815. doi: 10.1248/bpb.31.809. [DOI] [PubMed] [Google Scholar]
- Pasha TN, Farooq MU, Khattak FM, Jabbar MA, Khan AD. Effectiveness of sodium bentonite and two commercial products as aflatoxin absorbents in diets for broiler chickens. Anim Feed Sci Technol. 2007;132:103–110. doi: 10.1016/j.anifeedsci.2006.03.014. [DOI] [Google Scholar]
- Potluri S, Ventura HO, Mulumudi M, Mehra MR. Cardiac troponin levels in heart failure. Cardiol Rev. 2004;12:21–25. doi: 10.1097/01.crd.0000089981.53961.cf. [DOI] [PubMed] [Google Scholar]
- Rajadurai M, Prince PS. Preventive effect of naringin on isoproterenol-induced cardiotoxicity in Wistar rats: an in vivo and in vitro study. Toxicology. 2007;232:216–225. doi: 10.1016/j.tox.2007.01.006. [DOI] [PubMed] [Google Scholar]
- Rajkapoor B, Jayakar B, Kavimani S, Murugesh N. Effect of dried fruits of Carica papaya linn on hepatotoxicity. Biol Pharm Bull. 2002;25:1645–1646. doi: 10.1248/bpb.25.1645. [DOI] [PubMed] [Google Scholar]
- Rawal S, Kim JE, Coulombe R. Aflatoxin B1 in poultry: toxicology, metabolism and prevention. Res Vet Sci. 2010;89:325–331. doi: 10.1016/j.rvsc.2010.04.011. [DOI] [PubMed] [Google Scholar]
- Ruiz-Larnea MB, Leal AM, Liza M, Lacort M, deGroot H. Antioxidant effects of estradiol and 2-hydroxyestradiol on iron induced lipid peroxidation of rat liver microsome. Steroid. 1994;59:383–388. doi: 10.1016/0039-128X(94)90006-X. [DOI] [PubMed] [Google Scholar]
- Runnie I, Salleh MN, Mohamed S, Head RJ, Abeywardena MY. Vasorelaxation induced by common edible tropical plant extracts in isolated rat aorta and mesenteric vascular. J Ethnopharmacol. 2004;92:311–316. doi: 10.1016/j.jep.2004.03.019. [DOI] [PubMed] [Google Scholar]
- Rustom IYS. Aflatoxin in food and feed: occurrence, legislation and inactivation by physical methods. Food Chem. 1997;59:57–67. doi: 10.1016/S0308-8146(96)00096-9. [DOI] [Google Scholar]
- Schinella GR, Tournier HA, Prieto JM, Mordujovich de Buschiazzo P, Rios JL. Antioxidant activity of anti-inflammatory plant extracts. Life Sci. 2002;70:1023–1033. doi: 10.1016/S0024-3205(01)01482-5. [DOI] [PubMed] [Google Scholar]
- Scott MG, Heusel JW, LeGrys VA, Siggard-Anderson O. Renal electrolytes and blood gases. In: Burtis CA, Ashwood ER, editors. Tietz textbook of clinical chemistry. Philadelphia: W.B. Sawnders Company; 1999. pp. 1056–1092. [Google Scholar]
- Seigler DS, Pauli GF, Nahrstedt A, Leen R. Cyanogenic allosides and glucosides from Passiflora edulis and Carica papaya. Phytochem. 2002;60:873–882. doi: 10.1016/S0031-9422(02)00170-X. [DOI] [PubMed] [Google Scholar]
- Singal PK, Kirshenbaum LA. A relative deficit in antioxidant reserve may contribute in cardiac failure. Can J Cardiol. 1990;6:47–49. [PubMed] [Google Scholar]
- Stangeland T, Siv F, Remberg SF, Kare A, Lye KA. Total antioxidant activity in 35 Ugandan fruits and vegetables. Food Chem. 2009;113:85–91. doi: 10.1016/j.foodchem.2008.07.026. [DOI] [Google Scholar]
- Steel RG, Torrie GH. Principles and procedures of statistics and biometrical approach. 2. New York: McGraw-Hill Book Co.; 1960. pp. 71–117. [Google Scholar]
- Tamer L, Ercan B, Unlu A, Sucu N, Pekdemir H, Eskandari G, Atik U. The relationship between leptin and lipids in atherosclerosis. Indian Heart J. 2002;54:692–696. [PubMed] [Google Scholar]
- Tian J, Cai T, Yuan Z, Wang H, Liu L, Haas M, Maksimova E, Huang XY, Xie ZJ. Binding of Src to Na+/K+-ATPase forms a functional signaling complex. Mol Biol Cell. 2006;17:317–326. doi: 10.1091/mbc.E05-08-0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsakiris S, Angelogianni P, Schulpis KH, Behrakis P. Protective effect of l-cysteine and glutathione on rat brain Na+/K+-ATPase inhibition induced by free radical. Z Naturforsch C. 2000;55:271–277. doi: 10.1515/znc-2000-3-421. [DOI] [PubMed] [Google Scholar]
- Tsakiris S, Schulpis KH, Marinou K, Behrakis P. Protective effect of l-cysteine and glutathione on the modulated sukling rat brain Na+/K+-ATPase and Mg2+-ATPase activities induced by the in vitro gulactosaemia. Pharmacol Res. 2004;49:475–479. doi: 10.1016/j.phrs.2003.11.006. [DOI] [PubMed] [Google Scholar]
- Ungvari Z, Gupte SA, Recchia FA, Batkai S, Pacher P. Role of oxidative-nitrosative stress and downstream pathways in various forms of cardiomyopathy and heart failure. Curr Vasc Pharmacol. 2005;3:221–229. doi: 10.2174/1570161054368607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Breemen RB, Pajkovic N. Multitargeted therapy of cancer by lycopene. Cancer Lett. 2008;269:339–351. doi: 10.1016/j.canlet.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wangikar PB, Dwivedi P, Sinha N, Sharma AK, Telang AG. Teratogenic effects in rabbits of simultaneous exposure to ochratoxin A and aflatoxin B1 with special reference to microscopic effects. Toxicology. 2005;215:237–247. doi: 10.1016/j.tox.2005.06.022. [DOI] [PubMed] [Google Scholar]
- Forty-ninth report of the Joint FAO/WHO Expert Committee on Food Additives. WHO Technical Report Series. Geneva: WHO; 1998. [Google Scholar]
- Wild CP, Gong YY. Mycotoxins and human disease: a largely ignored global health issue. Carcinogenesis. 2010;31:71–82. doi: 10.1093/carcin/bgp264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee KC, Mukherjee D, Smith DE, Kline-Rogers EM, Fang J, Mehta RH, Almanaseer Y, Akhras E, Cooper JV, Eagle KA. Prognostic significance of an elevated creatine kinase in the absence of an elevated troponin I during an acute coronary syndrome. Am J Cardiol. 2003;92:1442–1444. doi: 10.1016/j.amjcard.2003.08.055. [DOI] [PubMed] [Google Scholar]